Back to Journals » Cancer Management and Research » Volume 11
Influence of serum total cholesterol, LDL, HDL, and triglyceride on prostate cancer recurrence after radical prostatectomy
Authors Cheng S, Zheng Q, Ding G, Li G
Received 12 February 2019
Accepted for publication 26 June 2019
Published 16 July 2019 Volume 2019:11 Pages 6651—6661
DOI https://doi.org/10.2147/CMAR.S204947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Sheng Cheng, Qiming Zheng, Guoqing Ding, Gonghui Li
Department of Urology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Purpose: The clinical impacts of serum lipid levels on prostate cancer recurrence after radical prostatectomy have been evaluated by several observational studies with conflicting results. We performed the present meta-analysis to summarize the evidence evaluating the role of serum lipid profile in prostate cancer patients.
Methods: We comprehensively searched the PubMed database for potentially relevant studies through January 2019. Pooled hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) for the highest versus the lowest level of serum lipid levels were calculated with the DerSimonian and Laird random-effects model.
Results: A total of 12 eligible studies with 10,978 prostate cancer cases were included in this study. The pooled HRs of prostate cancer recurrence after racial prostatectomy were 0.92 (95% CI 0.73–1.16, P=0.462), 0.87 (95% CI 0.56–1.35, P=0.535), 1.09 (95% CI 0.92–1.30, P=0.320), and 1.01 (95% CI 0.78–1.31, P=0.938) for serum total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride, respectively. Sensitivity analysis was conducted by excluding each study sequentially and the results showed that all the summary risk estimates were stable and not influenced by any single study.
Conclusion: The present meta-analysis indicated that serum lipid levels in patients undergoing radical prostatectomy were not associated with prostate cancer recurrence.
Keywords: serum lipids, prostate cancer, recurrence, radical prostatectomy, meta-analysis
Introduction
Prostate cancer is the most commonly diagnosed non-skin cancer and the second most common cause of cancer-related mortality in men in developed countries.1 Radical prostatectomy remains the gold-standard treatment for clinically localized prostate cancer.2 However, 20–40% of the patients undergoing radical prostatectomy will experience biochemical recurrence within 10 years.3,4 Well-established risk factors to predict prostate cancer recurrence include prostate-specific antigen (PSA) levels at diagnosis, tumor stage, Gleason score, and surgical margin status.5,6
Hyperlipidemia, a condition strongly related to obesity, currently affects about 20% of the adult population in the United States.7 Cholesterol is hypothesized to participate in prostate cancer pathogenesis as it plays a crucial role in the differentiation and growth of the prostate gland.8 The impact of dyslipidemia on the risk of prostate cancer recurrence has been evaluated by various epidemiologic studies. Some studies reported positive associations between elevated cholesterol,9 low-density lipoprotein (LDL),10 or triglyceride9 and the risk of prostate cancer recurrence, while others reported no associations11,12 or even inverse associations.13–15 Therefore, we aimed to investigate the effects of serum lipid levels, including total cholesterol, LDL, high-density lipoprotein (HDL), and triglyceride, on the prostate cancer recurrence after radical prostatectomy based on a meta-analysis of all eligible studies.
Materials and methods
Search strategy
We comprehensively searched PubMed database for potentially relevant studies through January 2019 using the following keywords: (Lipid level or cholesterol or high-density lipoprotein or HDL or low-density lipoprotein or LDL or triglyceride or dyslipidemia) and prostatectomy and (prospective or follow-up or followed up or cohort or longitudinal or nested case-control or case-cohort). In addition, we manually examined the reference lists of retrieved articles and related reviews for additional eligible studies. There was no limitation on publication date or language. We attempted to perform this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).16
Inclusion criteria
Studies included in this meta-analysis met all the following criteria: (i) It was a cohort study in prostate cancer patients after radical prostatectomy; (ii) the exposure of interest was serum lipid profile, including total cholesterol, HDL, LDL, or triglyceride; (iii) the endpoint of interest was prostate cancer recurrence; and (iv) the study provided the hazard ratios (HRs) with their 95% confidence intervals (CIs) for the highest compared with the lowest category of serum lipids. If multiple publications used the same study population, the study with the largest sample size was included in our meta-analysis.
Data extraction
The following information were extracted independently by two investigators (SC and QZ) using a standardized data-collection form: the first author’s last name, publication year, study location, study design (prospective or retrospective), length of follow-up, number of participants, rate of recurrence, age of participants, types of exposure, the HRs with their corresponding 95% CIs from the most fully adjusted model, and adjusted variables in the multivariate analysis.
Assessment of study quality
The study quality and risk of bias were evaluated by two independent investigators (SC and QZ) using the Newcastle–Ottawa Scale (NOS). NOS is a 9-star instrument designed to assess the characteristics of the study population, the comparability of the groups, and ascertainment of endpoint of interest. A study with no less than 7 stars was considered as a high-quality study.
Statistical analysis
The study by Allott et al (2016)9 assessed the association between serum cholesterol and prostate cancer recurrence in black and non-black men separately, which was considered as two independent studies. Risk estimates and their corresponding 95% CIs were not directly available in the study by Ohno et al,17 which were calculated from the data reported in the articles using the methods proposed by Parmar et al.18
Combined risk estimates with their corresponding 95% CIs for the highest versus the lowest level of total cholesterol, LDL, HDL, or triglyceride were calculated with the DerSimonian and Laird random-effects model,19 which takes into account both within-study and between-study variation. Heterogeneity across studies was tested by Q statistics (significance level set at P<0.10) and I2 statistic (a quantitative measure of inconsistency across studies). Prespecified subgroup analyses and meta-regression models were performed to investigate the potential sources of between-study heterogeneity.
A sensitivity analysis was performed by repeating meta-analysis after omitting each study in turn to determine the influence of a single study. Publication bias was tested by Begg’s funnel plot20 and Egger’s regression test.21 We also used the trim-and-fill method to evaluate publication bias.22 A two-tailed P<0.05 was considered significant, except where otherwise specified. All of the statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX).
Results
Study selection
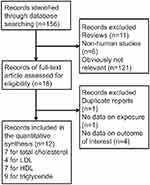
Figure 1 presents a flow chart of the study selection process. Searches of the electronic databases yielded 156 potentially eligible studies. After carefully reading the titles/abstracts, most studies were excluded because they were reviews (n=11), non-human studies (n=6), or obviously not related with our topic (n=121). After assessing the full-text of 18 potentially relevant articles, we identified 12 eligible studies,9–15,17,23–26 including 13 data sets for analysis; 1 study9 reported the results separately by racial differences. The main reasons for exclusion were as follows: duplicate reports (n=1), no data on exposure (n=1), and no data on outcome of interest (n=4).
Main characteristics of the included studies
The main characteristics of the 12 included studies are summarized in Table 1. These studies were published between 2010 and 2018, including 8 retrospective and 4 prospective cohort studies. Five studies were conducted in North America, 3 in Europe, and 4 in Asia. The total number of prostate cancer patients was 10,978. The length of the follow-up period ranged from 21 to 134.4 months. Total cholesterol was reported in 7 studies, LDL reported in 4 studies, HDL reported in 7 studies, and triglyceride reported in 9 studies. Definition of recurrence ascertainments was not consistent across studies; most studies used biochemical recurrence data, while some studies used either biochemical recurrence or receiving secondary treatment. NOS scores ranged from 6 to 9, with a mean of 7.75 (Table S1).
 |
Table 1 Main characteristics of the included studies |
Quantitative synthesis
The pooled HRs of prostate cancer recurrence after racial prostatectomy were 0.92 (95% CI 0.73–1.16, P=0.462) (Figure 2), 0.87 (95% CI 0.56–1.35, P=0.535) (Figure 3), 1.09 (95% CI 0.92–1.30, P=0.320) (Figure 4), and 1.01 (95% CI 0.78–1.31, P=0.938) (Figure 5) for serum total cholesterol, LDL, HDL, and triglyceride, respectively.
 |
Figure 2 A forest plot of hazard ratio for total cholesterol, which was reported in 7 studies (8 data sets). |
 |
Figure 3 A forest plot of hazard ratio for low-density lipoprotein (LDL), which was reported in 4 studies (5 data sets). |
 |
Figure 4 A forest plot of hazard ratio for high-density lipoprotein (HDL), which was reported in 7 studies (8 data sets). |
 |
Figure 5 A forest plot of hazard ratio for triglyceride, which was reported in 9 studies (10 data sets). |
In the subgroup analysis by study design and geographical region, significant associations were observed for total cholesterol in prospective studies and in Asia studies, for LDL in prospective studies, in retrospective studies, in North America studies, and in Europe studies, and for triglyceride in prospective studies and in Asia studies. There were no significant associations in the rest of the subgroups. Interaction analyses based on meta-regression models indicated that study design and geographical region were the significant sources of heterogeneity for LDL analysis (P=0.025) (Table 2). We also performed subgroup analyses by duration of follow-up, number of total patients, and NOS score. The results have been summarized in Table S2. A significant inverse association was observed between total cholesterol and prostate cancer recurrence in studies with long follow-up (≥36 months).
 |
Table 2 Subgroup analyses stratified by study design and region |
Obvious between-study heterogeneity was observed for total cholesterol (P=0.001, I2 = 71.6%), LDL (P=0.001, I2 = 78.40%), and triglyceride (P=0.001, I2 = 68.20%), except for HDL (P=0.295, I2 = 17.20%). Sensitivity analysis was conducted by excluding each study sequentially. As shown in Figure 6, all the summary risk estimates for total cholesterol, LDL, HDL, and triglyceride were stable and not influenced by any single study. There was no evidence of significant publication bias based on Begg’s test or Egger’s test for total cholesterol, LDL, and triglyceride, but not for HDL (Figure 7 and Table 3). The trim-and-fill analysis suggested that no trimming was performed and thus data were unchanged for HDL.
 |
Table 3 P-values of Begg’s test and Egger’s test |
 |
Figure 6 Sensitivity analysis was conducted by excluding each study sequentially and then repeating the meta-analysis to determine the influence of a single study. |
 |
Figure 7 Begg’s funnel plots for publication bias. If publication bias is present, the plot will be asymmetrical. |
Discussion
The present meta-analysis included 12 cohort studies with a total of 10,978 prostate cancer cases. The results indicated that serum lipid profile, including total cholesterol, LDL, HDL, and triglyceride, was not related with the prostate cancer recurrence after radical prostatectomy. To the best of our knowledge, this is the first meta-analysis aimed to evaluate the impact of dyslipidemia on oncologic outcomes in prostate cancer patients.
Several laboratory studies have investigated the impact of cholesterol levels on the growth of prostate tumors. Zhuang et al27 found that cholesterol-rich lipid rafts mediated epidermal growth factor (EGF)-induced AKT signaling activation, which promoted the survival of prostate cancer cells. Mostaghel et al28 reported that hypercholesterolemia increased intratumoral de novo steroidogenesis and thus was able to drive the growth of prostate tumors. Murtola et al29 proposed that cholesterol metabolism in prostate cancer had been reprogrammed to accelerate the cell growth of prostate cancer. However, epidemiologic evidence for the potential association between serum cholesterol and prostate cancer recurrence was mixed as discussed earlier. Considering that dyslipidemia was a modifiable risk factor and thus potentially had an important role in secondary prevention strategy for prostate cancer recurrence, we performed the present meta-analysis.
The role of serum lipid profile on other human cancers’ development has been assessed by various observational studies. A meta-analysis of prospective cohort studies by Lin et al30 indicated that serum total cholesterol and HDL were inversely associated with lung cancer risk, while triglyceride was positively associated with lung cancer risk. Touvier et al31 found that there was a modest but statistically significant inverse association between total cholesterol and more specifically HDL and breast cancer risk. As for prognosis analysis, a recent meta-analysis included 26 studies with a total of 24,655 individuals, which suggested that serum total cholesterol and HDL were significantly negatively associated with cancer survival.32 By contrast, two previous meta-analyses aimed to evaluate the association between blood lipid concentrations and prostate cancer risk did not support the hypothesis that dyslipidemia was related with the risk of prostate cancer.33,34 Therefore, the biological role of serum lipid profile played in tumorigenicity may be varied in human cancers.
This present study had several limitations that should be acknowledged. First, obvious heterogeneity was observed across included studies. Heterogeneity was generally caused by the inconsistency of study population, study design, methods of exposure (ie, the methods used to measure the serum metabolites) and outcome assessment, and so on. This can also explain why some studies demonstrated a positive association while others showed a negative association with total cholesterol, LDL, or HDL. Our subgroup analysis and interaction analysis suggested that study design and geographical region were the potential sources of heterogeneity. Second, the number of eligible studies for LDL analysis was relatively limited. In addition, the results of these studies were opposite, which made the summary risk estimate toward null. Further studies are needed to evaluate the role of LDL in the prognosis of prostate cancer. Third, a certain degree of publication bias was found for HDL analysis. Gray literature (eg, conference abstract) was hard to be found and small studies with null results were less likely to be published. Fourth, the cutoff points for highest versus lowest level of serum lipids varied across included studies, which might distort the summary analysis. Fifth, most of the included studies did not provide the risk estimates according to the patients’ Gleason score or tumor stage. Therefore, we were not able to investigate the hazard ratio of lipids with prostate cancer recurrence in context of the Gleason score and tumor stage. Finally, the length of follow-up in part of the included studies was relatively too short to identify enough number of recurrence cases, which reduced the statistical power.
This study also had some strengths. The sample size was relatively large with a total of 12 cohort studies and more than 10,000 prostate cancer patients, which improved the statistical power and reliability of pooled results. Sensitivity analyses performed by repeating meta-analysis after omitting each single study in turn indicated that the summary risk estimates were robust and not dominated by any individual study.
Conclusion
The present meta-analysis indicated that serum lipid levels in patients undergoing radical prostatectomy were not associated with prostate cancer recurrence.
Acknowledgments
This study was supported by grants from the Zhejiang Science and Technology Project (2017C33058).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Faria EF, Chapin BF, Muller RL, Machado RD, Reis RB, Matin SF. Radical prostatectomy for locally advanced prostate cancer: current status. Urology. 2015;86(1):10–15. doi:10.1016/j.urology.2015.03.012
3. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–439. doi:10.1001/jama.294.4.433
4. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910–914. doi:10.1097/01.ju.0000134888.22332.bb
5. Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51(5):1175–1184. doi:10.1016/j.eururo.2007.01.015
6. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. doi:10.1097/01.ju.0000045749.90353.c7
7. Sempos CT, Cleeman JI, Carroll MD, et al. Prevalence of high blood cholesterol among US adults. An update based on guidelines from the second report of the National Cholesterol Education Program Adult Treatment Panel. JAMA. 1993;269(23):3009–3014.
8. Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013;1835(2):219–229. doi:10.1016/j.bbcan.2013.01.002
9. Allott EH, Howard LE, Aronson WJ, et al. Racial differences in the association between preoperative serum cholesterol and prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2016;25(3):547–554. doi:10.1158/1055-9965.EPI-15-0876
10. Macleod LC, Chery LJ, Hu EY, et al. Metabolic syndrome, dyslipidemia and prostate cancer recurrence after primary surgery or radiation in a veterans cohort. Prostate Cancer Prostatic Dis. 2015;18(2):190–195. doi:10.1038/pcan.2015.12
11. Sanchis-Bonet A, Ortiz-Vico F, Morales-Palacios N, Sanchez-Chapado M. The association between metabolic syndrome and prostate cancer: effect on its aggressiveness and progression. Actas Urol Esp. 2015;39(3):154–160. doi:10.1016/j.acuro.2014.09.009
12. Komaru A, Kamiya N, Suzuki H, et al. Implications of body mass index in Japanese patients with prostate cancer who had undergone radical prostatectomy. Jpn J Clin Oncol. 2010;40(4):353–359. doi:10.1093/jjco/hyp164
13. Kang M, Jeong CW, Ku JH, Kwak C, Kim HH. Hypertriglyceridemia is a potential preoperative predictor for biochemical recurrence after radical prostatectomy. PLoS One. 2015;10(3):e0122438. doi:10.1371/journal.pone.0122438
14. Rantaniemi L, Tammela TLJ, Kujala P, Murtola TJ. Blood cholesterol, tumor clinical characteristics and risk of prostate cancer progression after radical prostatectomy. Scand J Urol. 2018;52(4):269–276. doi:10.1080/21681805.2018.1492967
15. Wettstein MS, Saba K, Umbehr MH, et al. Prognostic role of preoperative serum lipid levels in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate. 2017;77(5):549–556. doi:10.1002/pros.23296
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W264. doi:10.7326/0003-4819-151-4-200908180-00135
17. Ohno Y, Ohori M, Nakashima J, et al. Association between preoperative serum total cholesterol level and biochemical recurrence in prostate cancer patients who underwent radical prostatectomy. Mol Clin Oncol. 2016;4(6):1073–1077. doi:10.3892/mco.2016.831
18. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834.
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629
22. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463.
23. Allott EH, Farnan L, Steck SE, et al. Statin use, high cholesterol and prostate cancer progression; results from HCaP-NC. Prostate. 2018;78(11):857–864. doi:10.1002/pros.23644
24. Bhindi B, Xie WY, Kulkarni GS, et al. Influence of metabolic syndrome on prostate cancer stage, grade, and overall recurrence risk in men undergoing radical prostatectomy. Urology. 2016;93:77–85. doi:10.1016/j.urology.2016.01.041
25. Shiota M, Yokomizo A, Takeuchi A, et al. The feature of metabolic syndrome is a risk factor for biochemical recurrence after radical prostatectomy. J Surg Oncol. 2014;110(4):476–481. doi:10.1002/jso.23677
26. Post JM, Beebe-Dimmer JL, Morgenstern H, et al. The metabolic syndrome and biochemical recurrence following radical prostatectomy. Prostate Cancer. 2011;2011:245642. doi:10.1155/2011/245642
27. Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–2231.
28. Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7(1):e30062. doi:10.1371/journal.pone.0030062
29. Murtola TJ, Syvala H, Pennanen P, et al. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS One. 2012;7(6):e39445. doi:10.1371/journal.pone.0039445
30. Lin X, Lu L, Liu L, et al. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J Clin Lipidol. 2017;11(4):1073–1081. doi:10.1016/j.jacl.2017.05.004
31. Touvier M, Fassier P, His M, et al. Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr. 2015;114(3):347–357. doi:10.1017/S000711451500183X
32. Zhou P, Li B, Liu B, Chen T, Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: a systematic review and meta-analysis. Clin Chim Acta. 2018;477:94–104. doi:10.1016/j.cca.2017.11.039
33. Ma HQ, Cui LH, Li CC, Yu Z, Piao JM. effects of serum triglycerides on prostate cancer and breast cancer risk: a meta-analysis of prospective studies. Nutr Cancer. 2016;68(7):1073–1082. doi:10.1080/01635581.2016.1206582
34. YuPeng L, YuXue Z, PengFei L, et al. Cholesterol levels in blood and the risk of prostate cancer: a meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1086–1093. doi:10.1158/1055-9965.EPI-14-1329
Supplementary materials
 |
Table S1 Newcastle–Ottawa Scale scores of each included study |
 |
Table S2 Subgroup analyses stratified by duration of follow-up, No. of patients, and NOS score |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.