Back to Journals » Clinical and Experimental Gastroenterology » Volume 9
Influence of Saccharomyces boulardii CNCM I-745 on the gut-associated immune system
Authors Stier H, Bischoff S
Received 20 April 2016
Accepted for publication 18 July 2016
Published 13 September 2016 Volume 2016:9 Pages 269—279
DOI https://doi.org/10.2147/CEG.S111003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Heike Stier,1 Stephan C Bischoff2
1analyze & realize GmbH, Berlin, 2Department of Clinical Nutrition, University of Hohenheim, Stuttgart, Germany
Background: The probiotic Saccharomyces boulardii CNCM I-745 (also known as Saccharomyces cerevisiae HANSEN CBS 5926; in the following S. boulardii) has proven its effectiveness in preventive and therapeutic treatment of many gastrointestinal diseases, especially diseases associated with acute diarrhea. In particular, antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, traveller’s diarrhea, as well as acute diarrhea due to common viral and bacterial infections in children and adults.
Aim: The aim of this review is to summarize the experimental studies elucidating the molecular and immunological mechanisms by which these clinically proven effects are archived, with an emphasis on the gut-associated immune system. The main focus is laid on anti-inflammatory and immune-modulatory action of S. boulardii involved in bacterial or enterotoxin-mediated diarrhea and inflammation. An attempt is made to differentiate between the effects associated with cellular versus soluble factors and between prophylactic and therapeutic effects.
Methods: A literature search was performed in PubMed/PubMed Central for the effects of S. boulardii on the gut-associated immune system (focus acute diarrhea).
Results and conclusion: S. boulardii exhibits its positive effect by the direct effects on pathogens or their toxins as well as by influencing the host’s infection-induced signaling cascades and its innate and adaptive immune system. The combination of these mechanisms results in a reduction of the pathogens’ ability for adhesion or colonization and an attenuation of the overreacting inflammatory immune response. Thereby, the integrity of the intestinal epithelial cell layer is preserved or restored, and the diarrheic leakage of fluids into the intestinal lumen is attenuated.
Keywords: mode of action, probiotic, infectious gastrointestinal disease, diarrhea, safety
Introduction
Objective of this review
There is an expanding awareness of the role of the gut microbiome on immune function and response to pathogens. Saccharomyces boulardii is used worldwide for the prevention and treatment of infectious diarrhea of various etiologies. Meta-analyses have confirmed the clinical efficacy of S. boulardii in acute diarrhea of various causes in children1 and in adults.2–4
Some of the effects of these infectious types of diarrhea might be evoked by a direct influence of S. boulardii on the modulation of the exiting gut microbes.5 Further mechanisms are trophic effects on enterocytes,6–8 reduction of bacterial virulence by toxin and pathogen binding9–13 as well as interference with bacterial motility and translocation13,14 (for review, Pothoulakis15).
This review summarizes the current knowledge on how S. boulardii interferes with pathogen-induced signaling pathways and how it exhibits anti-inflammatory effects. Also it describe how S. boulardii interacts with the innate or adaptive immune system to achieve its protective and therapeutic effects.
S. boulardii CNCM I-745, a specific probiotic
S. boulardii is a yeast strain of the species Saccharomyces cerevisae and has been used as a probiotic for >50 years. Historically, it has been thought to be a different Saccharomyces species before genetic analysis classified S. boulardii as a strain of the S. cerevisae species. Therefore, the correct nomenclature for S. boulardii should be Saccharomyces (genus) cerevisiae (species) var boulardii (strain). Even though genetically very close, there are differences, which might be related to the number of genes involved in protein synthesis and stress response.16,17 As a consequence, S. boulardii exhibits a faster growth rate within the intestinal tract than S. cerevisae due to its increased temperature optimum and its higher acid resistance.18 Compared to probiotics such as Lactobacillus and Bifidobacterium, S. boulardii has the advantage to be naturally resistant against all antibiotics by being a yeast.
CNCM I-745 is a S. boulardii strain produced by Biocodex Laboratories (Gentilly, France) and very well characterized by numerous preclinical and clinical data. In an expert opinion, the use of CNCM I-745 in various clinical conditions is evaluated.19
Search method
A literature search was performed in PubMed/PubMed Central for the effects of S. boulardii on the gut-associated immune system (focus acute diarrhea; September to October 2015). Main search terms were “Saccharomyces boulardii” combined with “immune”, associated with either “gastro*”, “gut”, or “intestinal”. Publication languages other than “English”, “French”, Spanish”, or “German” were excluded.
Additional literature for specific topics (eg, gut-associated immune system or overview about diseases) and follow-up literature citations in the identified publications were added.
Interaction of S. boulardii and the immune system
Stimulating the immune activity in response to S. boulardii
Investigations on germ-free mice have shown the importance of the body’s own microbiota in the development of the immune system.20,21 Even though germ-free mice are an artificial system, which is not comparable to a healthy microbiome, it clearly demonstrates the importance of microbes for the development of the gut-associated immune system.
Therefore, it is not surprising that the probiotic S. boulardii modulates the host’s gastrointestinal immune system. Apart from its anti-inflammatory abilities during infections, S. boulardii can assist the host immune system by inducing the release of immunoglobulins and cytokines in response to the yeast itself.
Immunoglobulin induction by S. boulardii
Secretory immunoglobulin A (sIgA) release is the first-line of defense against pathogens in the intestine. It prevents adhesion and forces the clearance of pathogens through several mechanisms.22 sIgA release can be enhanced by S. boulardii in germ-free mice23,24 as well as in normal BALB/c mice25 or in the duodenal fluid of weanling rats.26 The release of sIgA was even further increased when mice received Clostridium difficile toxin A during S. boulardii treatment.25
Furthermore, in germ-free mice colonized with S. boulardii, there was an increase in total serum IgM as well as an increased number of Kupffer cells (liver macrophages). This leads to a more efficient clearing of enteropathogenic Escherichia coli (EPEC) from the blood stream compared to germ-free controls, coupled with a faster cytokine response.24 This demonstrates that S. boulardii is able to modulate the host immune system toward a more activated state by increasing the host’s resistance to enteropathogenic bacterial infections on a local level within the intestine, but also with systemic effects, for example, density of liver macrophages.
Induction of cytokines and immune cell maturation by S. boulardii
As early as 1986, Caetano et al were able to show in humans that S. boulardii can activate several cellular and humoral parameters involved in the nonspecific acute phase of defense against pathogens.27 They observed an increase of erythrocytes and leucocytes together with an increase in serum complement values in response to exposure to S. boulardii.
The putative immunomodulatory role of S. boulardii in the activation of dendritic cells (DCs) prior to infection was observed by a slight transcriptional upregulation for tumor necrosis factor alpha (TNFα) and C-C chemokine receptor type 7 mRNAs after coincubation of DCs with S. boulardii. This upregulation before infection might make the DCs more effective in antagonizing bacteria.28
Another study found that S. boulardii stimulated the production of several cytokines in DCs, including interleukin (IL)-1β, IL-12, IL-6, TNFα, as well as IL-10. In addition, S. boulardii induced high levels of the costimulatory molecules CD80 and CD86, indicative of DC maturation. Most likely, a heat-stable yeast cell wall-derived factor was responsible for the effects.29
These findings suggest that S. boulardii leads to a general unspecific immune system activation, which can be advantageous for fighting infections.
S. boulardii-mediated immune priming
The release of immunoglobulins and cytokines in response to the yeast itself helps to explain why the preexposure to S. boulardii is advantageous for fighting subsequent infections.12,30 Anti-inflammatory cytokines increased during early stages of infection due to S. boulardii strengthen the host anti-inflammatory abilities.13
In an early stage of Salmonella infection (0–90 minutes), S. boulardii treatment was observed to lead to an increase in interferon-γ (a macrophage-stimulating cytokine) and a downregulation of IL-10 (a macrophage-inhibiting cytokine) in the small intestine, even in areas with low bacterial population. Only later during infection, S. boulardii led to an upregulation of IL-10, normalizing the overreacting inflammatory response.14
β-Glucan, one possible factor for immunomodulation
Several pieces of evidence point toward a small, soluble, heat-stable factor derived from the cell wall of S. boulardii, which can induce at least some of the immunomodulatory effects. The yeast β-glucan fraction has been identified as one candidate in this respect.31 β-Glucans derived from fungi and yeast consist of a (1,3)-β-linked backbone with small number of (1,6)-β-linked side chains, which are essentially known for their immune-modulating effects.32 They are found in the cell walls of nearly all fungi, including S. boulardii. β-Glucans are microbe-associated molecular patterns detected by pattern recognition receptors. Important pattern recognition receptors for β-glucans are the dectin-1 receptor, the complement receptor 3, and Toll-like receptor, expressed on various immune cells, for example, monocytes, macrophages, and DCs and also on intestinal epithelial cells (IECs).33–35 Binding to dectin-1 provokes numerous responses including production of cytokines and chemokines in DCs and macrophages33 and forces IL-1β secretion.36 β-Glucan preparations from yeast have shown their immunomodulatory effects in human clinical trials.37 There is even a connection between S. boulardii binding via dectin-1 receptor and the possibility to interfere with colitis.38
Other, not yet identified components of S. boulardii may contribute to its various immunologic effects. More research is needed to elucidate all molecular mechanisms by which S. boulardii supports the host immune system during infections or in a preventive manner.
Summary of the immunomodulatory effects
The in vivo and in vitro results presented earlier demonstrate that S. boulardii is able to modulate host early immune response toward a more activated state. This increases the host’s resistance to microbial infections on a local level within the intestine, as well as systemically, for example, by increasing the amount of liver macrophages. In contrast, later during infections, S. boulardii helps to balance between pro- and anti-inflammatory immune responses by the modulation of various cytokines and chemokines and by inhibiting the maturation, migration, or proliferation of immune cells.
Anti-inflammatory action of S. boulardii during infections
There are many different mechanisms by which different pathogens cause diarrhea. Despite these different causalities, most cases of infectious diarrhea can be efficiently controlled by S. boulardii. The yeast interferes at various steps of the cascade of infection and diarrhea. One common feature is the induced inflammatory reaction of the host, which can be antagonized by S. boulardii.
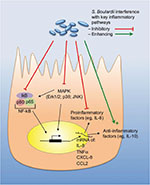
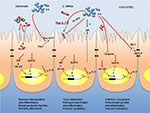
In the early stage of infection, proinflammatory cytokines are produced by the IECs, which contribute to the defense against invading pathogens. However, high levels of proinflammatory cytokines do not only attack the invaders but cause inflammation of the host tissue leading to tissue destruction. Therefore, suppression of the proinflammatory action by anti-inflammatory mediators can achieve a beneficial balance between both the reactions. A summary of the individual mechanisms of S. boulardii is provided in Table 1 and is illustrated in Figures 1 and 2.
Enterohemorrhagic E. coli
Gram-negative E. coli bacteria are part of the regular intestinal microbiome. However, the genetically different bacterium enterohemorrhagic E. coli (EHEC) causes diarrhea and hemorrhagic colitis and can lead to other severe complications. A specific protein, intimin, located within the bacterial cell wall, facilitates bacterial adhesion to IECs, which is a prerequisite for subsequent host cell invasion and tissue colonization (for review, Nguyen and Sperandio39).
Many of the EHEC pathogenic effects are caused by the cytotoxic Shiga toxin, which enters the host cell by receptor-mediated endocytosis. In addition, EHEC produces two hemolysin forms, which cause pore formation within the IECs and caspase-9-mediated apoptosis.40
Infection with EHEC causes inflammation and disruption of the tight junctions, leading to a breakdown of the barrier function of the intestinal epithelium. This, in turn, facilitates the invasion of IECs from their basolateral side.41 Additional apoptosis and necrosis of macrophages and lymphocytes (those host cells that are most dangerous to pathogens) worsen the EHEC pathogenicity.42,43
In vitro, the apoptosis program in human colon cells (T84) triggered by EHEC can be stopped by S. boulardii. This is achieved by interfering with the caspase-8 and caspase-9 pathways. In EHEC-infected T84 cells, the secretion of TNFα is upregulated, which might contribute to apoptosis. This increase was significantly reduced when T84 cells were preincubated with S. boulardii.42
Myosin light chain phosphorylation is correlated with an increase of tight-junction permeability. S. boulardii is able to preserve the barrier function of the epithelial cells after EHEC infections by the inhibition of myosin light chain phosphorylation.44
Furthermore, the secretion of the proinflammatory cytokine IL-8 was decreased by S. boulardii via inhibition of the NF-κB and MAPK signaling pathways. For this effect, S. boulardii cells must be present during the infection, as heat treatment and wash off the yeast cells no longer had this effect.44 Mechanistically, this was achieved by blocking phosphorylation as well as degradation of IκB, which is necessary for translocation of NF-κB,44 probably via Saccharomyces anti-inflammatory factor, a small molecular weight (<1 kDa), water soluble molecule, extracted from S. boulardii, and typically released into the culture media.45 Additionally, S. boulardii was found to inhibit TNFα transcription (Figure 2 and Table 1).42
EPEC
Another pathogenic E. coli, the EPEC causes serious and prolonged watery diarrhea, especially in children in developing countries. EPEC adheres to IECs and damages them by pore formation associated with cytotoxicity,46 as well as by causing apoptosis.47 In addition, tight junctions are disrupted.48
When T84 cells were infected with EPEC in the presence of S. boulardii, the tight junctions were preserved, the apoptotic program was prevented or at least delayed, and the number of intracellular EPEC significantly decreased. A common mechanism by which S. boulardii prevents (or delays) activation of the apoptosis program is the inhibition of the pathogen-induced caspase activation.49 EPEC induces phosphorylation of several proteins, including Src homology 2 domain containing protein (SHC). S. boulardii was able to reduce the degree of phosphorylation of most of these proteins, including SHC. SHC is known to be an upstream regulatory protein of the MAPK pathway, which explains the observed reduction of the EPEC-induced activation of the MAPK pathway by S. boulardii. Inhibition of the ERK1/2 MAPK pathway by S. boulardii also reduced EPEC internalization (Figure 2 and Table 1).49
Enterotoxigenic E. coli
Another E. coli infection leading to profuse watery diarrhea is the infection with enterotoxigenic E. coli (ETEC). It causes 840 million gastrointestinal infections or approximately 380,000 deaths worldwide each year (for review, Gupta et al50). In piglets, ETEC infection is the most common cause of inflammation and diarrhea, leading to reduced growth rate and increased mortality.51
In porcine intestinal cells, S. boulardii showed its anti-inflammatory abilities by decreasing the ETEC-induced gene expression of proinflammatory cytokines TNFα, IL-6, GM-CSF, and chemokines CCL2, CCL20, and CXCL8. In addition, S. boulardii was able to reduce ETEC adhesion to host intestinal cells28 and thus reduces the possibility of bacterial internalization and enhances the elimination of the pathogen. Feeding of piglets with S. boulardii reduced the bacterial translocation to mesenteric lymph nodes after ETEC infection (Table 1).52
Lipopolysaccharides
Bacterial endotoxin (lipopolysaccharides [LPS]) stimulation of human myeloid DCs induced the release of proinflammatory cytokines such as IL-6 and TNFα. S. boulardii culture supernatant, containing a <3kDa molecular weight compound,53 counteracted this inflammatory response, resulting in a reduction of IL-6 and TNFα and an increase in IL-10.23,53
After LPS stimulation, the same <3 kDa factor also inhibited activation and proliferation of native T-cells and the inflammation-associated migration of DC and T-cells. This was effectuated via suppression of C-C chemokine receptor type 7 expression, which is important for this migration.53 Furthermore, S. boulardii produces a protein phosphatase (63 kDa), which is able to reduce the inflammatory reaction and the toxicity of LPS by dephosphorylation (Table 1).54
Salmonella
Infection with Salmonella causes inflammation and necrosis of the intestine, leading to gastroenteritis, including diarrhea with life-threatening consequences. Salmonella adheres to the host IECs and invades them through the activation of host’s actin cytoskeleton by activating Rac1 GTPase.12 The invasion results in inflammatory reactions, including the production and release of proinflammatory cytokines (eg, IL-8), activation of the MAPK pathway, as well as induction of several transcription factors such as AP-1 and NFκB.55 These inflammatory responses cause diarrhea, lead to ulceration and destruction of the mucosa. Furthermore, Salmonella infections can cause systemic damage in case of translocation to liver, spleen, and lymph nodes (for review, Hurley et al56).
S. boulardii employs multiple mechanisms to interfere with Salmonella infection. In vitro, S. boulardii was found to reduce adhesion of Salmonella to IECs by (a probably mannose sensitive) binding of the yeast to the bacteria.12,30 In germ-free mice, Salmonella bound more frequently to S. boulardii than to epithelial cells.13,14 S. boulardii trapped the bacteria and thereby forced their elimination.
The yeast was able to interfere with host cell invasion of Salmonella by reducing Rac1 activation. This effect was more pronounced when HeLa cells were incubated overnight with S. boulardii before encountering a Salmonella infection.12 These two mechanisms – reduced adherence and inhibition of cytoskeleton changers – preserve IEC barrier function and inhibit bacterial translocation to the liver, which was shown in mice treated with S. boulardii.12,13
Furthermore, after overnight preincubation, S. boulardii or its supernatant could prevent the secretion of IL-8 via interference with the Salmonella-induced activation of MAPKs, ERK1/2, p38, and Jun N-terminal kinases and by the inhibition of phosphorylation of the IκB-α subunit necessary for NF-κB pathway. Additionally, the yeast could also directly (within a few hours) interfere with the IL-8 secretion process, without affecting the transcription machinery (Figure 2 and Table 1).12
The inhibitory effect of S. boulardii supernatant was completely abolished with heat treatment, indicating the presence of a heat-labile soluble factor, possibly Saccharomyces anti-inflammatory factor, mediating the inhibitory effect.12
During an ongoing Salmonella infection, S. boulardii was found to decrease the secretion of other inflammatory cytokines, namely, IL-6 and TNFα in vivo in mice. In contrast, anti-inflammatory cytokines increased in the early stages of infection due to S. boulardii, strengthening the host’s anti-inflammatory abilities.13 The effects of S. boulardii on the immune response in Salmonella-infected IEC and DC cultures have also been shown by Badia et al.30 At least partially, S. boulardii was able to inhibit the Salmonella-induced mRNA of TNFα expression.
Shigella flexneri
Shigella flexneri is a highly infectious human enteric pathogen, resulting in acute intestinal inflammation, abdominal cramps, severe diarrhea, and fever. The infection is associated with the disruption of tight junctions between the IECs, thereby disrupting the physical barrier and causing host cell invasion (for review, Ashida et al57 and Jennison and Verma58).
In vitro, the simultaneous treatment with S. boulardii during S. flexneri infection did not reduce the number of bacteria that invaded or attached to T-84 IECs, but was at least partially able to protect and restore the cellular barrier function, by restoring claudin-1 levels important for tight junctions.59 In response to S. flexneri infection, the epithelial cells released IL-8, the key cytokine to attract polymorphonuclear leukocytes from the blood into the subepithelial region. This cytokine release was reduced when S. boulardii or its cell-free culture supernatant was added simultaneously, but not when added after infection, again indicating the role of a soluble factor.59 As in other infectious diseases, S. boulardii acts via interference with phosphorylations of ERK1/2 (pERK1/2), and IκB. The protective effects of S. boulardii, specifically the reduction of inflammation and polymorphonuclear leukocyte infiltration,59 as well as the improved histopathology and reduced mortality,60 were confirmed in experimental models in mice (Table 1).
C. difficile
C. difficile-induced colitis and diarrhea is one of the most common nosocomial infections. Up to 25% of all hospitalized patients treated with antibiotics will develop antibiotic-associated diarrhea – C. difficile can be made accountable for 10%–20% of these cases. The pathogenic effects are caused by the release of toxin A and toxin B (for review Bartlett61).
S. boulardii was found to secrete a 54-kDa serine protease, which hydrolyzes toxin A and B as well as inhibits toxin binding to its intestinal glycoprotein receptor.9–11 Additionally, S. boulardii inhibited C. difficile growth and toxin production in vivo.62 This resulted in a restoration of protein synthesis and membrane integrity.10
Like other inflammatory intestinal infections, C. difficile causes the release of inflammatory cytokines. In a human colonocyte cell line, S. boulardii supernatant was able to inhibit the toxin A-stimulated IL-8 production. Comparable to the above-described infections, S. boulardii supernatant inhibited the activation of MAP kinases, such as Erk1/2 and Jun N-terminal kinases/SAPK, which are involved in the IL-8 signaling pathway. Pretreatment of a mouse ileal loop with S. boulardii supernatant inhibited toxin A-induced pro-inflammatory reactions and reduced toxin A-induced fluid secretion, as well as tissue damage (Figure 2 and Table 1).63
Rotavirus
Rotavirus infection accounts for hospitalization of up to 40% of the children <5 years of age with diarrhea.64 Apart from the administration of selected probiotics, including S. boulardii,65 no specific therapy for this viral infection is available. Rotavirus infects mature IECs. It seems that the chloride secretion, which is at least partially responsible for the diarrhea, is induced via an oxidative stress-dependent mechanism. In vitro, rotavirus-infected Caco-2 cells produce high levels of intracellular reactive oxygen species, in parallel with a decrease of the antioxidant glutathione, leading to chloride secretion, which was altered by S. boulardii.66 S. boulardii prevented chloride secretion by the inhibition of reactive oxygen species formation and reestablished balance of the GSH/GSSH redox system. A yeast-conditioned medium was sufficient to induce these effects (Table 1).66
Candida albicans
S. boulardii was also found to have positive effects on yeast infection. This has been investigated on intraepithelial lymphocytes infected by Candida albicans in vitro. S. boulardii interfered with proinflammatory cytokine production (interferon-γ, IL-1β), and it stimulated the anti-inflammatory cytokines IL-4 and IL-10.67
In a different study, S. boulardii decreased mRNA levels and TNFα, which had been increased by C. albicans infection, while stimulating mRNA production of the anti-inflammatory cytokine IL-10.31 TNFα reduction due to S. boulardii is probably mediated via a reduced Toll-like receptor 2 mRNA expression,68 a receptor known to be involved in yeast recognition (Table 1).69
Nitric oxide-related effects
In a castor oil-induced diarrhea model, S. boulardii was able to significantly reduce diarrhea. It has been shown that the induction of diarrhea is associated with nitric oxide (NO) overproduction. S. boulardii inhibited inducible NO synthase activity in a concentration-dependent manner. This activity remained stable after 15 minutes at 121°C, indicating a heat-stable factor.70 An S. boulardii-mediated decrease of NO levels in rat intestines was also observed in another study.71
Summary of the anti-inflammatory effects of S. boulardii
Independent of the pathogen, S. boulardii achieves its beneficial effects by inhibiting proinflammatory cytokine production or by enhancing anti-inflammatory mediators. Thereby, S. boulardii interferes with the host’s signal transduction cascades at various positions. Reduction of the proinflammatory response is one of the protective effects of S. boulardii against diarrheal pathogens. Depending on the infectious agent, soluble yeast-derived factors as well as S. boulardii cells are responsible for the effects.
S. boulardii also reduces the pathogen number by growth inhibition of the pathogens or by direct binding and inactivating toxins by enzymatic cleavage. The reduced adhesion together with the interference of the cytoskeleton-controlled bacterial internalization further reduces translocation and thus systemic damage. Finally, the yeast is able to preserve tight junction-mediated barrier function.
The positive effects of S. boulardii observed in these preclinical studies have been confirmed in many clinical trials, for example, for traveler’s diarrhea,72 rotavirus-induced gastroenteritis,65 or C. difficile infection.2,73,74
Safety
The safety of S. boulardii has been proven in numerous clinical investigations in healthy as well as severely ill patients.2,65,72–74 Adverse events reported in these investigations were either none65,75 or low on side effects.72 Nevertheless, all probiotics as well as the host’s own microbes bear the theoretical risk of epithelial translocation followed by systemic infection. The risk of developing fungemia due to the intake of S. boulardii is estimated to be 1 out of 5.6 million users.76 The reported cases of fungemia associated with S. boulardii intake were in extremely ill patients, either immunocompromised or with central venous catheters.76,77 For all other groups, the intake of S. boulardii is considered to be safe.
Clinical effects on mechanistic level
Even though the mode of action has been investigated in numerous in vitro and in vivo studies, the clinical effects are not fully understood on a mechanistic level.
S. boulardii has been clinically tested in several of the acute gastrointestinal conditions described earlier. Most of the clinical trials reported a statistically significant outcome in favor of S. boulardii. Meta-analysis showed a protective effect of S. boulardii in the treatment of acute diarrhea of various etiologies in children and adults, including antibiotic-associated diarrhea.75,78 S. boulardii is also effective in primary prevention of C. difficile infection, an important infection due to antibiotic treatment. However, it showed only limited effects in secondary C. difficile infection prevention.79
Even though there is a large body of evidence showing the overall positive effect of S. boulardii in these types of diarrhea, there is a small number of investigations where S. boulardii failed to show effectiveness.80 The reason for failure in those studies might be insufficient power, short study duration, or being underdosed. In order to understand these outcomes, it is not only important to critically analyze the intervention itself but also to perform mode of action investigations in human. There is only a limited number of investigations evaluating the immunological effects of S. boulardii in humans. Only one has been found,81 which was associated with immunological effects in acute diarrhea, the main focus of this review. In that study, the positive effect of S. boulardii in pediatric acute gastroenteritis was associated with enhanced immune response, indicated by increased serum IgA and decreased C-reactive protein levels. Furthermore, a significant increase in CD8 cells on day 7 was observed, indicating a late proinflammatory activity of S. boulardii, resulting in cytokine release and CD8 cell activation, which might help to limit the infection.81
Further knowledge about the mechanisms in human body will help to explain the effects that are not understood so far and to treat patients with the full capacity of S. boulardii.
Conclusion and future perspectives
S. boulardii has been used as a probiotic for >50 years. During this time, it has proven its effectiveness in many types of infectious diarrhea. It exhibits its positive effect by directly acting on pathogens and their toxins. It influences the host’s infection-induced signaling cascades and its innate and adaptive immune system. In total, these mechanisms result in the reduction of the pathogens’ ability for adhesion or colonization and an attenuation of the overreacting inflammatory immune response. This leads to a preserved or restored integrity of the IEC layer, and the diarrheic leakage of fluids into the intestinal lumen is attenuated.
Since S. boulardii interferes with bacterial infection at many stages, it becomes an interesting candidate as an antibiotic replacement in livestock farming to control pathogen-associated growth suppression. The ban of antibiotics for growth promotion requires alternatives. The first success was the introduction of S. boulardii to livestock feed.82,83
Immunomodulatory and anti-inflammatory effects of S. boulardii achieve positive outcomes in a mechanistically similar manner in various infections. Therefore, it is not surprising that S. boulardii may prove to be effective in other gastrointestinal diseases associated with inflammation, such as H. pylori infection,84,85 Inflammatory Bowel Disease,86 or colitis.31,87 Other digestion-related treatment areas might be obesity and type 2 diabetes.88
Even treatment of cancer patients with yeast is not totally out of scope. First results have shown that human B lymphomas were inhibited by rice fermented with S. boulardii.89 The yeast may also have a therapeutic or prophylactic role in intestinal neoplasia.90 Further research is needed to demonstrate clinical efficacy of S. boulardii for all these new possible applications.
Acknowledgments
We are grateful to Margret I Moré for reviewing the manuscript. The work of HS was funded by Medice Arzneimittel Pütter GmbH and Co KG.
Author contributions
HS performed the PubMed search. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All the authors approved the final version of the article, including the authorship list.
Disclosure
The work of HS was funded by Medice Arzneimittel Pütter GmbH and Co. KG. HS reports no other conflicts of interest in this work. SCB did not receive any funding for this article and also reports no conflict of interest in this work.
References
Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134(1):e176–e191. | ||
McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. | ||
McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5(2):97–105. | ||
Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6(6):374–382. | ||
Moré MI, Swidsinski A. Saccharomyces boulardii CNCM i-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis‚ a review. Clin Exp Gastroenterol. 2015;8:237–255. | ||
Buts JP. Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci. 2009;54(1):15–18. | ||
Buts JP, De Keyser N. Interaction of Saccharomyces boulardii with intestinal brush border membranes: key to probiotic effects? J Pediatr Gastroenterol Nutr. 2010;51(4):532–533. | ||
Jahn HU, Ullrich R, Schneider T, et al. Immunological and trophical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion. 1996;57(2):95–104. | ||
Pothoulakis C, Kelly CP, Joshi MA, et al. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104(4):1108–1115. | ||
Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67(1):302–307. | ||
Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64(12):5225–5232. | ||
Martins FS, Dalmasso G, Arantes RME, et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar typhimurium protects mice and modifies T84 cell response to the infection. PLoS One.2010;5(1):e8925. | ||
Martins FS, Vieira AlT, Elian SD, et al. Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microbes Infect. 2013;15(4):270–279. | ||
Pontier-Bres R, Munro P, Boyer L, et al. Saccharomyces boulardii modifies Salmonella typhimurium traffic and host immune responses along the intestinal tract. PLoS One. 2014;9(8):e103069. | ||
Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30(8):826–833. | ||
Edwards-Ingram L, Gitsham P, Burton N, et al. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2007;73(8):2458–2467. | ||
Cascio V, Gittings D, Merloni K, Hurton M, Laprade D, Austriaco N. S-Adenosyl-L-Methionine protects the probiotic yeast, Saccharomyces boulardii, from acid-induced cell death. BMC Microbiol. 2013;13:35. | ||
Fietto JL, Araujo RS, Valadao FN, et al. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol. 2004;50(8):615–621. | ||
Dinleyici EC, Kara A, Ozen M, Vandenplas Y. Saccharomyces boulardii CNCM I-745 in different clinical conditions. Expert Opin Biol Ther. 2014;14(11):1593–1609. | ||
Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol. 1998;6(1–2):13–18. | ||
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. | ||
Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(6):603–611. | ||
Martins FS, Silva AA, Vieira AT, et al. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191(8):623–630. | ||
Rodrigues AC, Cara DC, Fretez SH, et al. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol. 2000;89(3):404–414. | ||
Qamar A, Aboudola S, Warny M, et al. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun. 2001;69(4):2762–2765. | ||
Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35(2):251–256. | ||
Caetano JM, Parames M, Babo M, et al. Immunopharmacological effects of Saccharomyces boulardii in healthy human volunteers. Int J Immunopharmacol. 1986;8(3):245–259. | ||
Badia R, Zanello G, Chevaleyre C, et al. Effect of Saccharomyces cerevisiae var. Boulardii and beta-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88). Vet Res. 2012;43:4. | ||
Smith IM, Christensen JE, Arneborg N, Jespersen L. Yeast modulation of human dendritic cell cytokine secretion: an in vitro study. PLoS One. 2014;9(5):e96595. | ||
Badia R, Brufau MT, Guerrero-Zamora AM, et al. Beta-galactomannan and Saccharomyces cerevisiae var. boulardii modulate the immune response against Salmonella enterica serovar Typhimurium in porcine intestinal epithelial and dendritic cells. Clin Vaccine Immunol. 2012;19(3):368–376. | ||
Jawhara S, Habib K, Maggiotto F, et al. Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS One. 2012;7(7):e40648. | ||
Bohn JA, BeMiller JN. (1→3)-β-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohyd Poly. 1995(28):3–14. | ||
Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197(9):1119–1124. | ||
Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. | ||
Rice PJ, Adams EL, Ozment-Skelton T, et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther. 2005;314(3):1079–1086. | ||
Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-Beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2011;184(11):6335–6342. | ||
Stier H, Ebbeskotte V, Gruenwald J. Immune-modulatory effects of dietary yeast beta-1,3/1,6-D-glucan. Nutr J. 2014;13(1):38. | ||
Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–1317. | ||
Nguyen Y, Sperandio V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front Cell Infect Microbiol. 2012;2:90. | ||
Bielaszewska M, Aldick T, Bauwens A, Karch H. Hemolysin of enterohemorrhagic Escherichia coli: structure, transport, biological activity and putative role in virulence. Int J Med Microbiol. 2014;304(5–6):521–529. | ||
Cordeiro F, da Silva RI, Vargas-Stampe TL, Cerqueira AM, Andrade JR. Cell invasion and survival of Shiga toxin-producing Escherichia coli within cultured human intestinal epithelial cells. Microbiology. 2013;159(Pt 8):1683–1694. | ||
Dalmasso G, Loubat A, Dahan S, Calle G, Rampal P, Czerucka D. Saccharomyces boulardii prevents TNF-α-induced apoptosis in EHEC-infected T84 cells. Res Microbiol. 2006;157(5):456–465. | ||
Barnett Foster D, Abul-Milh M, Huesca M, Lingwood CA. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect Immun. 2000;68(6):3108–3115. | ||
Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immun. 2003;71(2):766–773. | ||
Sougioultzis S, Simeonidis S, Bhaskar KR, et al. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343(1):69–76. | ||
Guignot J, Segura A, Tran Van Nhieu G. The serine protease EspC from enteropathogenic Escherichia coli regulates pore formation and cytotoxicity mediated by the type III secretion system. PLoS Pathog. 2015;11(7):e1005013. | ||
Crane JK, Majumdar S, Pickhardt DF 3rd. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67(5):2575–2584. | ||
Glotfelty LG, Hecht GA. Enteropathogenic E. coli effectors EspG1/G2 disrupt tight junctions: new roles and mechanisms. Ann N Y Acad Sci. 2012;1258:149–158. | ||
Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68(10):5998–6004. | ||
Gupta SK, Keck J, Ram PK, Crump JA, Miller MA, Mintz ED. Part III. Analysis of data gaps pertaining to enterotoxigenic Escherichia coli infections in low and medium human development index countries, 1984–2005. Epidemiol Infect. 2008;136(6):721–738. | ||
Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6(1):17–39. | ||
Lessard M, Dupuis M, Gagnon N, et al. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge. J Anim Sci. 2009;87(3):922–934. | ||
Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156(1):78–87. | ||
Buts JP, Dekeyser N, Stilmant C, Delem E, Smets F, Sokal E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr Res. 2006;60(1):24–29. | ||
Hobbie S, Chen LM, Davis RJ, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159(11):5550–5559. | ||
Hurley D, McCusker MP, Fanning S, Martins M. Salmonella-host interactions – modulation of the host innate immune system. Front Immunol. 2014;5:481. | ||
Ashida H, Mimuro H, Sasakawa C. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Front Immunol. 2015;6:219. | ||
Jennison AV, Verma NK. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev. 2004;28(1):43–58. | ||
Mumy KL, Chen X, Kelly CP, McCormick BA. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G599–G609. | ||
Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81(3):251–256. | ||
Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334–339. | ||
Elmer GW, McFarland LV. Suppression by Saccharomyces boulardii of toxigenic Clostridium difficile overgrowth after vancomycin treatment in hamsters. Antimicrob Agents Chemother. 1987;31(1):129–131. | ||
Chen X, Kokkotou EG, Mustafa N, et al. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281(34):24449–24454. | ||
Forster J, Guarino A, Parez N, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123(3):e393–e400. | ||
Correa NB, Penna FJ, Lima FM, Nicoli JR, Filho LA. Treatment of acute diarrhea with Saccharomyces boulardii in infants. J Pediatr Gastroenterol Nutr. 2011;53(5):497–501. | ||
Buccigrossi V, Laudiero G, Russo C, et al. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS One. 2014;9(6):e99830. | ||
Fidan I, Kalkanci A, Yesilyurt E, et al. Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans. Mycoses. 2009;52(1):29–34. | ||
Jawhara S, Poulain D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med Mycol. 2007;45(8):691–700. | ||
Calich VL, Pina A, Felonato M, Bernardino S, Costa TA, Loures FV. Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis. FEMS Immunol Med Microbiol. 2008;53(1):1–7. | ||
Girard P, Pansart Y, Gillardin JM. Inducible nitric oxide synthase involvement in the mechanism of action of Saccharomyces boulardii in castor oil-induced diarrhoea in rats. Nitric Oxide. 2005;13(3):163–169. | ||
Soyturk M, Saygili SM, Baskin H, et al. Effectiveness of Saccharomyces boulardii in a rat model of colitis. World J Gastroenterol. 2012;18(44):6452–6460. | ||
Kollaritsch H, Holst H, Grobara P, Wiedermann G. Prophylaxe der Reisediarrhöe mit Saccharomyces boulardii. Ergebnisse einer plazebokontrollierten Doppelblindstudie [Prevention of traveler’s diarrhea with Saccharomyces boulardii. Results of a placebo controlled double-blind study]. Fortschr Med. 1993;111(9):152–156. German. | ||
Johnson S, Maziade PJ, McFarland LV, et al. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis. 2012;16(11):e786–e792. | ||
Tung JM, Dolovich LR, Lee CH. Prevention of Clostridium difficile infection with Saccharomyces boulardii: a systematic review. Can J Gastroenterol. 2009;23(12):817–821. | ||
Szajewska H, Mrukowicz J. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2005;22(5):365–372. | ||
Karpa KD. Probiotics for Clostridium difficile diarrhea: putting it into perspective. Ann Pharmacother. 2007;41(7):1284–1287. | ||
Lherm T, Monet C, Nougiere B, et al. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002;28(6):797–801. | ||
McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16(18):2202–2222. | ||
McFarland LV. Probiotics for the primary and secondary prevention of C. difficile infections: a meta-analysis and systematic review. Antibiotics. 2015;4(2):160–178. | ||
Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36(2):171–174. | ||
Ozkan TB, Sahin E, Erdemir G, Budak F. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J Int Med Res. 2007;35(2):201–212. | ||
Gil de los Santos JR, Storch OB, Gil-Turnes C. Bacillus cereus var. toyoii and Saccharomyces boulardii increased feed efficiency in broilers infected with Salmonella enteritidis. Br Poult Sci. 2005;46(4):494–497. | ||
Mountzouris KC, Dalaka E, Palamidi I, et al. Evaluation of yeast dietary supplementation in broilers challenged or not with Salmonella on growth performance, cecal microbiota composition and Salmonella in ceca, cloacae and carcass skin. Poult Sci. 2015;94(10):2445–2455. | ||
Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32(9):1069–1079. | ||
Sakarya S, Gunay N. Saccharomyces boulardii expresses neuraminidase activity selective for alpha2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. 2014;122(10):941–950. | ||
Dalmasso G, Cottrez F, Imbert V, et al. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131(6):1812–1825. | ||
Berg R, Bernasconi P, Fowler D, Gautreaux M. Inhibition of Candida albicans translocation from the gastrointestinal tract of mice by oral administration of Saccharomyces boulardii. J Infect Dis. 1993;168(5):1314–1318. | ||
Everard A, Matamoros Sb, Geurts L, Delzenne NM, Cani PD. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio. 2014;5(3):e01011–e01014. | ||
Ryan EP, Heuberger AL, Weir TL, Barnett B, Broeckling CD, Prenni JE. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J Agric Food Chem. 2011;59(5):1862–1870. | ||
Chen X, Fruehauf J, Goldsmith JD, et al. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in apcmin mice. Gastroenterology. 2009;137(3):914–923. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.