Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Influence of Hyperglycemia on the Prognosis of Patients with Diffuse Large B-Cell Lymphoma
Authors Zhou W , Li W, He C, Ma R, Gao Q , Wang Y, Feng L, Liu L
Received 19 April 2022
Accepted for publication 6 July 2022
Published 13 July 2022 Volume 2022:15 Pages 2039—2049
DOI https://doi.org/10.2147/DMSO.S370017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Weiling Zhou,1 Weijing Li,2 Cuiying He,2 Ruijuan Ma,2 Qian Gao,1 Yuan Wang,1 Lei Feng,1 Lihong Liu2
1Department of Endocrine and Metabolic Diseases, The Fourth Hospital of Hebei Medical University (Hebei Tumor Hospital), Shijiazhuang, People’s Republic of China; 2Department of Hematology, The Fourth Hospital of Hebei Medical University (Hebei Tumor Hospital), Shijiazhuang, People’s Republic of China
Correspondence: Lihong Liu, Department of Hematology, The Fourth Hospital of Hebei Medical University (Hebei Tumor Hospital), Shijiazhuang, People’s Republic of China, Tel +86 13831177920, Email [email protected]
Purpose: To explore the effects of primary and secondary hyperglycemia and the application of the hypoglycemic drug metformin on the prognosis of patients with diffuse large B-cell lymphoma (DLBCL).
Methods: We performed a retrospective analysis of 1767 DLBCL patients.Cox regression method was used for analysis to evaluate the prognostic factors, and the Kaplan-Meier method was used to draw a survival curve to analyze the effect of hyperglycemia and the hypoglycemic drug metformin on the progression-free survival (PFS) and overall survival (OS) of DLBCL patients.
Results: Our study showed that patients with hyperglycemia tend to have higher age (age> 60 years), high body mass index (BMI)(≥ 24kg/m2), late Ann Arbor stage (III–IV), high international prognostic index (IPI) (3– 5 score), high lactic dehydrogenase (LDH) level (> 250U/L), bulky disease and comorbidity. Hyperglycemia affects the survival time of the DLBCL population (PFS: adjusted HR 1.41, 95% CI: 1.16– 1.70, P < 0.001, OS: adjusted HR 1.33, 95% CI:1.09– 1.61, P=0.004).Compared with the non-hyperglycemia group, the secondary hyperglycemia increase affects the prognosis of the DLBCL population (P< 0.001). Compared with the secondary hyperglycemia group, the primary hyperglycemia group has a poor prognosis (P< 0.05). For patients with DLBCL and hyperglycemia (732 patients in total), the use of metformin can improve their PFS and OS (PFS: adjusted HR 0.69, 95% CI: 0.49– 0.96, P=0.028, OS: adjusted HR 0.68, 95% CI: 0.49– 0.95, P=0.024).
Conclusion: Hyperglycemia and secondary hyperglycemia are related to the poor prognosis of DLBCL population.For patients with DLBCL combined with hyperglycemia, the application of metformin can improve survival rate.
Keywords: hyperglycemia, diffuse large B-cell lymphoma, metformin, recrudescence, death, prognosis
Introduction
Lymphoma is a malignant tumor of the immune system that originates in lymph nodes and lymph node tissues. According to histopathological changes, it can be divided into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL, with an annual incidence of approximately 24% of NHL.1 Diabetes mellitus (DM) is a metabolic syndrome mainly manifested by chronic blood glucose elevation caused by the combined action of genetic and environmental factors. The number of people aged 20–79 years with diabetes worldwide has already reached 537 million in 2021 according to International Diabetes Federation, and this number is predicted to rise to 643 million by 2030 and 783 million by 2045.2 Studies have shown that diabetes is associated with an increased risk of multiple tumors in the liver, pancreas, endometrium, colorectal, breast, and bladder.3–9 Diabetes is associated with a higher risk of hematological malignancies, and is an independent risk factor for all-cause and specific cause mortality.10 Studies have shown that DM is associated with an increased risk of NHL.11,12 Metformin is a common hypoglycemic drug. Many studies have shown that the use of metformin in diabetic patients can reduce the incidence of cancer or reduce the mortality rate associated with cancer.13–17 Metformin inhibits tumor growth, proliferation, invasion and metastasis by decreasing blood glucose levels, attenuating insulin resistance, reducing inflammation and improving the tumor microenvironment.14 However, whether hyperglycemia, especially secondary hyperglycemia, affects the prognosis of DLBCL has not yet been reported, and there are few reports on whether metformin can help improve the prognosis of DLBCL patients.We aim to clarify the impact of hyperglycemia and the application of the hypoglycemic drug metformin on the prognosis of patients with DLBCL.
Methods
Cases
A retrospective collection of 1767 patients diagnosed with DLBCL in the Department of Hematology, the Fourth Hospital of Hebei Medical University from January 1, 2010 to June 1, 2020, all met the diagnostic criteria for DLBCL. Informed consent was obtained before data collection. Those who did not have complete clinical information or immunohistochemistry data, or who were lost to follow-up immediately after treatment were excluded from this study. The study was approved by the medical ethics committee of the the Fourth Hospital of Hebei Medical University (Hebei Tumor Hospital),(approval number, 2022KY384) and followed the principles of the Declaration of Helsinki.
Clinical Information
The collected clinical data include age, gender, height, weight, pathological diagnosis, Ann-Arbor staging, B symptoms, IPI score, LDH, β2 micro-globulin, blood glucose level, immunohistochemistry, therapy proposal, extranodal involvement, bulky disease, comorbidities (hypertension, cerebrovascular disease, hepatitis, tuberculosis, other malignant tumors) and whether taking metformin, Among them, pathological diagnosis is divided into germinal center B cell source (GCB type) and non-GCB (Non-GCB) type according to the Hans classification system.18 According to the results of PET-CT or CT, DLBCL patients were staged according to the Ann-Arbor staging system 19 B symptoms are defined as unexplained fever (body temperature above 38°C), night sweats, weight loss (unexplained weight within 6 months Reduce by more than 10%);IPI score according to the adverse factors (age> 60 years old, disease stage III/IV, LDH higher than normal, ECOG performance status score ≥ 2 points, number of extranodal involvement sites>1).20 Immunohistochemistry mainly includes whether or not Ki-67, BCL-2, BCL-6, C-MYC are positive and the positive ratio; Therapy proposal: All patients received CHOP or R-CHOP regimen, and some received radiotherapy and operation, CHOP regimen: cyclophosphamide 750 mg/m2 on day 1; doxorubicin 50 mg/m2 on day 1; vincristine 1.4 mg/m2 (maximum dose 2 mg/m2), on the 1st day; prednisone 60 mg/m2, on the 1st to 5th days.R-CHOP regimen: rituximab 375 mg/m2, day 1; cyclophosphamide 750 mg/m2, day 1; doxorubicin 50 mg/m2, day 1; vincristine 1.4 mg/m2 (maximum dose 2 mg/m2), on day 1; prednisone 60 mg/m2, on days 1 to 5. The specific drug dose is adjusted according to the patient’s body surface area, general condition and patient tolerance;Hyperglycemia mainly includes: ①The type 2 diabetes is clearly diagnosed according to the medical records of the previous outpatient or hospitalization (Dry mouth, polydipsia, and polyphagia and weight loss + Fasting plasma glucose (FPG)≥7.0mmol/L or random blood glucose≥11.1mmol/L or oral glucose tolerance test 2-hour blood glucose≥11.1mmol/L); ②FPG≥ 6.1 mmol/L during the evaluation before DLBCL treatment.21 Secondary hyperglycemia mainly includes: ①No previous history of DM and FPG <6.1 mmol/L at the time of evaluation before DLBCL treatment; ②During the treatment, At least two time’s of the first day of hospitalization FPG≥6.1mmol/L. Metformin group refers to taking single-drug metformin or metformin combined with other hypoglycemic drugs, non-metformin group refers to never taking metformin (other hypoglycemic drugs are not excluded).
The follow-up was be conducted by the telephone of our hospital’s follow-up center, outpatient review and inpatient review. The deadline for follow-up is August 15, 2021.Primary endpoint: Overall survival (OS) period is defined as the time from initial diagnosis to death, last follow-up or loss to follow-up due to any reason; progression-free survival (PFS) period is defined as the period from initial diagnosis to tumor recurrence, progression or last follow-up time.
Statistical Analysis
All data were statistically analyzed using SPSS for Windows (version 26.0, Armonk, NY: IBM Corp). Continuous variables were presented by mean±standard deviation. Categorical variables were presented as numbers and percentage.Group comparisons used t-tests on continuous, and x2 tests on categorical variables. The Cox regression risk model was used for univariate and multivariate analysis to evaluate independent risk predictors of DLBCL. The Kaplan-Meier method was used to draw the survival curve, and the Log rank test was used to compare differences between groups. P<0.05 indicates that the difference is statistically significant.
Results
1. A total of 1767 DLBCL patients were enrolled in this study. The mean age was 60.71 ± 14.66 years (17–96 years). 335 cases (19.0%) had hyperglycemia before the diagnosis of DLBCL (including 291 for type 2 diabetes and 44 for pre-diabetes), and 131 patients (7.4%) had high blood glucose before the pre-DLBCL assessment. 266 cases (15.1%) had secondary hyperglycemia,1035 patients (58.6%) had non-hyperglycemia. Patients with hyperglycemia are more likely to have higher age (age>60 years), higher BMI, late Ann-Arbor staging, high IPI score, high LDH level, bulky disease and comorbidities. (all P<0.05).(Table 1).
 |
Table 1 Baseline Clinical Characteristics of 1767 DLBCL Patients with or Without Hyperglycemia |
2. Those with DLBCL and hyperglycemia who are older than 60 years old mostly have primary hyperglycemia.Compared with secondary hyperglycemia, primary hyperglycemia had significant differences in age, β2 micro-globulin, C-MYC and metformin (P < 0.05).Compared with non-hyperglycemia group, the patients with secondary hyperglycemia tended to have higher age, higher BMI, late Ann Arbor stage, high IPI, high LDH level, bulky disease and comorbidities (P < 0.05). (Table 2).
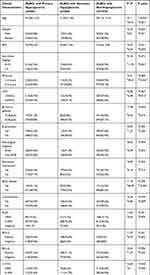 |
Table 2 Baseline Clinical Characteristics of 732 Patients with DLBCL with Hyperglycemia and 1035 Patients with DLBCL and Non-Hyperglycemia |
3. Cox univariate analysis of 1767 cases showed that hyperglycemia, age, BMI, Ann-Arbor staging, IPI, B symptoms, pathological diagnosis, LDH level, β2 micro- globulin, bulky disease,BCL-2 and C-MYC positive were all associated with OS and PFS (all P<0.05).Multivariate analysis of these factors showed that hyperglycemia, age, high BMI, late Ann-Arbor staging, high IPI, B symptoms, high LDH level,highβ2 micro-globulin, extranodal involvement, bulky disease,BCL-2 positive were all associated with worse prognosis (all P<0.05).(Tables 3 and 4).
 |
Table 3 Univariate and Multivariate Analysis of PFS in 1767 Patients with DLBCL |
 |
Table 4 Univariate and Multivariate Analysis of OS in 1767 Patients with DLBCL |
Cox univariate analysis of 732 patients with hyperglycemia showed that secondary hyperglycemia, metformin application, age,BMI, Ann-Arbor staging,IPI score, B symptoms,LDH level,β2 micro-globulin,C-myc were related to OS and PFS (all P<0.05).Multivariate analysis of these factors showed that secondary hyperglycemia no metformin application, high BMI,high IPI score, C-MYC positive were associated with poor prognosis (all P<0.05). (Tables 5 and 6).
 |
Table 5 Univariate and Multivariate Analysis of PFS in 732 Patients with DLBCL and Hyperglycemia |
 |
Table 6 Univariate and Multivariate Analysis of OS in 732 Patients with DLBCL and Hyperglycemia |
4. Survival analysis: the follow-up time for 1767 patients ended on August 5, 2021. The shortest follow-up time was 1 month, the longest time was 281 months, and the follow-up time was 35.8±33.4 months. 494 (28.0%) patients died and 875 (49.5%) patients experienced disease progression.The 5-year OS rate in hyperglycemia group was 57%, the 5-year OS rate in non-hyperglycemia group was 73%, the 5-year PFS rate in hyperglycemia group was 55%, and the 5-year PFS in non-hyperglycemia group was 72%. The median survival time in the hyperglycemia group was 109 months, and the median survival time in the non-hyperglycemia group was 184 months.
Compared with the non-hyperglycemia group, the hyperglycemia group were associated with worse prognosis, the differences in OS and PFS were statistically significant (all P<0.001) (Figure 1).
Compared with the secondary hyperglycemia group, the primary hyperglycemia group had a worse prognosis (all P <0.05);Compared with the non-hyperglycemia group, the secondary hyperglycemia also affected the prognosis of DLBCL patients (all P<0.001) (Figure 2).
The application of the hypoglycemic drug metformin can increase the PFS and OS of people with DLBCL and hyperglycemia (all P <0.05) (Figure 3).
Discussion
With the increase in the incidence of diabetes, the impact of diabetes on the prognosis of cancer has attracted more and more attention. This study retrospectively analyzed the prognosis of 1767 cases of DLBCL. Our study showed that the prevalence of type 2 diabetes with diffuse large B-cell lymphoma was 26.4%, of which 19.0% of patients had hyperglycemia before the diagnosis of DLBCL, and 7.4% of patients had hyperglycemia during the evaluation before DLBCL treatment. Among people over 60 years old, 20.3% of DLBCL patients had hyperglycemia, which was similar to the results of the Lam study.22 In our study, hyperglycemia significantly affected the PFS and OS of DLBCL patients (P <0.001), and the 5-year PFS and OS of DLBCL patients with hyperglycemia were significantly lower than non-hyperglycemia group (P <0.001), and Drozd-Sokolowska research was similar.23 Therefore, it is necessary to pay attention to people with hyperglycemia when diagnosing and treating DLBCL, especially those with unknown hyperglycemia. Yang and other studies found that compared with patients without diabetes, patients with T2DM had a higher risk of NHL, with a hazard ratio of 2.00 (95% CI: 1.32–3.03), which was consistent with the conclusions of this study.24 In addition, 12.1% of the population experienced increased blood glucose during DLBCL treatment. Compared with patients with non-hyperglycemia, secondary hyperglycemia also affected the prognosis of DLBCL patients (P <0.001), which was contrary to the results of Lamar.25 The sample size needs to be increased to further evaluate the relationship between the two.Compared with the secondary hyperglycemia group, the primary hyperglycemia group had a worse prognosis. The consideration of secondary hyperglycemia may be related to the application of hormones,26 excluding weight factors: This study found that compared with the non-hyperglycemia group, the secondary hyperglycemia had a higher BMI, indicating that overweight people are more likely to have secondary hyperglycemia, which in turn affects the prognosis of DLBCL people. So in the process of diagnosis and treatment, we need to pay attention to the weight management of overweight people.Through retrospective analysis, Anil showed that metformin combined with first-line chemotherapy can improve PFS and OS in diabetic patients, preclinical studies had shown metformin had the potential to re-sensitize drug-resistant lymphoma to chemoimmunotherapy.27 This study shows that for people with DLBCL and hyperglycemia, the use of the hypoglycemic drug metformin can increase the population’s PFS and OS (PFS: adjusted HR 0.69, 95% CI: 0.49–0.96, P=0.028, OS: adjusted HR 0.68, 95% CI: 0.49–0.95, P=0.024).Therefore, for people with DLBCL and hyperglycemia, if there is no obvious contraindication, metformin is the first choice for hypoglycemic therapy.
This is a large-scale clinical retrospective study with a large number of enrolled cases and a long follow-up time, which is representative to a certain extent. Moreover, this study is the first to suggest that secondary hyperglycemia also affects the prognosis of DLBCL patients. However, because this is a single-center study with a large time span, large-scale chromosome or FISH examination of lymphoma cells was not performed, and double-hit and triple-hit lymphoma models were not included. At the same time, this study did not exclude end-stage diabetes patients, multicenter and larger sample sizes are required to validate this result.
Conclusions
In short, our data suggested hyperglycemia was related to the poor prognosis of DLBCL population, and secondary hyperglycemia also affected the prognosis of DLBCL population, and hyperglycemia was an independent risk factor affecting the prognosis of DLBCL.This study also found that overweight people were more likely to have secondary hyperglycemia, and clinical attention should be paid to DLBCL patients with overweight.In addition, this study showed that the application of metformin could improve the survival rate of patients with DLBCL and hyperglycemia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–616. doi:10.1002/ajh.25460
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Tsilidis KK, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;11(1):350. doi:10.1136/bmj.g7607
4. Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi:10.1002/ijc.26165
5. Li D, Yeung SC, Has San MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–488. doi:10.1053/j.gastro.2009.04.013
6. Luo J, Beresford S, Chen C, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111(7):1432–1439. doi:10.1038/bjc.2014.407
7. Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: ameta-analysis and systematic review. World J Gastroenterol. 2015;21(19):6026–6031. doi:10.3748/wjg.v21.i19.6026
8. Chang YL, Sheu WH, Lin SY, et al. Good glycaemic control is associated with a better prognosis in breast cancer patients with type 2 diabetes mellitus. Clin Exp Med. 2018;18(3):383–390. doi:10.1007/s10238-018-0497-2
9. Tseng CH. Diabetes but not insulin increases the risk of lung cancer: a Taiwanese population-based stud. PLoS One. 2014;9(7):e101553. doi:10.1371/journal.pone.0101553
10. Gong IY, Cheung MC, Read S, et al. Association between diabetes and haematological malignancies: a population-based study. Diabetologia. 2021;64(3):540–551. doi:10.1007/s00125-020-05338-7
11. Wang Y, Liu X, Yan P, et al. Association between type 1 and type 2 diabetes and risk of non- Hodgkin’s lymphoma: a meta-analysis of cohort studies. Diabetes Metab. 2020;46(1):8–19. doi:10.1016/j.diabet.2019.04.006
12. Xu J, Wang T. Association of diabetes mellitus with non-Hodgkin lymphoma risk: a meta- analysis of cohort studies. Hematology. 2019;24(1):527–532. doi:10.1080/16078454.2019.1636485
13. Zhou J, Han S, Qian W, et al. Metformin induces miR-378 to downregulate the CDK1, leading to suppression of cell proliferation in hepatocellular carcinoma. Onco Targets Ther. 2018;11:4451–4459. doi:10.2147/OTT.S167614
14. Leng W, Jiang J, Chen B, Wu Q. Metformin and malignant tumors: not over the Hill. Diabetes Metab Syndr Obes. 2021;14:3673–3689. doi:10.2147/DMSO.S326378
15. Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res. 2014;7(9):867–885. doi:10.1158/1940-6207.CAPR-13-0424
16. Zhang Y, Chen R, Deng L, et al. The effect of metformin on the proliferation, apoptosis and CD133 mRNA expression of colon cancer stem cells by upregulation of miR 342-3p. Drug Des Devel Ther. 2021;15:4633–4647. doi:10.2147/DDDT.S336490
17. Zhang J, Wen L, Zhou Q, et al. Preventative and therapeutic effects of metformin in gastric cancer: a new contribution of an old friend. Cancer Manag Res. 2020;12:8545–8554. doi:10.2147/CMAR.S264032
18. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
19. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
20. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non- Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi:10.1056/NEJM199309303291402
21. Gao R, Liang JH, Man TS, et al. Diabetes mellitus predicts inferior survival in diffuse large B-cell lymphoma: a propensity score-matched analysis. Cancer Manag Res. 2019;11:2849–2870. doi:10.2147/CMAR.S185319
22. Lam C, Cronin K, Ballard R, et al. Differences in cancer survival among white and black cancer patients by presence of diabetes mellitus: estimations based on SEER -Medicare-linked data resource. Cancer Med. 2018;7(7):3434–3444. doi:10.1002/cam4.1554
23. Drozd-Sokolowska J, Zaucha JM, Biecek P, et al. Type 2 diabetes mellitus compromises the survival of diffuse large B- cell lymphoma patients treated with (R) -CHOP - The PLRG report. Sci Rep. 2020;10(1):3517. doi:10.1038/s41598-020-60565-7
24. Yang WS, Li HL, Xu HL, et al. Type 2 diabetes and the risk of non-Hodgkin’s lymphoma: a report from two population-based cohort studies in China. Eur J Cancer Prev. 2016;25(2):149–154. doi:10.1097/CEJ.0000000000000150
25. Lamar ZS, Dothard A, Kennedy L, et al. Hyperglycemia during first-line R-CHOP or dose adjusted R-EPOCH chemotherapy for non- Hodgkin lymphoma is prevalent and associated with chemotherapy alteration - a retrospective study. Leuk Lymphoma. 2018;59(8):1871–1877. doi:10.1080/10428194.2017.1410889
26. Dehghani M, Hobbi AM, Haghighat S, et al. Glucocorticoid induced diabetes and lipid profiles disorders amongst lymphoid malignancy survivors. Diabetes Metab Syndr. 2020;14(6):1645–1649. doi:10.1016/j.dsx.2020.08.027
27. Singh AR, Gu JJ, Zhang Q, et al. Metformin sensitizes therapeutic agents and improves outcome in pre-clinical and clinical diffuse large B-cell lymphoma. Cancer Meta b. 2020;8(1):10. doi:10.1186/s40170-020-00213-w
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



