Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Inhaled Corticosteroid Treatment Regimens and Health Outcomes in a UK COPD Population Study
Authors Bloom CI , Douglas I, Usmani OS , Quint JK
Received 9 December 2019
Accepted for publication 10 March 2020
Published 2 April 2020 Volume 2020:15 Pages 701—710
DOI https://doi.org/10.2147/COPD.S241568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Chloe I Bloom, 1 Ian Douglas, 2 Omar S Usmani, 1 Jennifer K Quint 1
1National Heart Lung Institute, Imperial College London, London SW3 6LR, UK; 2London School of Hygiene and Tropical Medicine, London, UK
Correspondence: Chloe I Bloom
National Heart Lung Institute, Imperial College London, Emmanuel Kaye Building, 1b Manresa Road, London SW3 6LR, UK
Email [email protected]
Background: Inhaled corticosteroids (ICS) are a prevailing treatment option for COPD patients but recent guidelines have relegated their use predominantly to patients with frequent exacerbations. Yet large numbers of patients worldwide are currently treated with ICS-containing regimens. We wished to determine in routine clinical practice how common ICS withdrawal is and the differences in health outcomes between patients managed on ICS-containing and non-ICS containing regimens.
Patients and Methods: COPD patients were identified from the UK primary care electronic healthcare records, between 2014 and 2018. Patients were grouped into three treatment regimens: long-acting beta-agonist (LABA) and inhaled corticosteroids (ICS), LABA and long-acting muscarinic antagonist (LAMA), and triple therapy (LABA, LAMA and ICS). Annual incidence of ICS withdrawal was measured. Multivariable logistic regression was used to identify patient factors associated with withdrawal. Multivariable Poisson regression was used to assess the association of exacerbations and hospitalised pneumonia between the ICS-containing regimens (LABA-ICS and triple therapy) and patients prescribed LABA-LAMA.
Results: Of 117,046 patients, around three-quarters were prescribed ICS-containing inhalers but ICS withdrawal occurred annually in only approximately 2– 3% of patients. Exacerbations in the past year, but not a past history of pneumonia, were associated with ICS withdrawal. A total of 31,034 patients using three treatment regimens (LABA-ICS, LABA-LAMA or triple therapy) were assessed for their relative risk of exacerbations and pneumonia; the exacerbation risk was slightly lower in LABA-ICS users but the same in triple therapy users, as compared to LABA-LAMA users (LABA-ICS adjusted IRR=0.82 (95% CI 0.73– 0.93), triple adjusted IRR=0.99 (95% CI 0.88– 1.11)). There was no difference in the pneumonia risk (LABA-ICS adjusted IRR=0.96 (95% CI 0.71– 1.31), triple adjusted IRR=1.16 (95% CI 0.87– 1.57)).
Conclusion: Use of ICS-containing inhaled medication is prevalent across the UK while ICS withdrawal from established treatment was relatively uncommon. Exacerbations and pneumonia risk was similar between the ICS-containing and non-ICS containing treatment regimens.
Keywords: inhalers, real world, corticosteroids, primary care
Corrigendum for this paper has been published
Introduction
The mainstay of pharmacological treatment of COPD is long-term inhaled medication. A key goal is to reduce the frequency and severity of exacerbations. Beyond the short-acting bronchodilators, there are three classes of inhaled medication: long-acting beta-agonists (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends different inhaled treatment depending on the patient's lung function, recent exacerbation history and current symptoms.1 In 2017, GOLD had their five-year major revision and proposed that when regular monotherapy with a long-acting bronchodilator is insufficient, a LABA–LAMA combination should be prescribed first-line in preference over ICS-LABA (unless a patient has asthma or asthma/COPD overlap).2 ICS should only be added to long-acting bronchodilators in patients with frequent exacerbations. However, in real life, inhaler prescribing practices are known to be out of synchrony with guideline recommendations leading to many COPD patients worldwide being prescribed ICS, often without clinical indication.3–8
The two major concerns over ICS use are their efficacy to reduce exacerbations, over long-acting bronchodilators, and their long-term safety profile. Evidence from randomised-controlled trials suggests that LABA-ICS is not superior to LABA-LAMA but may increase the risk of pneumonia.9–11 Although trials have shown triple therapy does offer some advantage over LABA-LAMA, the greatest benefit is for patients with raised eosinophil counts or an asthma co-diagnosis.12
Due to safety concerns, in particular the risk of developing pneumonia, several trials have investigated discontinuation of ICS,13–15 including trials with a more “real-life” setting.16,17 Even with this evidence, while modern guidelines have shifted towards advocating ICS use only in those with a higher exacerbation risk, there still remains little clarity as to who, or how, patients should be stepped down from triple therapy, or switched from LABA-ICS, to LABA-LAMA. In the UK, ICS withdrawal is ostensibly a growing clinical practice that is encouraged in primary care.18 Therefore, this study sought to clarify how often COPD patients in UK primary care have their ICS withdrawn and the patient factors associated with this practice. In addition, we assessed the risk in a real-world population of exacerbations and hospitalised pneumonia associated with triple therapy and LABA-ICS, as compared to LABA-LAMA.
Methods
Data Source
We used the Clinical Practice Research Database (CPRD-GOLD), a nationally representative database of de-identified electronic healthcare records. CPRD contains information on diagnoses, symptoms, prescriptions and test results on over 11 million patients, from over 670 GP practices across the UK.19 Patient’s data were linked to Hospital Episode Statistics (HES) database as HES contains information on all admissions to National Health Service hospitals in England. Patients’ data were linked to Office of National Statistics (ONS) for mortality data. Approximately 60% of patients in CPRD have individual-level linkage to HES, ONS and IMD.
Study Population
All patients included in this historical open cohort study were aged >35 years old, had a history of smoking, had a COPD diagnosis as defined using validated algorithm of clinical codes alone20 and were prescribed inhaled airways medication (Supplementary Figure 1). All patients with at least 1 year of data between 1st January 2014 and 1st January 2019 were included in the prescription analysis. Only patients prescribed one of the three treatment regimens (see below), and had HES linked data, were included in the outcomes analysis (Supplementary Figure 1). Patients were included from the latest date of the following: January 1st 2014, research acceptable date (CPRD quality control), continuous records date, 35th birthday, or prescription for inhaled airways medication. Follow-up was censored at the earliest date of the following: January 1st 2019, date transferred out of CPRD, last data collection or death. For the prescription analysis, data were only included if there was a full calendar year.
Inhaler Regimens and Outcomes
Inhaler treatments included were inhaled corticosteroid (ICS), long-acting beta-agonist (LABA), and long-acting muscarinic antagonist (LAMA) and short-acting beta-agonist (SABA). Inhaler regimens included were LABA-ICS, LAMA-LABA or triple therapy (LABA, LAMA and ICS). All regimens could be prescribed as a combination inhaler or as multiple single components. Patients were only eligible for the statistical analysis if they had at least two prescriptions of their inhaler regimen within 91 days, in the year before their study start date. Withdrawal was defined as ≥6 months of no ICS-containing inhaler prescription.
Exacerbations were identified using a validated algorithm (algorithm uses a combination of treatment with oral corticosteroids, antibiotics, and codes for exacerbation and symptoms).21,22 Exacerbations recorded within 14 days after the index 1 were considered part of the same exacerbation. Pneumonia was defined as a hospital admission for pneumonia using ICS-10 (J18); only the first pneumonia during follow-up was recorded.
Covariates
Body mass index (BMI) was measured as kg/m2. COPD severity was classified using 2019 GOLD spirometry staging classification and the MRC Dyspnoea Scale; the patient’s most recent spirometry and MRC recordings were used. The following variables were defined using only data in the year before a patient’s study start date: “past exacerbations”, “pneumonia” and “SABA use” (frequency of SABA prescriptions). Incident inhaler use was defined as inhalers prescribed for COPD patients started within 3 months of the patient’s study start date. Prevalent inhaler use was defined as inhalers prescribed for at least 1 year. A history of bronchiectasis, ischaemic heart disease (IHD), heart failure, ischaemic cerebrovascular disease (stroke), anxiety, depression or asthma were recorded using appropriate (available upon request). A history of asthma was defined using validated Read codes.23
Statistical Analysis
Inhaler prevalence was calculated as the percentage of patients prescribed each regimen per year, 2014 to 2018, by the total COPD cohort in the database that year. The incidence of ICS withdrawal was calculated as those patients that were withdrawn divided by the total number using ICS-containing regimen at the beginning of that year. Association between patient factors and ICS withdrawal was assessed using multivariable logistic regression model. The proportion that was re-started on ICS within a year of withdrawal was also calculated.
Baseline characteristics and rates were tabulated for each inhaler regimen for patients in the outcomes analysis. To calculate rate ratios of exacerbations between the treatment regimens, and to take into account multiple exacerbations, a repeated measures Poisson regression model was used. To assess the association between inhaler regimen and pneumonia the data were modelled using Poisson regression and time to first pneumonia during follow-up. Both models were adjusted for age, gender, BMI, smoking history, COPD severity, SABA use, past exacerbations, pneumonia and comorbidities (asthma, bronchiectasis, heart failure, IHD, stroke, anxiety and depression). All statistical analyses were performed using STATA 14.2.
Ethical Approval
The protocol for this research was approved by the Independent Scientific Advisory Committee (ISAC) for MHRA Database Research (protocol number 18_185) and the approved protocol was made available to the journal and reviewers during peer review. Generic ethical approval for observational research using CPRD with approval from ISAC has been granted by a Health Research Authority (HRA) Research Ethics Committee (East Midlands – Derby, REC reference number 05/MRE04/87). Linked pseudonymised data were provided for this study by CPRD. Data are linked by NHS Digital, the statutory trusted third party for linking data, using identifiable data held only by NHS Digital. Select practices consent to this process at a practice level with individual patients having the right to opt-out.
Results
Temporal Changes in Prescription Regimens
The proportion of patients prescribed an ICS-containing regimen slowly declined from 83.7% in 2014, to 72.2% in 2018, this was predominantly due to the decrease in LABA-ICS prescriptions (Figure 1). The proportion prescribed LABA-LAMA regimen greater than quadrupled during this time period, from 2.2% in 2014 to 10.8% in 2018.
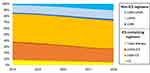 |
Figure 1 COPD inhaler prescriptions between 2014 and 2018 by ICS-containing or non-ICS containing inhalers. |
The percentage of patients that had ICS withdrawn was very low, around 2–3%, but in general, this increased slightly between 2014 and 2018 (Figure 2). Of note, those switched to a non-ICS regimen, 46.9% were switched back to an ICS-containing regimen within 1 year.
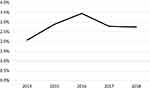 |
Figure 2 Percentage of patients that ICS withdrawn from their treatment regimen between 2014 and 2018. |
Patient Characteristics by Treatment Regimen
A total of 31,034 patients were eligible for the outcomes analysis; two-thirds of patients were prescribed triple therapy (60.7%), followed by LABA-ICS (36.9%) and LAMA-LABA (2.4%) (Table 1). Patients’ prescribed triple therapy had higher proportion with MRC score ≥3, GOLD spirometry ≥3, ≥1 exacerbation and history of pneumonia. Patients prescribed LABA-ICS or triple therapy had much higher proportion with asthma co-diagnosis and those with eosinophils >0.3 x109/L.
 |
Table 1 Patient Characteristics by Treatment Regimen |
Factors Associated with Switching from an ICS-Containing Regimen to a Non-ICS Containing Regimen
The factors significantly associated with having ICS withdrawal were being underweight, obese, a past history of a stroke or having had at least one COPD exacerbation in the past year (Table 2). Factors found to be significantly associated with not withdrawing ICS were GOLD spirometry greater than mild, any SABA use, an asthma co-diagnosis and comorbid heart failure. A history of hospitalised pneumonia was not significantly associated with ICS withdrawal.
 |
Table 2 Multivariable Analysis to Assess Factors Potentially Associated with Switching from ICS-Containing Regimen to Non-ICS Containing Regimen |
Comparing Health Outcomes Between Patients on Triple Therapy, ICS-LABA and LABA-LAMA Regimens
Patients prescribed triple therapy had over 60% higher rates of exacerbations, per person year, than those prescribed LAMA-LABA or LABA-ICS, but the rate between dual therapy regimens was similar (Table 3). After adjusting for all confounders, there was no difference in the risk of exacerbations between patients using triple therapy and patients using LAMA-LABA, but there was a slightly reduced risk of exacerbations in patients prescribed LABA-ICS compared to LAMA-LABA (LABA-ICS: adjusted IRR=0.82 95% CI 0.73–0.93, triple: adjusted IRR=0.99 95% CI 0.88–1.11) (Table 4).
 |
Table 3 Rates of Exacerbations for Each Treatment Regimen |
 |
Table 4 Association Between Treatment Regimens and Exacerbations After Adjusting for Multiple Risk Factors |
Patients prescribed triple therapy had around 50% higher rates of hospitalised pneumonia, per person year, than those prescribed LAMA-LABA or LABA-ICS, but the rate between dual therapy regimens was similar (Table 5). After adjusting for all confounders, there was no significant difference in the risk of hospitalised pneumonia between patients using triple therapy and patients using LAMA-LABA, but there was a non-significant increased risk of hospitalised pneumonia in patients prescribed triple therapy compared to LAMA-LABA (LABA-ICS: adjusted IRR=0.96 95% CI 0.71–1.31, triple: adjusted IRR=1.16 95% CI 0.87–1.57; compared to the reference group of LAMA-LABA users) (Table 6).
 |
Table 5 Rates of First Pneumonia for Each Treatment Regimen |
 |
Table 6 Association Between Treatment Regimens and Hospitalised Pneumonia Events After Adjusting for Multiple Risk Factors |
Discussion
Over two-thirds of COPD patients between 2014 and 2018 were prescribed ICS-containing inhaled therapy, in particular triple therapy; similar to the proportion seen in Sweden but higher than that found in the Balearic Islands (Spain).24,25 In keeping with guideline changes and recommendations during this time period, the proportion prescribed non-ICS inhaled therapy steadily increased, especially dual bronchodilator (LABA-LAMA) treatment. Only a very small proportion of patients on established ICS treatment had their ICS withdrawn, of whom nearly half had ICS re-introduced within a year. Surprisingly, one of the most influential factors in ICS withdrawal were one or more exacerbations in the year prior, even though this is the group of patients that are expected to benefit from ICS. This could be because they were seen more frequently by a physician or because there was concern over the known association between ICS and respiratory tract bacterial infections.26–29 However, a past history of hospitalised pneumonia was not associated with ICS withdrawal and many resumed ICS use.
Encouragingly, and as we have shown before in a different UK primary care patient cohort,3 patients that were prescribed triple therapy, had more severe disease and were more likely to exacerbate than those prescribed dual therapy. But after taking into account major risk factors, including disease severity and history of exacerbations, the risk of exacerbations was similar between all three regimens. Although the risk in the LABA-ICS treated patients was marginally lower, the confidence intervals crossed those treated with triple therapy; it is possible a larger sample size would find the effect estimate not to be statistically different and there may have been some residual confounding. There was also no significant difference in the risk of hospitalised pneumonia between regimens, even though the estimate was slightly higher in the triple therapy treated patients, but not LABA-ICS treated patients. The meaning of this is unclear, as both triple and LABA-ICS regimens clearly contain ICS, but the total amount of ICS use may have varied, furthermore, in this sample size the marginally elevated estimate was not statistically significant.
The 2017 Cochrane Systematic Review examined multiple randomised-controlled trials comparing LABA-ICS to LABA-LAMA, and found that patients using LAMA-LABA had fewer exacerbations and a lower pneumonia risk, but the evidence was only of low or moderate quality.9 The 2016 FLAME trial randomised patients to either LABA-LAMA or ICS-LABA and found that LABA-LAMA was superior in terms of exacerbation rates,10 although, other studies using a different LABA–LAMA combination found equivocal results.11 Another large trial, 2018 IMPACT study, also compared triple therapy to either LABA-LAMA or ICS-LABA, and again found lower rates of exacerbations with triple therapy.30 Several trials have compared triple therapy to LAMA-LABA but a meta-analysis in 2018 found only modest improvements in efficacy against exacerbations from triple therapy, except in those with raised eosinophil counts where the benefits were more substantiated, and no difference in pneumonia risk.12 Unfortunately, results from trials do not always predict the effectiveness of inhaled treatment in routine clinical practice.31,32 Firstly, effectiveness is influenced by inhaler technique, which is optimised in trials,33,34 and secondly, over 90% of COPD patients in primary care do not meet trial eligibility criteria.32 Thirdly, changing inhaler devices rely on a good patient-pharmacist or patient-doctor/nurse relationship, which often does not happen.35 Furthermore, many patients are inappropriately prescribed ICS-LABA, the consequence of changing these patients to LABA-LAMA has not been studied in trials. Therefore, data from everyday clinical practice are also required, ideally a real-world pragmatic trial, but even observational studies using routinely collected data help inform on the general COPD population.
The main strengths of this study are the size of the population included, longevity of their follow-up, representativeness of the database and the accuracy and completeness of prescription records. However, although this study was large, pneumonia is an infrequent event and as such may require even larger studies of real world populations. Study limitations to be considered are the lack of information on prescription dispensing, or adherence to medication. Due to the low numbers of patients withdrawing from ICS, even in such a large database, the direct impact of this practice could not be assessed. Studies using electronic healthcare records can suffer from misclassification; in this study, we have used a validated definition of COPD and COPD exacerbations, however, it is possible some diagnosed exacerbations were actually a pneumonia or both a pneumonia and an exacerbation of their COPD. Furthermore, many patients had a co-diagnosis of asthma, it was not possible in this study to investigate misclassification, a follow-up study would be warranted that addressed a population of patients only given a COPD diagnosis. There may also have been residual confounding, factors that influenced the physician’s decision to start a patient on particular regimen that was not recorded.
Conclusion
ICS-containing regimens are common prescribed in COPD patients, often inappropriately, yet withdrawal from ICS is uncommon. The factors associated with withdrawal were not as expected which may be related to the lack of clear recommendations in guidelines. Health outcomes, exacerbations and pneumonia, after controlling for multiple known risk factors, were analogous amongst all three treatment regimens.
Disclosure
OSU and JKQ are co-last authors contributed to this study. CIB reports funding from AstraZeneca and Asthma UK which were received by JKQ, outside the submitted work. JKQ's research group reports funding from outside the submitted work from AstraZeneca, Asthma UK, The Health Foundation, MRC, Wellcome Trust, BLF, GlaxoSmithKline, Insmed, Bayer, IQVIA, and Boehringer Ingelheim. JKQ reports funding from outside the submitted work from AstraZeneca, GlaxoSmithKline, Chiesi, Bayer, Teva, and Boehringer Ingelheim for Advisory board participation or travel. ID reports grants outside the submitted work from NIHR, GlaxoSmithKline, MRC, ABPI. OSU reports from outside the submitted work grants from AstraZeneca, Edmond Pharma and GlaxoSmithKline; personal fees from Aerocrine, Boehringer Ingelheim, Cipla, Mundipharma, Napp, Sandoz, Takeda and Zentiva. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease Pocket Guide to Copd Diagnosis, Management, and Prevention a Guide for Health Care Professionals; 2017.
2. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–555. doi:10.1164/rccm.200703-456SO
3. Bloom CI, Elkin S, Quint J. Changes in COPD inhaler prescriptions in the United Kingdom, 2000 to 2016. Int J Chron Obstruct Pulmon Dis. 2019;14:279–287. doi:10.2147/COPD.S190086
4. Drivenes E, Ostrem A, Melbye H. Predictors of ICS/LABA prescribing in COPD patients: a study from general practice. BMC Fam Pract. 2014;15:42. doi:10.1186/1471-2296-15-42
5. Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi:10.1016/j.rmed.2015.12.004
6. Casas Herrera A, Montes de Oca M, Menezes A, et al. Respiratory medication used in COPD patients from seven Latin American countries: the LASSYC study. Int J Chron Obstruct Pulmon Dis. 2018;13:1545–1556. doi:10.2147/COPD
7. Tavakoli H, Johnson KM, FitzGerald JM, et al. Trends in prescriptions and costs of inhaled medications in chronic obstructive pulmonary disease: a 19-year population-based study from Canada. Int J Chron Obstruct Pulmon Dis. 2019;14:2003–2013. doi:10.2147/COPD
8. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi:10.2147/COPD.S91694
9. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). In: Horita N, editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2017. doi:10.1002/14651858.CD012066.pub2
10. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi:10.1056/NEJMoa1516385
11. Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a Phase 3 COPD study. Eur Respir J. 2016;48:1030–1039. doi:10.1183/13993003.00216-2016
12. Cazzola M, Rogliani P, Calzetta L, Matera MG. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018;52:1801586. doi:10.1183/13993003.01586-2018
13. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294. doi:10.1056/NEJMoa1407154
14. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy De-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–339. doi:10.1164/rccm.201803-0405OC
15. Rossi A, van der Molen T, Olmo RD, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44:1548–1556. doi:10.1183/09031936.00126814
16. Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77. doi:10.1186/1465-9921-15-77
17. Vogelmeier C, Worth H, Buhl R, et al. ‘Real-life’ inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494. doi:10.2147/COPD.S125616
18. Primary Care respiratory Society. Stepping down inhaled corticosteroids in COPD. Available from: https://www.pcrs-uk.org/resource/stepping-down-inhaled-corticosteroids-copd.
19. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–836. doi:10.1093/ije/dyv098
20. Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4:e005540–e005540. doi:10.1136/bmjopen-2014-005540
21. Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin. Epidemiol. 2016;8:771–782. doi:10.2147/CLEP
22. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11:e0151357. doi:10.1371/journal.pone.0151357
23. Nissen F, Morales DR, Mullerova H, et al. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD). BMJ Open. 2017;7:e017474. doi:10.1136/bmjopen-2017-017474
24. Sulku J, Janson C, Melhus H, et al. A cross-sectional study assessing appropriateness of inhaled corticosteroid treatment in primary and secondary care patients with COPD in Sweden. Int J COPD. 2019;14:2451–2460. doi:10.2147/COPD
25. Román-Rodríguez M, van Boven JFM, Vargas F, et al. Factors associated with inhaled corticosteroids prescription in primary care patients with COPD: a cross-sectional study in the Balearic Islands (Spain). Eur. J. Gen. Pract. 2016;22:232–239. doi:10.1080/13814788.2016.1212011
26. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur. Respir. J. 2009;34:641–647. doi:10.1183/09031936.00193908
27. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–166. doi:10.1164/rccm.200611-1630OC
28. Calverley PMA, Stockley RA, Seemungal TAR, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139:505–512. doi:10.1378/chest.09-2992
29. Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50:1700037. doi:10.1183/13993003.00037-2017
30. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi:10.1056/NEJMoa1713901
31. Price D, Haughney J, Sims E, et al. Effectiveness of inhaler types for real-world asthma management: retrospective observational study using the GPRD. J Asthma Allergy. 2011;4:37–47. doi:10.2147/JAA.S17709
32. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219–223. doi:10.1136/thx.2006.066837
33. Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1333–1343. doi:10.1164/rccm.201604-0733OC
34. Sulaiman I, Seheult J, MacHale E, et al. Irregular and ineffective: a quantitative observational study of the time and technique of inhaler use. J Allergy Clin Immunol Pract. 2016;4:900–909.e2. doi:10.1016/j.jaip.2016.07.009
35. Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1. doi:10.1186/1471-2466-9-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
