Back to Journals » International Journal of General Medicine » Volume 15
Influence of KDR Genetic Variation on the Effectiveness and Safety of Bevacizumab in the First-Line Treatment for Patients with Advanced Colorectal Cancer
Received 14 February 2022
Accepted for publication 21 April 2022
Published 16 June 2022 Volume 2022:15 Pages 5651—5659
DOI https://doi.org/10.2147/IJGM.S362366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fei Wang,1 Gang Liu2
1Department of Oncology, The Seventh Medical Center of People’s Liberation Army General Hospital, Beijing, People’s Republic of China; 2Department of General Surgery, The First Medical Center of People’s Liberation Army General Hospital, Beijing, People’s Republic of China
Correspondence: Gang Liu, Department of General Surgery, the first Medical Center of People’s Liberation Army General Hospital, No. 28 FuXing Road, HaiDian District, Beijing, 100048, People’s Republic of China, Tel +86 18600310565, Email [email protected]
Objective: Bevacizumab is usually considered a first-line anti-tumor therapy, which inhibits tumor growth by downregulating the vascular endothelial growth factor (VEGF) that further silences the activity of the kinase insert region receptor (KDR) gene. In the current study, we investigated the treatment response of bevacizumab in advanced colorectal cancer (CRC) patients bearing 889 C>T mutation in the KDR gene.
Methods: A total of 135 advanced CRC patients were treated with bevacizumab along with chemotherapy at the seventh medical center of the People’s Liberation Army general hospital from January 2012 to June 2021 and were analyzed retrospectively. The KDR genotyping and mRNA expression analyses were performed in 57 patients.
Results: The KDR genotyping revealed 97 (71.85%) cases with CC genotype, 34 (25.19%) cases with CT, and 4 (2.96%) cases with TT genotype, while the minor allele frequency of 889 C>T was found as 0.16. The median progression-free survival (PFS) of the patients with CT/TT genotype and CC genotype was found to be 6.1 and 9.7 months, respectively (P = 0.009). The median overall survival (OS) of the two genotypes was 13.7 and 19.7 (P = 0.025), respectively. Multivariable Cox regression analysis of PFS, CT/TT genotype was found to be an independent factor for PFS (odds ratio (OR) = 1.88, P = 0.023). Additionally, the mRNA expression of KDR in 57 biopsies taken from patients with CT/TT genotypes was significantly higher than that of patients with CC genotype (P < 0.001). Additionally, in terms of safety, 55 patients experienced grade 2 or higher fatigue (incidence rate 40.74%) after receiving bevacizumab along with chemotherapy.
Conclusion: The 889 C>T mutation in KDR gene affects the KDR expression in colorectal cancer patients, thereby affecting the effectiveness of bevacizumab therapy.
Keywords: colorectal cancer, bevacizumab, kinase insert domain receptor, polymorphism, clinical outcomes
Introduction
Colorectal cancer (CRC) is one of the leading causes of the morbidity and mortality in Chinese population, with approximately 375,000 new cases with 191,000 deaths annually were recorded in China.1 In recent years, due to the advancement in treatment strategies, the survival rate of patients with CRC was dramatically improved specifically, bevacizumab and aflibercept showed significant enhancement in overall survival of CRC patients.2–4 In addition, the first edition of IDEA research in 2018 National Comprehensive Cancer Network (NCCN) guidelines recommended Xelox adjuvant chemotherapy for three months to low-risk stage III colon cancer patients. However, several influencing factors were still being recognized that contributed to the long-term survival of the patients. Consequently, it was necessary to further investigate more accurate detection and treatment methods for CRC.
Angiogenesis is very important for the development of tumors, which creates a new vascular system through the sprouting of capillaries in the human blood vessel network in tumors.5,6 It is generally believed that tumor cells secrete different vascular growth factors to stimulate the growth of surrounding microvessels. Among all secreted vascular growth factors, the vascular endothelial growth factor (VEGF) is a leading endothelial cell mitogen, which specifically integrates with the kinase insert domain containing receptor (KDR) and transmits the angiogenesis signals.7,8 Several VEGF inhibitors were developed, including Bevacizumab, a humanized monoclonal antibody that specifically acts on VEGF-A to block VEGF signaling pathways to block or slow down tumor angiogenesis. Therefore, it is believed that KDR could be a clinically important biomarker for CRC, thus its genetic polymorphisms could be involved in the effectiveness of bevacizumab.9,10
The KDR gene is located on chromosome 4q12 and contains 30 exons, and different genetic mutations have been reported in different ethnic groups, and the differences in its mRNA expression level were also often found among different populations.11 Previously, the 889C>T SNP in the coding region of the KDR gene showed a greater impact on the prognosis of patients with liver cancer treated with sorafenib.12 However, so far no clinical study of bevacizumab has been conducted on CRC patients bearing KDR mutations in Chinese population. Therefore, we explored for the first time whether KDR 889 C>T SNP could affect the effectiveness of bevacizumab as first-line treatment for advanced CRC patients.
Materials and Methods
Experimental Design and Treatment Strategies
We performed a retrospective analysis on a total of 135 patients with advanced CRC treated with bevacizumab in the Department of Medical Oncology, the seventh medical center of PLA general hospital from January 2012 to June 2021. The subjects inclusion criteria were as follows: age ≥18, Eastern cooperative oncology group (ECOG) performance status of 0–2 score, patients received bevacizumab in combination with chemotherapy as first-line treatment, and at least one measurable lesion. Based on the following criteria, the subjects were excluded from the study: familial adenomatous polyposis, other hereditary CRC syndromes, and patients who lost the follow-up. The primary study endpoints were progression-free survival (PFS) and the secondary endpoints were objective response rate (ORR), overall survival (OS) and safety. We used response evaluation criteria in solid tumors (RECIST) 1.1 criteria to analyze the effectiveness of the bevacizumab in patients with different genotypes of KDR. The bevacizumab was administered to the patients with the following dosage plans: 1. Oxaliplatin (Jiangsu Hengrui Medicine Co. Ltd.) plus Leucovorin calcium (J Jiangsu CHIATAI TIANQING Pharmaceuticals group) and 5-fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co. Ltd.) regimen (FOLFOX) in combination with bevacizumab (Shanghai Roche Pharmaceuticals Ltd.), bevacizumab 5mg/kg intravenous by infusion on the first day of each week then FOLFOX was administered for two weeks. 2. Oxaliplatin plus Capecitabine regimen (XELOX) in combination with bevacizumab, bevacizumab 7.5 mg/kg injected intravenously on the first day then the Xelox regimen was given regularly for three-week. The doses of the therapy were adjusted after observing hematological or non-hematological toxicities in the patients. The current study was approved by the ethics committee of PLA general hospital, and informed written consent was taken from each patient or family member when potentially life-threatening adverse events were observed in the patients and the treatment was discontinued. However, these patients were still included in our study. All experimental and sampling procedures were performed by strictly following the guidelines of Helsinki declaration of 1964 and its latest amendments.
Blood Sampling and Genotyping
Approximately 4 mL of peripheral blood samples were collected from all patients, and genomic DNA was extracted by the phenol-chloroform method, and stored at −20°C. The 889C>T polymorphism of the KDR gene was genotyped by real-time PCR. The primers for KDR 89C>T were directly purchased from Thermofisher Scientific. The TaqMan genotyping assay was performed for allelic discrimination of selected polymorphism by using ABI TaqMan genotyping kit at real-time PCR (Applied Biosystems, Foster City, CA), and each sample was genotyped according to the manufacturer’s instructions. Negative control was set as a reference for the authenticity of the analysis, and the samples were performed at least thrice to ensure the accuracy of the results.
Tissue Sampling and KDR mRNA Expression Analysis
The tissue specimens of 57 patients with CRC from 135 patients were collected during the tissue biopsy process and immediately transferred into RNAlater (Qiagen, Germany) microtubes. Then, RNeasyTM Mini Kit (Qiagen) was used to extract RNA by following the manufacturer’s instructions. The quality and concentration of total RNA were evaluated by using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). To remove any contamination of DNA, all RNA samples were incubated for 30 minutes at 37°C in solution containing DNAse, RNAse inhibitor, DNAse buffer, and DEPC-treated water. Then, ethylenediaminetetraacetic acid (EDTA) was added, and samples were incubated at 65°C for 10 min to remove the DNAse.
The cDNA was synthesized using Prime Script II reverse transcriptase (TaKaRa, Japan) at 37°C for 15 min followed by 85°C for 10 seconds to inactivate the reverse transcriptase. The specific primers for KDR (F: CTACTGATTTTTGCCCTTGTTC, R: TAGTCATTGTTCCCAGCATTTC), and GAPDH (F: CATGGCCTCCAAGGAGTAA
G, R: GCTTGAGCACAGGGTACTTTA) were designed using Allele ID software version 7.5 (Premier Biosoft International, Palo Alto, CA, USA) and blasted with NCBI Primer. Real-time PCR was performed on a Roche light cycler 480 machine. SYBR Premix Ex Taq II (TaKaRa, Japan) was used to detect gene expressions. All reactions were performed in triplicate to minimize the machine error and to confirm the accuracy of results. Negative controls with no cDNA template were also included in all runs to identify possible contamination. The expression of GAPDH mRNA was used as an internal reference. KDR mRNA was calculated by relative quantification method 2 −ΔΔCt.13
Statistical Analysis
All variables in this study were analyzed using SPSS 22.0 statistical analysis software (IBM, Armonk, NY). Using baseline clinical data, the distribution of discrete variables and different genotypes at the 889C>T locus were examined by the Chi-square test. To analyze the continuous variables and different genotypes, Mann–Whitney U (between two groups) with non-parametric test was used. Kaplan–Meier curves were drafted by GraphPad Prism 7.0 to contrast the differences in PFS and OS of patients with different genotypes, and the differences between the curves were compared with the Log rank test. PFS was calculated from the date of bevacizumab treatment to the patient’s tumor progression or death in any case. OS was calculated from the beginning of enrollment into the group to death or the last follow-up. In multivariable analysis, the Cox risk ratio model was used to construct PFS, and the backward LR selection step was used to correct potential confounding variables. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients and Genotyping of 889C>T
The baseline clinical data of the recruited 135 patients are shown in Table 1. The median age of the patients was 58 (31–75) years, in total 86 (63.7%) of the patients were male and 49 (36%) were female. The cases of colon and rectal cancers were identified 87 (64.44%) and 48 (35.56%), respectively, and overall 93 (68.64%) and 11 (8.47%) of the tumors were moderate and differentiated, respectively. A total of 47 (34.81%) cases were identified to have right-side tumors, while 88 (65.19%) were detected with left-sided tumors, and overall 87 (64.44%) cases with tumor burden >3. Approximately 95 (70.37%) and 12 (8.88%) of patients had liver and peritoneal metastases, respectively. Overall, 70 cases were found to have mutations in other genes, such as RAS mutation in 50 (37.04%) and BRAF mutations in 20 (14.82%) cases, and 94 (69.63%) of the patients had an ECOG score of 0. In the combination regimens, 71.11% of patients were treated with bevacizumab with Xelox (capecitabine plus oxaliplatin).
 |
Table 1 Comparison of Baseline Characteristics of 135 Patients with CRC Patients According to 889 C>T Genotype Status |
KDR 889C>T TaqMan genotyping assays showed that 97 (71.85%) cases were detected with wild-type homozygous (CC genotype), 34 (25.19%) cases with heterozygous (CT genotype), while 4 (2.96%) cases were found to have mutant-type homozygous (TT genotype). The minimum allele frequency was recorded as 0.16; however, Hardy Weinberg equilibrium was observed (P = 0.636) for the distribution frequency of these three genotypes. As shown in Table 1, 889C>T had a balanced distribution of baseline characteristics.
Influence of KDR 889C>T on the Effectiveness of Bevacizumab
Given that there were relatively few patients with TT genotype (4 patients), thus TT and CT genotypes were combined (38 cases) for predictive analysis. The ORR of the overall population was observed at 40.74%. However, 19 patients showed a complete response (CR), 36 patients with a partial response (PR), 43 patients had stable disease (SD) and 37 patients showed progression disease (PR) according to RECIST version 1.1. The ORR was 40.74%, and disease control rate (DCR) was 72.59% (Figure 1). The median PFS of patients with CT/TT genotype and CC genotype were 6.1 and 9.7 months, respectively (P = 0.009) (Figure 2). In addition, a Cox risk ratio model was introduced to incorporate risk factors (such as age, gender, ECOG score, tumor types and location, metastatic site, RAS/BRAF mutation status and 889C>T) which might potentially influence the PFS. However, after the multivariate adjustment, the statistically significant independent effect was still confirmed of KDR 889C>T polymorphism on PFS (OR = 1.88, P = 0.023). In the Cox model, other statistically significant variables were age (OR = 1.91, P = 0.015), RAS mutation status (OR = 2.00, P = 0.004), liver metastatic site (OR = 4.27, P < 0.001), tumor location (OR = 2.07, P = 0.022) and ECOG score (OR = 2.27, P < 0.001) (Table 2). Furthermore, overall survival was also carried out in this study, the median OS of patients with CT/TT genotype and CC genotype were 13.7 and 19.7 months, respectively, this difference was statistically significant (P = 0.012) (Figure 3).
 |
Table 2 Multivariable Cox Regression Analysis of Progression Free Survival According to Baseline Characteristics and Polymorphism |
 |
Figure 1 The percentage of effectiveness of the 135 patients. |
 |
Figure 2 Comparison of progression free survival of 135 CRC patients according to KDR889 C>T status. |
 |
Figure 3 Comparison of overall survival of 135 CRC patients with different genotypes according to KDR 889 C>T status. |
The Adverse Reaction of the Patients
Moreover, 55 patients (incidence rate 40.74%) experienced grade 2 or higher fatigue after receiving bevacizumab in combination with chemotherapy. The common drug-related adverse reactions during bevacizumab treatment were recorded in patients as neutropenia (32.7%), hypertension (30.2%), leucopenia (28.2%), thrombocytopenia (24.3%), proteinuria (21.6%), hand-foot syndrome (15.8%), AST/ALT elevations hypertension (14.3%). CC and CT/TT types were not significantly associated with any of grade 2 or above adverse reactions.
Influence of 889C>T Polymorphism on the Expression of KDR mRNA
The expression of KDR mRNA was ascertained in 57 CRC tissues obtained through an open cut biopsy procedure. The correlation between different genotypes of 889C>T and KDR expression levels in patients’ tissues was analyzed. Among 57 patients (biopsied), 40 patients showed CC genotypes, 16 patients had CT genotypes, and 1 patient was found to have TT genotype. The distribution frequencies of the three genotypes were also determined by the Hardy Weinberg balance (P = 0.675). Since the number of patients with TT and CT genotypes was 17 cases, by comparing the KDR mRNA relative expression with the normal CC genotype, patients with TT and CT genotypes were found to have significantly higher KDR mRNA expression (P < 0.001) (Figure 4).
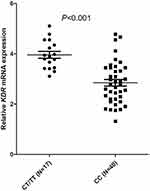 |
Figure 4 Comparison of relative KDR mRNA expression according to KDR 889 C>T status. |
Discussion
In the current study, we retrospectively recruited 135 patients with advanced CRC who have been treated with bevacizumab as the first line of treatment. Among these 28.1% patients were found to have KDR 889C>T polymorphism, the correlation analysis between the effectiveness of the bevacizumab and KDR 889C>T polymorphism demonstrated that patients with CT/TT genotype were significantly associated with poor PFS and OS. The KDR mRNA expression analysis in 57 cancer tissue specimens showed a significantly higher KDR mRNA expression level in patients with TT and CT KDR 889C>T genotypes. We speculated that different mutant genotypes of 889C>T influence the effectiveness of bevacizumab and the prognosis of patients with advanced CRC.
Bevacizumab is a humanized monoclonal antibody that specifically inhibits the activity of VEGF-A, thus it has achieved great success in the treatment of different cancers including colorectal cancer, lung cancer, ovarian cancer, etc.14–17 In recent years, many studies have been conducted on the biological targets of bevacizumab, such as somatic mutations, VEGF expression, germ cell mutations, and so on, but most of the studies did not produce meaningful results.18,19 As a vascular targeting drug, bevacizumab does not act on tumor cells but affects the vascular endothelial growth factor that normally exists in vivo. Therefore, polymorphisms in their target genes may have a greater impact on their effectiveness. A series of previous studies investigated the effectiveness of bevacizumab against mutant VEGF-A or its receptor genes.20,21 Another study revealed that the KDR polymorphisms 889C>T were significantly related to the effectiveness of bevacizumab combined with the FOLFIRI regimen in Chinese advanced colorectal cancer patients.20,22 However, there were few studies have been conducted on the polymorphisms of KDR specifically in CRC and their effect on the effectiveness of bevacizumab and the prognosis of the patients.
Regarding genotypic results, our exploration was as semblable as the previous investigation by Mario Scartozzi’s et al.23 They included 148 advanced liver cancer Caucasian patients who received sorafenib treatment. Their results showed that the genotype distribution frequency of 889C>T site in 148 patients was 22%, and the median PFS and OS of CT/TT genotype patients were significantly less than those of CC patients, which was consistent with the results of our research but our patients had higher frequency of the 889C>T mutant genotype. However, they did not thoroughly explore the possible underlying mechanisms of this site on the effectiveness of sorafenib in the treatment of liver cancer. In addition, Armin Gerger et al included 132 patients treated with FOLFOX or XELOX combined with bevacizumab in advanced colorectal cancer,24 and they identified that patients with CT/TT genotype at 889C>T had poor ORR, but there were no significant differences regarding PFS and OS. Wang et al included 118 patients treated with bevacizumab in advanced colorectal cancer and suggested that patients with CT/TT genotype at 889C>T site had significantly higher ORR, PFS and OS,25 which was partly similar to our study. These results indicated that the 889C>T site might influence the effectiveness of bevacizumab, but potential mechanisms were unknown. Additionally, they also reported some major adverse reactions above grade 2 during treatment were neutropenia, hypertension, leucopenia, etc. Different genotypes did not show any association with the adverse reactions as we report the same.
In addition, the correlation between 889C>T and mRNA expression was explored in 57 cancer tissue specimens, the results disclosed that the expression of KDR gene mRNA was significantly increased in CT/TT patients, which was partly similar to Babyshkina N,26 and they also found that the KDR gene protein expression was higher in CT/TT patients. KDR was the most important receptor with the strongest binding ability to VEGF-A, and its expression level could play a vital role in the process of angiogenesis. It has been shown that the higher expression level of KDR in tumor cells make it easier for tumor cells to regenerate blood vessels, which contributes to tumor cells relapsing and metastasizing.27,28 Therefore, relevant clinical research results indicated that the higher the expression level of the KDR gene was associated with the worse PFS and OS of patients with NSCLC.29 We have found similar results in current CRC patients.
Since the polymorphic locus changes belong to germ cell-line mutations, we performed the genotyping through DNA extracted from the blood, and the blood sampling is easy to perform at any stage of the disease, which facilitates clinical outcomes. In conclusion, this study excavated that the KDR 889 C>T may be an element that had an independent influence on the treatment effect of bevacizumab in advanced CRC patients. KDR is an important target in vascular targeted therapy, and some genetic mutations may also have a greater impact on the treatment of vascular targeting drugs.30
In addition, our study had some limitations. First, the sample size included in the study was only 135 cases, which were too small to evaluate the prognosis of CRC; however, we still managed to perform OS and clinical effectiveness of the bevacizumab by combining the mutant samples. Secondly, the consequence of retrospective analysis had some biasness that could not be avoided. However, our study sufficiently evaluated the predictive significance of the KDR 889C>T, and also revealed as the reasons for predictive differences caused by this mutation. This might have potential clinical significance for the predictive evaluation of bevacizumab in patients with advanced CRC and other cancers. Therefore, it is highly recommended to identify specific KDR or other genetic genotypes of the patients before applying vascular targeted drug therapy or reasonable medication as precision medicine.
Conclusion
KDR is an important target for vascular targeted therapy, and some genetic mutations in this gene may also have a greater impact on the effectiveness of vascular targeting drugs. In the current study, we determined that the KDR 889 C>T may have an independent influence on the treatment effects of bevacizumab in advanced CRC patients.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Chen WQ, Zheng RS, Baade P, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Geng F, Wang Z, Yin H, Yu J, Cao B. Molecular targeted drugs and treatment of colorectal cancer: recent progress and future perspectives. Cancer Biother Radiopharm. 2017;32(5):149–160. doi:10.1089/cbr.2017.2210
3. Taieb J, Aranda E, Raouf S, Dunn H, Arnold D. Clinical and regulatory considerations for the use of bevacizumab biosimilars in metastatic colorectal cancer. Clin Colorectal Cancer. 2021;20(1):42–51.e3. doi:10.1016/j.clcc.2020.10.005
4. Scartozzi M, Vincent L, Chiron M, Cascinu S. Aflibercept, a new way to target angiogenesis in the second line treatment of metastatic colorectal cancer (mCRC). Target Oncol. 2016;11(4):489–500. doi:10.1007/s11523-016-0447-4
5. Osaki T, Serrano JC, Kamm RD. Cooperative effects of vascular angiogenesis and lymphangiogenesis. Regen Eng Transl Med. 2018;4(3):120–132. doi:10.1007/s40883-018-0054-2
6. Colunga T, Dalton S. Building blood vessels with vascular progenitor cells. Trends Mol Med. 2018;24(7):630–641. doi:10.1016/j.molmed.2018.05.002
7. Balikova I, Postelmans L, Pasteels B, et al. Genetic biomarkers in the VEGF pathway predicting response to anti-VEGF therapy in age-related macular degeneration. BMJ Open Ophthalmol. 2019;4(1):e000273. doi:10.1136/bmjophth-2019-000273
8. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. doi:10.1038/nrc3627
9. Hagstrom SA, Ying GS, Maguire MG, et al. VEGFR2 gene polymorphisms and response to anti-Vascular endothelial growth factor therapy in age-related macular degeneration. Ophthalmology. 2015;122(8):1563–1568. doi:10.1016/j.ophtha.2015.04.024
10. Kanat O, Ertas H. Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy. World J Clin Oncol. 2019;10(2):52–61. doi:10.5306/wjco.v10.i2.52
11. Jauhri M, Gupta V, Shokeen Y, Minhas S, Bhalla S, Aggarwal S. KDR mutation: a high-frequency rare mutation and its correlation with other somatic mutations in Indian colorectal cancer patients. J Next Gen Sequencing Appl. 2017;4(2):1000148. doi:10.4172/2469-9853.1000148
12. Wang W, Ma XP, Shi Z, et al. Epidermal growth factor receptor pathway polymorphisms and the prognosis of hepatocellular carcinoma. Am J Cancer Res. 2015;5(1):396–410.
13. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi:10.1038/nprot.2008.73
14. Ruan GY, Ye LX, Liu GF, An J, Jalid S, Sun P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: an updated systematic review and meta-analysis. Onco Targets Ther. 2018;11:521–528. doi:10.2147/OTT.S155581
15. Zirlik K, Duyster J. Anti-angiogenics: current situation and future perspectives. Oncol Res Treat. 2018;41(4):166–171. doi:10.1159/000488087
16. Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized Phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi:10.1200/JCO.2013.51.4489
17. Chu TQ, Chen JH, Han BH. Current progression of bevacizumab in advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2018;40(10):793–800. doi:10.3760/cma.j.issn.0253-3766.2018.10.013
18. Jia MQ, Guo YX, Zhu DY, et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-kappaB pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt A):1622–1633. doi:10.1016/j.bbadis.2018.01.021
19. Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388(10043):518–529. doi:10.1016/S0140-6736(15)01088-0
20. Cui W, Li F, Yuan Q, Chen G, Chen CL, Yu B. Role of VEGFA gene polymorphisms in colorectal cancer patients who treated with bevacizumab. Oncotarget. 2017;8(62):105472–105478. doi:10.18632/oncotarget.22295
21. Angelucci A, Monache SD, Cortellini A, Padova MD, Ficorella C. “Vessels in the storm”: searching for prognostic and predictive angiogenic factors in colorectal cancer. Int J Mol Sci. 2018;19(1):299. doi:10.3390/ijms19010299
22. Kim SY, Kim TW. Current challenges in the implementation of precision oncology for the management of metastatic colorectal cancer. ESMO Open. 2020;5(2):e000634. doi:10.1136/esmoopen-2019-000634
23. Scartozzi M, Faloppi L, Baroni GS, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135(5):1247–1256. doi:10.1002/ijc.28772
24. Papachristos A, Kemos P, Katsila T, et al. VEGF-A and ICAM-1 gene polymorphisms as predictors of clinical outcome to first-line bevacizumab-based treatment in metastatic colorectal cancer. Int J Mol Sci. 2019;20(22):5791. doi:10.3390/ijms20225791
25. Wang HX, Mei X, Gong TX, et al. The impact of genetic variation of KDR on clinical outcomes of advanced colorectal cancer patients treated by first line bevacizumab based regimens. Zhonghua Yi Xue Za Zhi. 2018;98(34):2737–2742. doi:10.3760/cma.j.issn.0376-2491.2018.34.012
26. Babyshkina N, Zavyalova M, Tarabanovskaya N, et al. Predictive value of vascular endothelial growth factor receptor type 2 in triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Mol Cell Biochem. 2018;444(1–2):197–206. doi:10.1007/s11010-017-3244-1
27. Jinesh GG, Manyam GC, Mmeje CO, Baggerly KA, Kamat AM. Surface PD-L1, E-cadherin, CD24, and VEGFR2 as markers of epithelial cancer stem cells associated with rapid tumorigenesis. Sci Rep. 2017;7(1):9602. doi:10.1038/s41598-017-08796-z
28. Lian L, Li XL, Xu MD, et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19(1):183. doi:10.1186/s12885-019-5322-0
29. Jantus-Lewintre E, Sureda BM, Larriba JL, et al. Prospective exploratory analysis of angiogenic biomarkers in peripheral blood in advanced NSCLC patients treated with bevacizumab plus chemotherapy: the ANGIOMET study. Front Oncol. 2021;11:695038. doi:10.3389/fonc.2021.695038
30. Lim YH, Odell ID, Ko CJ, Choate KA. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151(11):1240–1243. doi:10.1001/jamadermatol.2015.1925
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
