Back to Journals » International Journal of General Medicine » Volume 14
Influencing Factors and Prognostic Value of 18F-FDG PET/CT Metabolic and Volumetric Parameters in Non-Small Cell Lung Cancer
Authors Zhang L, Ren Z, Xu C, Li Q, Chen J
Received 18 May 2021
Accepted for publication 28 June 2021
Published 21 July 2021 Volume 2021:14 Pages 3699—3706
DOI https://doi.org/10.2147/IJGM.S320744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lixia Zhang,1 Zhe Ren,2 Caiyun Xu,1 Qiushuang Li,3 Jinyan Chen1
1Department of Nuclear Medicine, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310006, People’s Republic of China; 2Department of Chest Surgery, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310006, People’s Republic of China; 3Department of Clinical Evaluation Centers, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310006, People’s Republic of China
Correspondence: Lixia Zhang Tel +86-0571-86620310
Email [email protected]
Objective: This study aims to explore factors influencing metabolic and volumetric parameters of [18F]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging in non-small cell lung cancer (NSCLC) and the predictive value for prognosis of NSCLC.
Methods: Retrospective analysis was performed on 133 NSCLC patients who received 18F-FDG PET/CT imaging. After 18F-FDG injection at 3.7 MBq/kg, 1 h early imaging and 2 h delayed imaging were performed. The metabolic and volumetric parameters such as SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were measured. The tumor markers including CFYRA21-1, NSE, SCC-ag and the immunohistochemical biomarkers including Ki-67, P53 and CK-7 were examined. All patients were followed up for 24 months, and the 1-year and 2-year overall survival rate (OS) were recorded.
Results: There were significant differences in metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak and TLG) between adenocarcinoma and squamous cell carcinoma of NSCLC. SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were correlated with tumor marker NSE and TNM stage. MTV and TLG were related to CYFRA21-1, and only MTV was associated with SCC-ag. SUVpeak and SULmax were related to P53. In addition, early SULpeak and delayed MTV were significant prognostic factors of 1-year OS, while early SUVpeak, delayed TLG and delayed MTV were predictive factors of 2-year OS in NSCLC.
Conclusion: The metabolic and volumetric parameters of 18F-FDG PET/CT were related to a variety of factors such as NSE, CFYRA21-1, SCC-ag, P53 and TNM stage, and have a predictive value in prognosis of NSCLC.
Keywords: NSCLC, 18F-FDG, PET/CT, metabolic parameters, volumetric parameters
Introduction
In recent years, the incidence and mortality of lung cancer ranks first in the world.1,2 Non-small cell lung cancer (NSCLC) is a common type of lung cancer, with high malignancy, strong invasiveness and easy distant metastasis, and accounting for 85–90% of all lung cancers.3 Most NSCLC patients are already in stage III or stage IV at the time of diagnosis.4 Thus, early diagnosis and accurate clinical staging of NSCLC are of great significance in formulating treatment plans, prolonging survival and improving quality of life.
At present, with the development of medical imaging technology, imaging examination has an important value in the diagnosis of cancer.5 Positron emission tomography (PET) is the most advanced noninvasive molecular imaging technology, using isotopes of the basic elements (such as 11C, 13N, 15O, etc.) to dynamically and quantitatively observe the biochemical metabolism and other biological characteristics of tumor tissue at the molecular level.6,7 PET has high sensitivity, but compared with conventional computed tomography (CT) or magnetic resonance imaging (MRI), its image resolution is reduced, which is not conducive to accurate location of the lesion. Therefore, in clinical application, PET often combined with CT or MRI.8 Usually, PET/CT is required for NSCLC patients.9
[18F]fluorodeoxyglucose (18F-FDG) is the most commonly used molecular probe for PET, which is a glucose analogue that can indirectly reflect glucose metabolism and tumor cell proliferation.10 In order to enable PET to more accurately evaluate tumors, the study of 18F-FDG PET metabolism is particularly important.
PET imaging can provide a variety of metabolic and volumetric parameters, such as standard uptake value (SUV), standard uptake value of lean body mass (SUL), metabolic tumor volume (MTV), total lesion glycolysis (TLG), etc., and provide parameters of early and delayed imaging according to different imaging time.11 In the 18F-FDG PET/CT study, SUVmax is the most commonly used quantitative parameter, which can reflect the metabolic activity of tumors. SUVpeak refers to the ROI (spherical ROI of 1cm3 volume) SUV value of a tissue sphere with a diameter of about 1.2 cm. Compared with SUVmax, SUVpeak takes into account the tumor FDG uptake in a certain volume, and is a more robust and accurate parameter than SUVmax. For obese patients, it is suggested that SUV modified by SUL should be used. SUL is a very popular metabolic parameter in recent years, and it is more objective for metabolic quantitative analysis and treatment response evaluation of lung cancer. Some schemes of treatment response evaluation recommend the use of SUVpeak or SULpeak of the tumor. MTV is a volume parameter that can be measured quantitatively, which can reflect the metabolic volume of lesions on the basis of anatomical location. Based on tumor metabolic volume, TLG is a comprehensive parameter that can reflect both tumor metabolic activity and tumor metabolic volume. In this study, we explored the influencing factors and diagnostic value of metabolic and volumetric parameters of 18F-FDG PET/CT in NSCLC.
Materials and Methods
Clinical Data
A total of 133 patients, underwent PET/CT imaging in our center and diagnosed as NSCLC by surgery or puncture pathology, were analyzed retrospectively from May 2017 to December 2018. These patients included 91 males and 42 females, aged 65.72 ±9.61 years (38–85 years). Patients with a history of other malignant tumors, with any other type of malignant tumor, with diabetes, and NSCLC patients treated with surgery, radiotherapy, chemotherapy, targeted therapy and traditional Chinese medicine treatment were excluded from the study. The basic characteristics of the patients are shown in Table 1. Early and delayed metabolic and volumetric parameters SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were measured at 1 h and 2 h after 18F-FDG injection at 3.70 MBq/kg. The tumor markers (CFYRA21-1, NSE, SCC-ag) were collected within 2 weeks before and after PET/CT examination, and the immunohistochemical biomarkers (Ki-67, P53, CK-7) were achieved through surgery or puncture pathology. The patients were followed up for 1-year and 2-year overall survival rate (OS). This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Zhejiang Hospital of Traditional Chinese Medicine (2018-X-0135). All patients signed informed consent before PET/CT imaging.
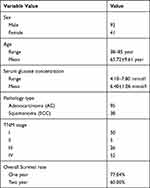 |
Table 1 Key Characteristics of Patients |
PET/CT Imaging
The patients fasted for more than 4 hours and the blood glucose value was 6.40 ±1.06 mmol/mL (4.10–7.80 mmol/mL) before PET/CT examination. The 18F-FDG was provided by Shanghai Atom Kexing Pharmaceutical Co., Ltd, with pH 6.0–7.0 and radiochemical purity >95%. The national drug standard is H20051183. The Siemens biograph mCT flow PET/CT instrument was used. Early imaging was performed 1 hour after injection of 18F-FDG. The patients were kept on a supine and motionless position with hands on his or her head. First, spiral CT scanning was performed with a thickness of 5 mm and pitch 0.75:1, and then PET scan was performed using 3D Flowmotion acquisition mode. The scan range was from the top of the skull to the middle of the thigh. The CT acquisition conditions are as follows: the tube voltage is 120 kV, the tube current is adjusted according to the tissue density between 35 and 280 mAs, the layer thickness is 5 mm and the pitch is 0.8. PET emission scanning adopts 3D mode. Delayed imaging was performed 2 hours after injection of tracer, and the acquisition parameters were the same as those of early imaging. After the acquisition, all the data were transferred to the Sygno TrueD workstation and reconstructed by iterative method. The early imaging data were iteratively reconstructed by point flight technology (TOF) and TrueX, and the attenuation of PET images were corrected by plain scan CT transmission scan data. The reconstruction slice thickness was 3 mm and the interval was 3 mm. The TrueD software was used to analyze and diagnose the images in cross-section, coronal plane, sagittal plane and MIP. The region of interest (ROI) (VOI), was delineated by post-processing software. The metabolism and volume parameters SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were measured by 3D measurement software.
Statistical Processing
Statistical Package for the Social Sciences (SPSS) program version 26.0 (IBM, Armonk, NY) software was used for data statistical analysis. The measurement data that accorded with normal distribution were expressed by mean±standard deviation, and the differences among groups were compared with t-test or analysis of variance. Those data that did not accord with normal distribution were expressed by median (minimum value, maximum value), and the differences among groups were compared with non-parametric test. The linear regression method was used to analyze the relationship between blood glucose, age, tumor markers, immunohistochemical biomarkers, pathological type and metabolic and volumetric parameters. Nonparametric test was used to analyze the differences of metabolic and volumetric parameters in different TNM stages and pathological types. Logistic regression analysis was used to find the influencing factors of 1-year survival rate or 2-year survival rate, and then the predictive model was established. ROC curve was drawn to evaluate the validity of the predictive model. P < 0.05 means statistical significance.
Results
Comparison of Metabolic and Volumetric Parameters in Different Pathological Types of NSCLC
There were significant differences in metabolic and volumetric parameters between lung adenocarcinoma (AC) and squamous cell carcinoma (SCC) (P < 0.05). As shown in Table 2, SUVmax, SUVpeak, SULmax, SULpeak and TLG of SCC were all higher than those of AC (P < 0.05).
 |
Table 2 Metabolic and Volumetric Parameters of Different Pathological Types of NSCLC |
The Relationship Between Metabolic and Volumetric Parameters of 18F-FDG PET/CT Imaging and Blood Glucose, Age in NSCLC
All patients fasted for more than 4 hours, and the blood glucose concentration was controlled at 4.10–7.80 mmol/L. In this range, metabolism and volume parameters such as SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were not related to blood glucose concentration (P > 0.05, data not shown). Moreover, SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG of NSCLC patients were also not related to patients age (P > 0.05, data not shown).
The Relationship Between Metabolic and Volumetric Parameters of 18F-FDG PET/CT Imaging and Serum Tumor Markers
The serum levels of tumor markers NSE, CYFRA21-1 and SCC-ag in patients with NSCLC were detected by electrochemical luminescence assay. We also analyzed the relationship between these serum tumor markers and metabolic and volumetric parameters of 18F-FDG PET/CT imaging. As shown in Table 3, the metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG) were correlated with tumor marker NSE (P < 0.05). The MTV and TLG were related to tumor marker CYFRA21-1 (P < 0.05). Only MTV was associated with SCC-ag (P < 0.05).
 |
Table 3 The Relationship Between Metabolic and Volumetric Parameters and Serum Tumor Markers |
The Relationship Between Metabolic and Volumetric Parameters of 18F-FDG PET/CT Imaging and Immunohistochemical Biomarkers
As shown in Table 4, the early SUVpeak and early SULmax were related to immunohistochemical biomarker P53 (P < 0.05) while other parameters were not related to P53. Furthermore, no significant was also found between all metabolic and volumetric parameters and Ki-67 or CK-7 (P > 0.05, data not shown).
 |
Table 4 The Relationship Between Metabolic and Volumetric Parameters and P53 |
The Relationship Between Metabolic and Volumetric Parameters of 18F-FDG PET/CT Image and TNM Stage in NSCLC
As shown in Table 5, among the metabolic and volumetric parameters, early SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG were significantly correlated with TNM staging (r= 0.501, 0.525, 0.505, 0.532, 0.612, 0.547). These data suggested that TNM stage was the influencing factors of metabolic and volumetric parameters of NSCLC 18F-FDG PET/CT imaging.
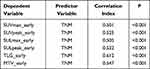 |
Table 5 The Correlation of Metabolic and Volumetric Parameters and TNM Stage |
The Prognostic Value of 18F-FDG PET/CT in NSCLC
The 1-year and 2-year OS of 133 NSCLC cases were 77.04% and 60%, respectively. Early SULpeak and delayed MTV are the influencing factors of 1-year OS, and based on these two indicators, the logistic regression predictive value is obtained. According to the predictive value and gold standard, the ROC curve is plotted (Figure 1A). The area under the curve (AUC) is 0.780 and the cutoff value is 0.875 (P=0.043). The early SUVpeak, delayed TLG and delayed MTV are the influencing factors of 2-year OS, and the logistic regression predictive value is obtained based on these three indicators. According to the predictive value and golden standard, the ROC curve is plotted (Figure 1B). The AUC was 0.816 and the cut-off value was 0.759 (P=0.035).
Discussion
PET/CT is a kind of multimodal imaging which integrates metabolic imaging and anatomical imaging. It has been recommended for diagnosis, differential diagnosis, staging, curative effect evaluation, prognosis evaluation and recurrence monitoring of lung cancer. The most commonly used molecular probe in clinic is 18F-FDG, which can reflect the metabolic activity, tissue perfusion and cell proliferation of tumor cells.12 In this study, we explored the metabolic and volumetric parameters, such as SUV, SUL, MTV and TLG of 18F-FDG PET/CT in NSCLC.
The reports on whether there are differences in metabolic and volumetric parameters of PET/CT imaging among different pathological types of lung cancer are not consistent. Some studies have reported that there is no significant difference in SUVmax among AC, SCC and small cell lung cancer.13 Wei D Hu et al14 reported that the SUVmax, MTV and TLG of SCC were higher than those of AC. In this study, we found that there were significant differences in metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak and TLG) between AC and SCC. The metabolic and volumetric parameters of SCC were all higher than those of AC, similar to the study of Wei D Hu et al. It is considered that these differences were related to the rapid growth rate, short doubling time and high expression of Glut-1 in SCC.15,16
It is well known that hyperglycemia reduces 18F-FDG uptake and SUV in tumors. In this study, all patients fasted for more than 4 hours, and the blood glucose concentration was controlled at 4.10–7.80 mmol/L. In this range, metabolism and volume parameters (SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG) were not related to blood glucose concentration, suggesting that the blood glucose concentration is not the influence factor of metabolism and volume parameters of 18F-FDG PET/CT imaging when the blood glucose concentration is in the normal range. However, insulin injection before examination is not recommended to control blood glucose, as it will cause an increase in muscle uptake of 18F-FDG,17 which may affect the accuracy of parameters such as SUVmax.
Tumor markers are some chemicals released into the blood during the occurrence and development of tumors, which play an important role on tumor screening, diagnosis, curative effect evaluation, recurrence monitoring and so on. The concentrations of the tumor markers CYFRA21-1, SCC-ag and NSE in serum is also a simple and feasible method to predict NSCLC.18 Dogan et al19 reported that serum CYFRA21-1 was significantly correlated with all volumetric tumor parameters, such as SUVmax, MTV and TLG. A study of PET/CT in squamous cervical cancer by Nakamura20 showed that serum SCC-ag had a statistically significant association with tumor stage and tumor maximum size, but had no correlation with the SUVmax. However, a recent study21 showed that there was a significant correlation between serum SCC-ag and SUVmax in Stage IIB-IVB squamous cervical cancer. There is no study exploring the relationship between SCC-ag, NSE and volumetric tumor parameters in NSCLC. Our study found that metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG) in NSCLC were related to tumor marker NSE, while MTV and TLG were related to tumor marker CYFRA21-1. Only MTV were related to tumor marker SCC-ag. Moreover, immunohistochemical markers Ki-67, CK7 and P53 were also widely used to enhance diagnostic accuracy.22,23 Many literatures have shown a significant correlation between SUVmax and Ki-67 expression,24 but Liu LP et al25 reported that there was no correlation between SUVmax and Ki-67 expression. In this study, it was found that all metabolism and volume parameters were not related to Ki-67 and CK7, which were similar to those reported by Liu LP et al. So far, P53 gene has been found to be the most closely related to human tumor.26 It has been reported that SUVmax is related to the expression of P53 gene.27 This study found that P53 expression was related to SUVpeak and SULmax, but not related to MTV and TLG. As this study belongs to a single-center study, further accumulation of cases and multicenter studies are needed to clarify the relationship.
In addition, we also explore the application of metabolic and volumetric parameters of 18F-FDG PET/CT in evaluating TNM staging of NSCLC. Ivayla Apostolova et al28 reported that SUVmax and MTV were related to T stage and N stage in NSCLC. The retrospective analysis results of 107 NSCLC cases by Li M et al29 showed that the tumor stage increased with the increase of SUVmax in the primary tumor, and the possibility of lymph node metastasis and distant metastasis also increased. Wei D Hu et al14 reported that MTV and TLG were positively correlated with the stage of adenocarcinoma except for SUVmax, and only MTV was positively correlated with the stage of squamous cell carcinoma. Compared with other parameters, MTV and TLG both measure tumor volume and metabolic activity in 3D dimensions, which can provide more information about tumor aggressiveness and is independent of tumor stage and other clinical factors. Lee et al30 first assessed the prognosis of NSCLC through MTV, and analyzed 19 cases (18 cases of NSCLC, 1 case of small cell lung cancer) lung cancer patients (stage I-stage IV). It was found that for every 25 mL increase in MTV, the risk of disease progression and death increased by 2.8 times. In this study, nonparametric test analysis was used to find that there were differences in metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak, MTV and TLG) in patients with different TNM stages. TNM stages were all influence factors of metabolic and volumetric parameters in NSCLC 18F-FDG PET/CT.
In recent years, it has been found that the survival predictive value of MTV and TLG is better than that of SUVmax. Huang et al31 found that 1-year and 2-year OS were respective 83% and 52.8% through the study of 53 patients with locally progressive NSCLC, which were the influencing factors of OS. Ohri N et al32 studied 214 patients with III stage NSCLC and found that MTV was an independent predictor of OS. M Dosani et al33 through the analysis of 134 patients with NSCLC. It was found that TLG and MTV were more likely to predict OS than SUVmax. In this study, the 1-year and 2-year OS of NSCLC patients were 77.04% and 60%, respectively. It was found that early SULpeak and delayed MTV were the influencing factors of 1-year OS, and early SUVpeak, delayed TLG and delayed MTV are the influencing factors of 2-year OS. To sum up, SUVpeak, SULpeak, TLG and MTV in 18F-FDG PET/CT imaging are the influencing factors of annual and 2-year OS in NSCLC, and have important predictive value for survival. According to the predicted value of the model and gold standard, the predictive value of 2-year OS is better than that of 1-year OS.
Conclusion
In NSCLC 18F-FDG PET/CT imaging, the metabolic and volumetric parameters (SUVmax, SUVpeak, SULmax, SULpeak, and TLG, except MTV) of squamous cell carcinoma were all significantly higher than those of adenocarcinoma. Tumor markers (NSE, CFYRA21-1 and SCC-ag), immunohistochemical biomarker P53, tumor stage (T, N, M and TNM stage) are influencing factors of metabolic and volumetric parameters. Early SULpeak, early SUVpeak, delayed TLG and delayed MTV have predictive value in prognosis of NSCLC.
Funding
This work received no funding.
Disclosure
The authors declare no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Slotman BJ, Senan S. Radiotherapy in small-cell lung cancer: lessons learned and future directions. Int J Radiat Oncol Biol Phys. 2011;79(4):998–1003. doi:10.1016/j.ijrobp.2010.10.039
3. Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proce. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013
4. Brainard J, Farver C. The diagnosis of non-small cell lung cancer in the molecular era. Modern Pathol. 2019;32(Suppl 1):16–26. doi:10.1038/s41379-018-0156-x
5. Wang C, Wang Z, Zhao T, et al. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials. 2018;157:62–75. doi:10.1016/j.biomaterials.2017.12.002
6. Abrantes AM, Pires AS, Monteiro L, et al. Tumour functional imaging by PET. Biochimica et biophysica acta Mol dis. 2020;1866(6):165717. doi:10.1016/j.bbadis.2020.165717
7. Valladares A, Beyer T, Rausch I. Physical imaging phantoms for simulation of tumor heterogeneity in PET, CT, and MRI: an overview of existing designs. Med Phys. 2020;47(4):2023–2037. doi:10.1002/mp.14045
8. Riola-Parada C, García-Cañamaque L, Pérez-Dueñas V, Garcerant-Tafur M, Carreras-Delgado JL. Simultaneous PET/MRI vs PET/CT in oncology. A systematic review. Rev Esp Med Nucl Imagen Mol. 2016;35(5):306–312. doi:10.1016/j.remn.2016.06.001
9. Kaseda K. Recent and Current Advances in FDG-PET Imaging within the Field of Clinical Oncology in NSCLC: a Review of the Literature. Diagnostics. 2020;10(8). doi:10.3390/diagnostics10080561
10. Kandathil A, Sibley RC
11. Sarikaya I, Sarikaya A. Assessing PET Parameters in Oncologic (18) F-FDGStudies. J Nucl Med Technol. 2020;48(3):278–282. doi:10.2967/jnmt.119.236109
12. Pugachev A, Ruan S, Carlin S, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62(2):545–553. doi:10.1016/j.ijrobp.2005.02.009
13. Ha S, Choi H, Cheon GJ, et al. Autoclustering of Non-small Cell Lung Carcinoma Subtypes on (18) F-FDGPET Using Texture Analysis: a Preliminary Result. Nucl Med Mol Imaging (2010). 2014;48(4):278–286. doi:10.1007/s13139-014-0283-3
14. Hu WD, Wang HC, Wang YB, Cui LL, Chen XH. Correlation study on 18F-FDG PET/CT metabolic characteristics of primary lesion with clinical stage in lung cancer. Quarterly j Nuclear Med Mol Imaging. 2019;65(2):172–177. doi:10.23736/S1824-4785.19.03146-7
15. Honda O, Johkoh T, Sekiguchi J, et al. Doubling time of lung cancer determined using three-dimensional volumetric software: comparison of squamous cell carcinoma and adenocarcinoma. Lung Cancer. 2009;66(2):211–217. doi:10.1016/j.lungcan.2009.01.018
16. Choi WH, YooIe R, Kim TJ, Lee KY, Kim YK. Is the Glut expression related to FDG uptake in PET/CT of non-small cell lung cancer patients? Tech Health Care. 2015;23(Suppl 2):S311–318. doi:10.3233/THC-150967
17. Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi:10.1007/s00259-014-2961-x
18. Thakur MK, Gadgeel SM. Predictive and Prognostic Biomarkers in Non-Small Cell Lung Cancer. Semin Respir Crit Care Med. 2016;37(5):760–770. doi:10.1055/s-0036-1592337
19. Dogan I, Karyagar S, Karyagar SS, Kahraman C, Alver A. Relationship between pretreatment levels of serum Cyfra 21.1, CEA and PET metabolic parameters in NSCLC. Ann Nucl Med. 2014;28(9):829–835. doi:10.1007/s12149-014-0877-y
20. Nakamura K, Okumura Y, Kodama J, Hongo A, Kanazawa S, Hiramatsu Y. The predictive value of measurement of SUVmax and SCC-antigen in patients with pretreatment of primary squamous cell carcinoma of cervix. Gynecol Oncol. 2010;119(1):81–86. doi:10.1016/j.ygyno.2010.04.020
21. Shou H, Yasuo Y, Yuan S, Lou H, Ni J. Association of pretreatment SUV(max) of cervix and SCC-antigen with FIGO2018 stage in Stage IIB-IVB squamous cervical cancer and relationship to prognosis. Int j Gynaecol Obstetrics. 2021;152(1):112–117. doi:10.1002/ijgo.13465
22. Bellizzi AM. An Algorithmic Immunohistochemical Approach to Define Tumor Type and Assign Site of Origin. Adv Anat Pathol. 2020;27(3):114–163.
23. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–109. doi:10.1016/j.semcancer.2017.11.019
24. Kaida H, Kawahara A, Hayakawa M, et al. The difference in relationship between 18F-FDG uptake and clinicopathological factors on thyroid, esophageal, and lung cancers. Nucl Med Commun. 2014;35(1):36–43. doi:10.1097/MNM.0000000000000019
25. Liu LP, Zhang XX, Cui LB, et al. Preliminary comparison of diffusion-weighted MRI and PET/CT in predicting histological type and malignancy of lung cancer. Clin Respir J. 2017;11(2):151–158. doi:10.1111/crj.12316
26. Zeng H, Xie P, Meng X, et al. Risk factors for brain metastases after prophylactic cranial irradiation in small cell lung cancer. Sci Rep. 2017;7:42743. doi:10.1038/srep42743
27. Zhang Z, Wang H, Ding Q, et al. The tumor suppressor p53 regulates autophagosomal and lysosomal biogenesis in lung cancer cells by targeting transcription factor EB. Biomed Pharmacother. 2017;89:1055–1060. doi:10.1016/j.biopha.2017.02.103
28. Apostolova I, Ego K, Steffen IG, et al. The asphericity of the metabolic tumour volume in NSCLC: correlation with histopathology and molecular markers. Eur J Nucl Med Mol Imaging. 2016;43(13):2360–2373. doi:10.1007/s00259-016-3452-z
29. Li M, Liu N, Hu M, et al. Relationship between primary tumor fluorodeoxyglucose uptake and nodal or distant metastases at presentation in T1 stage non-small cell lung cancer. Lung Cancer. 2009;63(3):383–386. doi:10.1016/j.lungcan.2008.06.004
30. Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69(2):328–333. doi:10.1016/j.ijrobp.2007.04.036
31. Huang W, Fan M, Liu B, et al. Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. J Nucl Med. 2014;55(10):1584–1590. doi:10.2967/jnumed.114.142919
32. Ohri N, Duan F, Machtay M, et al. Pretreatment FDG-PET metrics in stage III non-small cell lung cancer: ACRIN 6668/RTOG 0235. J Natl Cancer Inst. 2015;107(4):djv004–djv004. doi:10.1093/jnci/djv004
33. Dosani M, Yang R, McLay M, et al. Metabolic tumour volume is prognostic in patients with non-small-cell lung cancer treated with stereotactic ablative radiotherapy. Current Oncol. 2019;26(1):e57–e63. doi:10.3747/co.26.4167
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

