Back to Journals » Cancer Management and Research » Volume 13
Influence of Severe Gastrointestinal Complications in Primary Gastrointestinal Diffuse Large B-Cell Lymphoma
Authors Shen Y , Ou J, Wang B, Wang L, Xu J, Cen X
Received 4 December 2020
Accepted for publication 13 January 2021
Published 4 February 2021 Volume 2021:13 Pages 1041—1052
DOI https://doi.org/10.2147/CMAR.S295671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Ye Shen,* Jinping Ou,* Bingjie Wang, Lihong Wang, Junhui Xu, Xinan Cen
Department of Hematology, Peking University First Hospital, Beijing, 100034, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinan Cen
Department of Hematology, Peking University First Hospital, No. 8, Xishiku Street, Xicheng District, Beijing, 100034, People’s Republic of China
Email [email protected]
Background: This study assessed the clinical characteristics of gastrointestinal bleeding (GIB), obstruction (GIO), and perforation (GIP) in patients with primary gastrointestinal diffuse large B-cell lymphoma (PGI-DLBCL) and the influence on long-term survival.
Methods: A retrospective analysis was performed of 148 patients with PGI-DLBCL admitted to Peking University First Hospital from August 1994 to May 2018. The clinical characteristics of GIB, GIO, and GIP before and after chemotherapy were recorded. The associated overall survival and progression-free survival were analyzed.
Results: Among 148 patients, 56.8% had gastrointestinal complications (GICs), including GIB, GIO, GIP, and multiple complications, and 22.6% of them occurred after chemotherapy, mostly during the first 4 cycles. The most common clinical manifestations of patients with GICs were abdominal pain or discomfort (79.8%), hematemesis or melena (22.6%), and abnormal bowel habits (17.9%). Patients with Eastern Cooperative Oncology Group (ECOG) score ≥ 2, tumor mass ≥ 10 cm, or intestinal involvement had significantly higher risk of severe GICs as initial manifestations. Among 130 patients who received chemotherapy, B symptoms, tumor mass ≥ 10 cm, and Lugano stage (IIE, IV) strongly correlated with GICs after chemotherapy (P < 0.05). Rituximab did not increase the risk of GICs. GICs which occurred before or after chemotherapy reduced the objective response rate at the end of chemotherapy. The prognosis of patients was significantly worsened by GIP, GIB, or multiple complications after chemotherapy (P < 0.05). GIB at presentation or GIO before or after chemotherapy had no prognostic value (both P > 0.05).
Conclusion: GICs adversely affect the quality of life, prolong the length of hospitalization, and shorten the long-term survival of patients with PGI-DLBCL.
Keywords: diffuse large B-cell lymphoma, gastrointestinal complication, bleeding, obstruction, perforation
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for ~32.5% of all non-Hodgkin’s lymphoma every year, and it is the most common subtype.1 Among patients diagnosed as DLBCL, approximately 32% involve primary extra-nodal sites, while the gastrointestinal tract is the most common primary extra-nodal site (34%).2 Gastrointestinal complications (GICs), including gastrointestinal bleeding (GIB), obstruction (GIO) and perforation (GIP), cause particular diagnostic and management problems in primary gastrointestinal (PGI) DLBCL, and DLBCL is the most easily perforated gastrointestinal lymphoma.3 Although unusual, severe GICs in PGI-DLBCL can lead to delaying and complicating chemotherapy, decreasing the quality of life, prolonging hospitalization, and even mortality. However, the available data related to GICs in PGI-DLBCL are insufficient.4–7 Therefore, the present study explored two main issues in PGI-DLBCL: the clinical characteristics of severe GICs before and after chemotherapy, and the influence of severe GICs on overall survival (OS) and progression-free survival (PFS).
Subjects and Methods
Subjects
This retrospective study was approved by the Medical Ethics Committee of Peking University First Hospital and Institute and conducted in accordance with the Declaration of Helsinki. For inclusion, patients met the diagnostic criteria for primary gastrointestinal lymphoma as defined by Dawson et al,8 and the World Health Organization’s classification of lymphoid neoplasms;9 and had not received any lymphoma treatment before enrollment. Patients with any of the following were excluded: infected with human immunodeficiency virus; younger than 18 years; or participation in other clinical trials.
Thus, 157 patients were initially included in this study from August 1994 to May 2018. However, eight patients were excluded because of incomplete clinical data. Stage and therapy responses were evaluated by imaging and endoscopic examination. The stage of PGI-DLBCL was based on the definitions of the Lugano Staging System.10 Therapy responses were accessed according to the Lymphoma Response Criteria.11
Therapy Regiments
Patients were mainly treated with the following three therapeutic modalities: surgery, chemotherapy, and radiotherapy. The CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens with or without rituximab were the most common treatments. Patients were followed up every three months for the first three years after chemotherapy, every six months in the fourth and fifth years, and then every year. The last follow-up date was 31 July 2018.
Definition of Severe GICs
Severe GICs were confirmed by clinical tests, imaging, or surgery. Furthermore, severe GICs were recorded from initial manifestation to the last follow-up or death. Severe GIB was defined as overt bleeding (hematemesis, melena, and/or bloody stools) or occult bleeding (positive occult blood and drop in hemoglobin of ≥2 g/dL),12 and hemoglobin < 10 g/dL was required simultaneously. GIO, including partial obstruction and complete obstruction, was defined as the absence of passage of flatus or feces, which was confirmed by imaging, endoscopy, or surgery.13 GIP was considered to be the presence of free air under the diaphragm on abdominal imaging, or intestinal perforation during laparotomy, with or without localized abscess or peritonitis.3 During the course of the disease, if two or more of the above complications occurred, this was considered as multiple complications.
Statistical Analysis
The statistical software used in this study was IBM SPSS Statistics 22.0. Correlations between variables were assessed by Pearson’s chi-squared test or Fisher’s exact test. Univariate survival analysis was carried out by Kaplan-Meier and Log rank tests. Differences were considered statistically significant at P < 0.05.
Results
Clinical Characteristics
A total of 148 patients with PGI-DLBCL were included, of which 84 suffered from severe GICs. Among these 84 patients, abdominal pain or discomfort (79.8%, 67/84) was the predominant symptom, followed by hematemesis or black stools (22.6%, 19/84) and abnormal bowel habits (17.9%, 15/84). Furthermore, 11 patients had complications before and after chemotherapy, and there were 95 complication events among them (Table 1). Of the 95 cases of severe GICs, there were 76 and 19 cases as the initial manifestation and after chemotherapy, respectively. Of the 76 patients with severe GICs as the initial manifestation, there were 35, 19, 4, and 18 with specifically GIB, GIO, GIP, and multiple complications. Of the 19 patients with severe GICs after chemotherapy, there were 6, 5, 2, and 6 patients with GIB, GIO, GIP, and multiple complications.
 |
Table 1 Demographic and Clinical Characteristics of Severe GICs Before and After Chemotherapy |
Before chemotherapy, between patients with and without GICs, there were significant differences in performance status (ECOG ≥2, P = 0.021), tumor size (≥10 cm, P = 0.040) and major involved sites (intestine, P = 0.001; Table 1). After chemotherapy, B symptoms (P = 0.012), tumor size (≥10 cm, P = 0.030), and Lugano stage (IIE-IV, P = 0.042) were observed more frequently in patients with GICs than in those without GICs. Rituximab did not increase the risk of GICs. Before and after chemotherapy, severe GICs negatively correlated with objective response rate at the end of chemotherapy.
Subgroup Analysis Based on Complication Types
The median follow-up time was 36.70 months (range from 0.2 to 269.8 months), and the 3-year PFS and OS of the entire cohort were 55.9% and 66.6%, respectively. Before chemotherapy, patients with GICs had similar OS as did those without GICs (3-year OS, 62.1% cf. 71.2%, P = 0.161; Figure 1B), but they had worse PFS (3-year PFS, 46.6% cf. 65.7%, P = 0.023; Figure 1A). After chemotherapy, patients with GICs had significantly shorter 3-year PFS and OS compared with those without GICs (3-year PFS: 21.1% cf. 66.3%, P < 0.001; 3-year OS: 36.8% cf. 75.9%, P = 0.001; Figure 1C and D).
 |
Figure 1 GICs, gastrointestinal complications. PFS (A and C) and OS (B and D) in patients having GICs before and after chemotherapy. |
GIB
Of the 148 followed patients, 35 presented with GIBs as the initial manifestation, with a median age of 64 years (18–85 years). Their mean minimum hemoglobin concentration in the presence of GIB was 8.7 g/dL. Among the 35 patients with GIB at presentation, 74.3% (26/35) showed upper GIBs confirmed by endoscopy or radiology or the attending clinician. Furthermore, 13 and 20 patients were treated with surgery plus chemotherapy and chemotherapy, respectively. In this 33-patient cohort, surgery plus chemotherapy did not have a significant effect on either PFS or OS relative to chemotherapy alone (P = 0.585, P = 0.615). Furthermore, patients with GIB at the initial presentation had similar survival outcomes as did those without GICs (3-year PFS, 59.5% cf. 65.7%, P = 0.581; 3-year OS, 76.1% cf. 71.2%, P = 0.816; Figure 2A and B).
 |
Figure 2 GIB, gastrointestinal bleeding. PFS (A and C) and OS (B and D) in patients having GIB before and after chemotherapy. |
Among the 130 patients, 6 developed GIB after chemotherapy, with a median age of 70 years (60–85 years; Table 2). They had the mean minimum hemoglobin concentration of 6.7 g/dL in the presence of GIB, and 3 of them had GIB at the initial presentation. Only one patient achieved complete remission; the other five died of tumor progression. Patients with GIB after chemotherapy had worse 3-year PFS and OS compared with those who had no GICs after chemotherapy (3-year PFS, 16.7% cf. 66.3%, P < 0.001; 3-year OS, 16.7% cf. 75.9%, P < 0.001; Figure 2C and D).
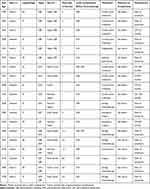 |
Table 2 Clinical Characteristics of 19 Patients with PGI-DLBCL Who Developed GICs After Chemotherapy |
GIO
Nineteen of 148 patients had GIO as the initial manifestation with a median age of 59 years (19–86 years). Among them, 96.3% (18/19) developed intestinal obstruction, with the ileocecal region most frequently involved (42.1%, 8/19). Moreover, 15 and 4 patients received surgery plus chemotherapy and chemotherapy, respectively. In this 19-patient cohort, surgery plus chemotherapy had no statistically significant effect on OS in comparison with chemotherapy (P = 0.100), but it showed significant clinical benefit on PFS (P < 0.001). Patients with GIO as the initial presentation had similar 3-year PFS and OS as did those without GICs (3-year PFS, 38.8% cf. 65.7%, P = 0.058; 3-year OS, 58.4% cf. 71.2%, P = 0.451; Figure 3A and B).
 |
Figure 3 GIO, gastrointestinal obstruction. PFS (A and C) and OS (B and D) in patients having GIO before and after chemotherapy. |
Five of the 130 patients experienced GIO after chemotherapy, with a median age of 48 years (18–68 years; Table 2). Among them, 80% (4/5) developed intestinal obstruction, with the ileocecal region most frequently involved (40.0%, 2/5). Four of those five patients were still in complete remission at the last follow-up, but the other one patient eventually died of disease progression. Patients with GIO after chemotherapy had similar 3-year PFS and OS as did those without GICs (3-year PFS, 60.0% cf. 66.3%, P = 0.933; 3-year OS, 80.0% cf. 75.9%, P = 0.466; Figure 3C and D).
GIP
Four of the 148 patients had GIP as the initial manifestation, with a median age of 77.5 years (73–83 years). 75% (3/4) of them developed gastric perforation. All of them presented with ECOG ≥2 (100%, 4/4). Two patients underwent surgery plus chemotherapy, and 1 maintained complete remission, but the other died of disease progression. One patient died after surgical treatment. The other one patient did not undergo surgery or chemotherapy due to extensive tumor invasion and cachexia status, and he died nine days later.
Two of the 130 patients had GIP during the second and fourth chemotherapy cycles, and both of them were intestinal perforation (Table 2). After emergency surgery and chemotherapy, one patient attained complete remission and the other died of disease progression.
Multiple Complications
Eighteen of the 148 patients had multiple complications before chemotherapy, with a median age of 58 years (34–83 years). GIB plus GIO was the most common combination at initial presentation (83.3%, 15/18). The small bowel (35.3%, 6/17) was the most common obstruction site. Twelve patients received surgery plus chemotherapy, three received chemotherapy, and three received surgery. In this 18-patient cohort, patients treated with surgery plus chemotherapy experienced significantly longer PFS and OS in comparison with those who received chemotherapy alone or surgery alone (P < 0.001). Patients with multiple complications as the initial manifestation had shorter PFS and OS than did those without complications (3-year PFS, 33.3% cf. 65.7%, P = 0.003; 3-year OS, 44.4% cf. 71.2%, P = 0.025; Figure 4A and B).
 |
Figure 4 MC, multiple complications. PFS (A and C) and OS (B and D) in patients having multiple complications before and after chemotherapy. |
Six of the 130 patients had multiple complications after chemotherapy, with a median age of 58 years (34–83 years; Table 2), and all of them were men. Three of them had GICs before chemotherapy. GIB plus GIO was the most common combination after chemotherapy (66.7%, 4/6). All those six patients eventually died, despite aggressive resuscitation. Patients with multiple complications after chemotherapy had worse PFS and OS than did those without complications after chemotherapy (3-year PFS, 0.00% cf. 66.3%; 3-year OS, 0.00% cf. 75.9%; both P < 0.001; Figure 4C and D).
Discussion
In the past 20 years, diagnosis, staging, and treatment of PGI-DLBCL have undergone profound changes, and treatment has shifted away from surgery. However, the management of severe GICs remains a clinical challenge. To accumulate evidence-based medicine, we retrospectively analyzed the clinical features and influences of severe GICs in 148 patients with PGI-DLBCL. Our results showed that, among the 84 patients with severe GICs, the most common clinical manifestations were abdominal pain or discomfort, hematemesis or black stools, and changes in bowel habits. Patients with ECOG ≥2, tumor mass (≥10 cm), or involvement of the intestine were more likely to present with GICs. Patients with GICs as the initial manifestation had a lower objective response rate at the end of chemotherapy and had significantly worse PFS compared with those without GICs initially, while such a disadvantage showed no significant effect on OS. This may be attributed to delayed chemotherapy, intolerance of chemotherapy, insufficient dose of chemotherapy drugs, or the development of new complications. In addition, B symptoms, tumor mass (≥10 cm) and Lugano stage (IIE, IV) significantly correlated with GICs after chemotherapy. However, rituximab did not increase the risk of GICs.14 GICs after chemotherapy reduced the objective response rate at the end of chemotherapy and had a significant negative influence on both PFS and OS.
In this study, the incidence rates of GIB before and after chemotherapy were 23.6% and 4.6% respectively. The reasons for GIB may include active lymphoma, chemotherapy-induced ischemia, larger tumor size, and infections (particularly Cytomegalovirus and Candida).15,16 Most of the affected cases were upper GIB, which is consistent with one previous study, and in that study, tumor with upper GIB was more aggressive than lower GIB tumors.17 In the present study, GIB after chemotherapy usually occurred during the first 3 cycles of chemotherapy. GIB before chemotherapy did not significantly affect OS or PFS. Because bleeding is more alerting than other non-specific symptoms (such as abdominal pain or bloating), it can provide opportunities for early diagnosis and treatment. In contrast, even though most GIB after chemotherapy appeared to improve, these patients had significantly shorter OS and PFS than those without GICs after chemotherapy. As reported by Spectre et al,15 GIB after chemotherapy may be considered a risk factor for poor prognosis in PGI-DLBCL. In addition, for patients in the current study with GIB initially, surgery had no significant effect on either PFS or OS. This may be because surgery will cause serious damage to the physiology of the gastrointestinal tract, increase the risk of perioperative GICs, delay chemotherapy, and cause malabsorption syndrome, thereby reducing the quality of life of patients and affecting the prognosis.18
In the current study, the incidence rates of GIO before and after chemotherapy were 12.8% and 3.8%, respectively. Several factors may contribute to GIO, including benign causes (eg, local edema, adhesions, and intestinal paralysis caused by chemotherapy), or malignant causes (recurrent cancer).15,16 Patients with tumor mass or intestinal involvement are prone to GIO, and the ileocecal region is the most common obstruction site. GIO after chemotherapy usually occurred during the first 2 cycles of chemotherapy. In addition, our results suggested that GIO occurred before or after chemotherapy had no significant effect on PFS or OS, which is similar with one previous study.15 It may be that mechanical obstruction leads to earlier diagnosis and early treatment. Furthermore, we found that patients who developed GIO after chemotherapy usually had a history of surgery before chemotherapy, and most of their symptoms could be relieved after conservative treatment. Therefore, GIO after chemotherapy in this study may develop from benign causes. However, it can be observed that GIO can really affect the quality of life and delay chemotherapy.
In this study, the incidence rates of GIP before and after chemotherapy were 2.7% and 1.5%, respectively, which was lower than in other reports.3,19 GIP after chemotherapy occurred in the second and fourth cycles. Perforation can be caused by spontaneous tumor necrosis, chemotherapy, or cytotoxic drug-induced ulcer progression, usually in the context of corticosteroids or non-steroidal anti-inflammatory drug treatment.16 Perforation can delay and complicate chemotherapy, and adversely affect the long-term survival of patients who may die from the perforation or its complications.3,19 The data of GIP in our study is limited.
The incidence rates of multiple complications before and after chemotherapy were 12.2% and 4.6%, respectively. No other studies have detailed multiple complications. It is important to note this situation, because multiple complications have a significant adverse effect on the long-term survival of patients whether before or after chemotherapy. In this study, patients with advanced stage or tumor mass were more likely to have multiple complications. Multiple complications after chemotherapy usually developed during the first 4 cycles of chemotherapy. GIB plus GIO was the most common combination. The mortality rates of patients with multiple complications before or after chemotherapy were 66.7% and 100%, even though some complications improved at that time after aggressive resuscitation. The significantly lower overall survival may indicate that tumors are highly invasive and require high vigilance and reasonable management.
Therefore, more attention and optimal treatment should be adopted to reduce the incidence of fatal GICs and improve the GICs that have already occurred. Firstly, for patients with high-risk GIB, such as those with advanced age, tumor mass, history of peptic ulcers, or using antiplatelet agents, a proton pump inhibitor is recommended to prevent GIB during chemotherapy. Furthermore, based on positron emission tomography/computed tomography (PET-CT) and the Ki-67 proliferation index, we tend to appropriately reduce the dose of drugs (such as corticosteroids and anthracyclines) that may damage the mucosa in the first 2 chemotherapy cycles. Helicobacter pylori infection should be screened and treated if necessary.20,21 When active GIB is suspected, the patient should be managed as any other high-risk GIB. The Rockall score and Glasgow-Blatchford bleeding score can be utilized to evaluate risk of adverse outcomes and the need for intervention in upper GIB.22,23 Experienced endoscopists must be involved from the beginning,24,25 but should be wary of rebleeding after endoscopic management.17 Transcatheter arterial embolization is the next choice for identifying GIB sites, but also shows limited efficacy to manage GI lymphoma-related bleeding due to high rebleeding at new sites.26 Surgery is required to treat rebleeding when necessary.25,27 Secondly, patients with a heavy tumor burden, intestinal involvement, or a surgical history should be monitored for GIO. Vincristine, which may induce intestinal paralysis,28 can be postponed in the first cycle in older or unfit patients. Other preventive measures include maintaining electrolyte balance, care taken with opioids and drugs inhibiting bowel motility, and a low-fiber diet. A suction probe can be installed in patients with high-risk of occlusion. Surgery does not improve the long-term survival of patients with GIO.29 Unless strangulation is suspected and requires emergency surgery, conservative management with analgesia, intravenous infusion, nutritional support, and rhino-endoscopic suction should be used initially. The chemotherapy regimen could be adjusted if necessary. Thirdly, increased vigilance and active surveillance are needed in patients with ECOG ≥2 or beyond the initial treatment time, because they have a high risk for GIP. Significantly hypermetabolic foci on PET-CT or a higher Ki-67 level in the pathology indicate that tumor cells proliferated vigorously and are sensitive to chemotherapy, which may lead to tumor lysis syndrome and thus GIP. A drug dose that may easily cause mucosal damage and tumor lysis syndrome can be appropriately reduced to balance the destruction due to chemotherapy and mucosal repair in the first 2 cycles. Protective measures need to be adopted, such as an adjunctive proton pump inhibitor. Patients with new abdominal pain during treatment should receive a prompt physical examination and evaluation of free air in the abdomen with appropriate imaging. A delay in diagnosis and treatment results in increased mortality. Surgery is recommended as long as the patient is in good health. All in all, our clinical experience suggests that the most appropriate style of intervention depends on tumor location, complication type, tumor burden, and severity of GICs. Of course, more high-quality evidence is needed to find the optimal treatment for patients with GICs.
This is a single-center retrospective study on a limited scale, especially with a small number of GIB, GIO, GIP, and multiple complications after chemotherapy. A prospective trial is warranted to explore appropriate predictors of GIB, GIO, and GIP. It was recently reported that ibrutinib plus lenalidomide and rituximab in relapsed/refractory non-germinal center B-cell-like DLBCL showed an encouraging result.30 Whether a chemotherapy-free regimen can decrease the occurrence of GICs associated chemotherapy in patients with PGI-DLBCL is worth exploring.
Conclusion
Patients with PGI-DLBCL usually develop GIB, GIO, or GIP, whether these present as the initial manifestation or after chemotherapy. Gastrointestinal complications greatly degrade quality of life, prolong hospitalization, and reduce the long-term survival of patients. The current results warrant further validation by larger studies.
Abbreviations
DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; GIB, gastrointestinal bleeding; GIC, gastrointestinal complication; GIO, gastrointestinal obstruction; GIP, gastrointestinal perforation; OS, overall survival; PET-CT, positron emission tomography/computed tomography.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of Peking University First Hospital and Institute (number 2017–1304). The requirement for written informed consent was waived off due to the retrospective nature of the study. All data used in this manuscript were anonymized.
Acknowledgments
Ye Shen and Jinping Ou are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, specifically in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the national cancer data base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. doi:10.1002/ajh.24086
2. Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the surveillance, epidemiology and end results database. Am J Hematol. 2014;89(3):310–314. doi:10.1002/ajh.23638
3. Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24(9):2439–2443. doi:10.1093/annonc/mdt188
4. Koch P, Del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma–results of the prospective German multicenter study GIT NHL 01/92. J clin oncol. 2001;19(18):3874–3883. doi:10.1200/JCO.2001.19.18.3874
5. Schmidt WP, Schmitz N, Sonnen R. Conservative management of gastric lymphoma: the treatment option of choice. Leuk Lymphoma. 2004;45(9):1847–1852. doi:10.1080/1042819042000219476
6. Gou HF, Zang J, Jiang M, Yang Y, Cao D, Chen XC. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol. 2012;29(1):227–234. doi:10.1007/s12032-010-9783-x
7. Abbott S, Nikolousis E, Badger I. Intestinal lymphoma--a review of the management of emergency presentations to the general surgeon. Int J Colorectal Dis. 2015;30(2):151–157.
8. Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80–89. doi:10.1002/bjs.18004921319
9. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
10. Rohatiner A, d’Amore F, Coiffier B. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5(5):397–400. doi:10.1093/oxfordjournals.annonc.a058869
11. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J clin oncol. 2007;25(5):579–586. doi:10.1200/JCO.2006.09.2403
12. JW E, Eikelboom JW, Bosch J. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2019;157(2):403–412.e405. doi:10.1053/j.gastro.2019.04.041
13. Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13(3):432–437. doi:10.3748/wjg.v13.i3.432
14. Kassam SA, Goldstone AH, Hoffbrand AV, Perez-Machado M, Mcnamara CJ. Rituximab does not increase the complication rate, particularly perforation, when added to chemotherapy for patients with primary b-cell lymphomas of the gastrointestinal tract. Blood. 2005;106(11):4742. doi:10.1182/blood.V106.11.4742.4742
15. Spectre G, Libster D, Grisariu S, et al. Bleeding, obstruction, and perforation in a series of patients with aggressive gastric lymphoma treated with primary chemotherapy. Ann Surg Oncol. 2006;13(11):1372–1378. doi:10.1245/s10434-006-9069-x
16. Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61(2):179–192. doi:10.1136/gutjnl-2011-300563
17. Schatz RA, Rockey DC. Gastrointestinal bleeding due to gastrointestinal tract malignancy: natural history, management, and outcomes. Dig Dis Sci. 2017;62(2):491–501. doi:10.1007/s10620-016-4368-y
18. Koch P, Probst A, Berdel WE. Treatment results in localized primary gastric lymphoma: data of patients registered within the german multicenter study (GIT NHL 02/96). J Clin Oncol. 2005;23(28):7050–7059. doi:10.1200/JCO.2005.04.031
19. Chin CK, Tsang E, Mediwake H, et al. Frequency of bowel perforation and impact of bowel rest in aggressive non-Hodgkin lymphoma with gastrointestinal involvement. Br J Haematol. 2019;184(5):826–828. doi:10.1111/bjh.15173
20. Karstensen JG, Ebigbo A, Aabakken L, et al. Nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) cascade guideline. Endoscopy International Open. 2018;6(10):E1256–e1263. doi:10.1055/a-0677-2084
21. Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18(11):1202–1207. doi:10.3748/wjg.v18.i11.1202
22. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321. doi:10.1136/gut.38.3.316
23. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–1321. doi:10.1016/S0140-6736(00)02816-6
24. Laine L. Timing of endoscopy in patients hospitalized with upper gastrointestinal bleeding. N Engl J Med. 2020;382(14):1361–1363. doi:10.1056/NEJMe2002121
25. Oakland K, Chadwick G, East JE, et al. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British society of gastroenterology. Gut. 2019;68(5):776–789. doi:10.1136/gutjnl-2018-317807
26. Zheng L, Shin JH, Han K, et al. Transcatheter arterial embolization for gastrointestinal bleeding secondary to gastrointestinal lymphoma. Cardiovasc Intervent Radiol. 2016;39(11):1564–1572. doi:10.1007/s00270-016-1422-2
27. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(10):a1–46. doi:10.1055/s-0034-1393172
28. Yasu T, Ohno N, Kawamata T, Kurokawa Y. Vincristine-induced paralytic ileus during induction therapy of treatment protocols for acute lymphoblastic leukemia in adult patients. Int J Clin Pharmacol Ther. 2016;54(6):471–473. doi:10.5414/CP202584
29. Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007;136(4):521–538. doi:10.1111/j.1365-2141.2006.06444.x
30. Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–1036. doi:10.1182/blood.2018891598
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
