Back to Journals » International Journal of General Medicine » Volume 14
Influence of GSTP-1 Polymorphism on the Prognosis of Patients with High-Grade Glioma Who Received Temozolomide Plus Radiotherapy Adjuvant Treatment
Authors Zhi DB, Wang ZY, Xie T, Tu WW
Received 30 July 2021
Accepted for publication 11 November 2021
Published 22 December 2021 Volume 2021:14 Pages 10173—10183
DOI https://doi.org/10.2147/IJGM.S328810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
De-Bao Zhi,1,* Zhi-Yu Wang,2,* Tong Xie,1 Wen-Wen Tu1
1Department of Surgical Care Unit, Xuhui District Central Hospital, Shanghai, 200031, People’s Republic of China; 2Department of Neurosurgery, Xuhui District Central Hospital, Shanghai, 200031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: De-Bao Zhi Tel +86 18616568331
Email [email protected]
Background: Glutathione S-Transferase P 1 (GSTP-1) gene plays an important physiological role in the body. The present study was conducted to identify the clinical implication of GSTP-1 gene polymorphism on the prognosis of patients with high-grade glioma (HGG) who received temozolomide plus radiotherapy adjuvant treatment.
Methods: This study recruited a total of 186 patients with HGG who were treated with temozolomide plus radiotherapy adjuvant regimen (retrospectively). Baseline clinical characteristics were obtained and the prognostic data of the patients were collected. Peripheral blood specimen of patients was preserved for genotyping of GSTP-1 polymorphism during hospitalization. Correlation analysis was carried out accordingly. Additionally, fresh peripheral blood specimens that were available for mRNA expression analysis were collected for the mRNA expression analysis.
Results: The median progression-free survival (PFS) and overall survival (OS) of the 186 patients with HGG who received temozolomide plus radiotherapy regimen was 8.5 months (95% CI: 5.95– 11.05) and 15.5 months (95% CI: 11.49– 19.51), respectively. The prevalence of 313A>G among 186 patients with glioma was AA genotype: 126 cases (67.7%), AG genotype: 54 cases (29.1%), GG genotype: 6 cases (3.2%), minor allele frequency of 313A>G was 0.18. Association analysis suggested that the median PFS of patients with AA and AG/GG genotypes was 11.2 and 5.0 months, respectively (χ2=11.17, P=0.001). Furthermore, the median OS of patients with AA and AG/GG genotypes was 18.9 and 10.5 months, respectively (χ2=12.684, P< 0.001). Besides, when adjusted for PFS in multivariate Cox regression analysis, AG/GG genotype was an independent factor for PFS (HR=0.48, P=0.006). The mRNA expression results indicated that mRNA expression of GSTP-1 in patients with AG/GG genotypes of 313A>G was significantly higher than that of patients with AA genotype (P< 0.001).
Conclusion: GSTP-1 polymorphism 313A>G might be used as a potential biomarker to predict the prognosis of patients with HGG who received temozolomide plus radiotherapy adjuvant treatment.
Keywords: high-grade glioma, temozolomide, GSTP-1, polymorphism, prognosis
Introduction
Gliomas originate from glial or precursor cells and develop into astrocytoma, oligodendroglioma, ependymoma and oligoastrocytoma,1 which is estimated to be the most common primary tumor in the brain and accounts for approximately 80% of central nervous system malignancies.2 It was reported that there are approximately 50,000 new cases in China annually.3 Gliomas are classified into four grades according to the WHO category, among which grade I and grade II gliomas were divided into low-grade glioma, and grade III and grade IV gliomas were recognized as high-grade glioma (HGG).4 Although considerable breakthroughs in immunotherapy for many kinds of tumors were observed in recent years, study progress that has dramatically improved the prognosis for patients with HGG is still scanty and limited drugs have been approved by the FDA in the treatment of glioma currently.5 Given that the unique structure in the central nervous system prevents numerous antitumor drugs from entering the brain, many challenges still exist regarding the efficacy of anti-glioma drugs.6 Therefore, surgical resection combined with chemoradiotherapy still plays an important role for patients with HGG currently.7 Unfortunately, due to the infiltrative growth of glioma, radiation damage of radiotherapy to the surrounding normal brain tissue and resistance to chemotherapeutic drugs, considerable challenges have been observed which compromise the prognosis of patients with glioma.8 For HGG, the median overall survival (OS) of patients with grade III glioma is approximately 3 years, whereas patients with grade IV glioma conferred a worse prognosis with the median OS of 15 months.9 Therefore, it is urgently necessary to identify the mechanism of drug resistance to postoperative radiotherapy plus temozolomide and to investigate the potential biomarkers that could predict the prognosis of patients with HGG who received concurrent chemoradiotherapy at present.10
Apart from surgical resection, radiotherapy is also an important adjuvant treatment for patients with HGG. A previous study exhibited that patients with HGG significantly benefited from 30 doses of 60Gy radiotherapy compared with 20 doses of 45Gy clinically.11 And a subsequent randomized clinical trial indicated that no dramatic survival benefit was observed in HGG when radiotherapy dose was increased to 60Gy.12 As a result, total dosage of 54–60Gy and 1.8–2.0Gy/fraction is still the standard recommended dosage for the treatment of HGG currently.13 Regarding chemotherapy, as a novel oral alkylating agent, temozolomide has a broad spectrum of antitumor activity and is absorbed through oral administration with almost 100% bioavailability into the circulatory system throughout the body. Simultaneously, under physiological pH state, temozolomide is rapidly converted to the active product MTIC, thus playing a cytotoxic role in vivo.14
Glutathione S-transferases (GSTs) family is comprised of eight isoforms that detoxify the xenobiotic substrates by catalyzing their conjugation to reduce glutathione.15 Among them, Glutathione S-Transferase P 1 (GSTP-1) is the most widely reported isoform in various diseases.16 GSTP-1 is located at 11q13.2, contains 7 exons, and is 3.2kb in length with the ability to protect cells from carcinogens and cytotoxins. Individual difference was observed among different study populations.17 A previous study suggested that GSTP-1 gene played an important role in biodetoxification or biotransformation by binding to reduced glutathione.18 With regard to the pharmacogenomics research results of GSTP-1, relatively limited studies were performed to identify the clinical significance of GSTP-1 polymorphism in Chinese population. Interestingly, a recent study investigated the correlation between genetic variations of GSTP-1 and the clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer (NSCLC) and concluded that 313A>G polymorphisms could influence the platinum-induced clinical outcomes.19 Additionally, another previous foreign study indicated that 313A>G was associated with prognosis of patients with glioma who received concurrent chemoradiotherapy.20 These research indicated that GSTP-1 polymorphism could be of potential clinical significance to predict the prognosis of patients who received chemoradiotherapy. However, currently, no study has investigated the association between GSTP-1 polymorphism and the survival of patients with HGG who were treated with chemoradiotherapy in Chinese population.
Consequently, the present study was conducted to identify the implication of GSTP-1 polymorphism on the prognosis of patients with HGG who received temozolomide plus radiotherapy adjuvant treatment.
Materials and Methods
Study Design and Patient Enrollment
The present study was designed as a real-world retrospective trial, patients with HGG who underwent surgical resection and temozolomide plus radiotherapy treatment from January 2012 to December 2020 in the Surgical Care Unit of Xuhui District Central Hospital participated in this study retrospectively. The main inclusion criteria included: 1) histologically confirmed diagnosis of glioma; 2) grade III or IV stage according to the WHO classification criteria; 3) Eastern Cooperative Oncology Group (ECOG) performance status ≤2 score; 4) appropriate liver and kidney function, adequate blood routine function (neutrophil count ≥1.5×109/L, platelet count ≥100×109/L); 5) patients underwent surgical resection and temozolomide plus radiotherapy adjuvant treatment. The key exclusion criteria were: 1) concomitant other cancer or serious diseases that might compromise the survival of the patients; 2) failed to receive postoperative temozolomide plus radiotherapy treatment. The flow chart of this retrospective study was illustrated in Figure 1. Eventually, a total of 186 patients with HGG were enrolled. The primary purpose of this study was to identify the association between GSTP-1 polymorphism status and prognosis of patients with HGG who received temozolomide plus radiotherapy adjuvant regimen. This study was approved by the ethics committees of Xuhui District Central Hospital. All the patients were provided with written informed consent prior to study commencement and signed the consent form accordingly, and this study was conducted in accordance with the Declaration of Helsinki.
 |
Figure 1 The flow chart of this retrospective study of patients with high-grade glioma who received temozolomide plus radiotherapy treatment. |
Treatment Regimens
Treatment regimen of the patients with HGG was recorded retrospectively. Implementation of radiotherapy was as follows: all the radiotherapy was performed within 6 weeks after surgical resection according to the patients’ physical conditions with the total dosage of 54 or 60Gy in 30 fractions, 1.8 or 2.0Gy/F, once daily, the first 5 days of each week, for a total of 6 weeks. Additionally, usage and dosage of temozolomide were as follows: concurrent with radiotherapy, starting on the same day as radiotherapy, 75mg/m2/day, for a total of 42 days. During the administration, temozolomide could be suspended based on the patients’ tolerance, no more than 49 days. Then, 4 weeks after the completion of concurrent chemoradiotherapy, patients received 6 cycles of temozolomide monotherapy as adjuvant chemotherapy, dosage of the first cycle was 150mg/m2, followed by each cycle of 200mg/m2, days 1–5, every 28 days as one cycle. Treatment duration of temozolomide was adjusted according to the patient’s tolerance during the therapy.
Specimen Collection and Genotyping of GSTP-1 Polymorphism
Approximately 3mL peripheral blood specimen of each patient, prior to chemoradiotherapy administration, was collected using EDTA anticoagulant tubes for the subsequent polymorphism analysis. Genomic DNA was extracted from the blood specimen using phenol-chloroform method and then stored at −20°C for subsequent usage. The polymorphisms of GSTP-1 gene were collected from the NCBI database with the minor allele frequency (MAF)>0.1 among Chinese population and according to the previous report that the polymorphisms of GSTP-1 was of clinical significance,21 which were 313A>G (rs1695), 7905T>C (rs4891) and 7188G>A (rs749174). Prognostic analysis was performed first in the study. As shown in Table 1, only 313A>G was observed with positive relevance to PFS in the preliminary analysis. Therefore, the present study performed the analysis between 313A>G and prognosis subsequently. 313A>G was genotyped using restriction fragment length polymorphisms polymerase chain reaction (PCR-RFLP). Genotyping of each sample was performed with procedures according to the instructions. The PCR products were amplified first and then the product was incubated and digested with restriction enzymes. The PCR product was 286 bp. The forward primer was 5ʹ- CCCCAGTGACTGTGTGTTGA-3ʹ, the reverse primer was 5ʹ- GCACCCTGACCCAAGAAG-3ʹ. A total of 2 μL PCR products were digested using the restriction enzyme BsmBI (Thermo Fisher Scientific, USA). The genotypes were distinguished through the size of the band: AA genotype (one 286 bp band); AG genotype (three bands: one 286 bp band, one 150 bp band and one 136 bp band); GG genotype (two bands: one 150 bp band and one 136bp band). Additionally, a negative reference was set during analysis to eliminate the interference of confounding factors.
 |
Table 1 Details of the Polymorphisms of GSTP-1 Included in This Study and the Preliminary Analysis Between Genotype Status and PFS |
Collection of Peripheral Blood Mononuclear Cells (PBMC) and GSTP-1 Gene mRNA Expression Analysis
Given that GSTP-1 gene was related to the pharmacokinetics of chemotherapeutic drugs rather than the gene of tumor predisposition and development, we chose peripheral blood mononuclear cells (PBMC) instead of tumor tissue to investigate the GSTP-1 gene mRNA expression. Initially, the matched PBMC specimens were collected from 79 subjects among the 186 patients with HGG prior to temozolomide combined with radiotherapy administration. Unfortunately, extraction of PBMC specimens from 8 patients was not successful and 6 patients failed the RNA extraction experiment. Therefore, a total of 65 specimens were available for GSTP-1 mRNA expression analysis ultimately. Total RNA samples were extracted using TRIzol reagents (Takara Biotechnology) according to the manufacturer's instructions and stored at −80°C for mRNA expression analysis. A total of 500ng RNA extracted from PBMC specimens was used as the templates for reverse-transcription polymerase chain reaction. Relative quantitative determination of GSTP-1 gene mRNA expression was performed using the Light Cycler® 480 (Roche, Shanghai, China) with SYBR Premix EX Taq system. The forward primer of GSTP-1 gene was 5ʹ-GTAGTTTGCCCAAGGTCAAG −3ʹ, the reverse primer was 5ʹ- AGCCACCTGAGGGGTAAG −3ʹ. GAPDH mRNA expression was used as an internal reference. GSTP-1 mRNA expression was calculated by relative quantification method 2 −ΔΔCt.22
Statistical Analysis
All variables in this study were analyzed using SPSS software version 25.0. Chi-squared test was used to determine whether the genotype status of polymorphism corresponded with Hardy-Weinberg equilibrium. Statistical difference of proportion variables and continuous variables according to 313A>G genotype status was analyzed using the chi-squared test and the Mann–Whitney U nonparametric test, respectively. Regarding the prognostic data, Kaplan-Meier survival curves were drawn using Stata 14.0 to estimate the difference in progression-free survival (PFS) and OS of the patients according to the genotype status of 313A>G. The difference between the survival curve was calculated using the Log rank test. PFS was defined from the date of adjuvant chemoradiotherapy administration to the date of tumor progression or death of the patients, whichever occurred first. OS was defined from the date of adjuvant chemoradiotherapy administration to the date of death of the patients from any cause. For those without survival events by the end of the study, survival end points were censored at the date of last follow-up. Cox regression analysis was introduced for PFS in multivariable analysis. P<0.05 was accepted as significant statistical difference.
Results
Baseline Characteristics of the 186 Patients with HGG According to Genotype Status of 313A>G Polymorphism
Baseline characteristics of the 186 patients with HGG were exhibited in Table 2, the median age of the 186 patients was 62 years (range: 29–81 years). Males and females were observed in 117 patients and 69 patients, respectively. ECOG 0 score and 1–2 score was noted in 76 and 110 patients, respectively. Patients underwent biopsy or partial resection and total resection was observed in 113 cases and 73 cases, respectively. In the 73 patients who underwent total resection it was indicated that >98% of tumor mass was resected and in the 113 patients who received biopsy or partial resection, >70% of tumor mass was resected. Interestingly, the most common WHO grade of the patients included was grade IV (84.4%). Additionally, patients with IDH positive and negative mutation were observed in 51 cases and 88 cases, respectively. However, 47 patients were not available for IDH mutation status. Furthermore, a total of 28 patients were treated with 54Gy radiotherapy and 158 patients were administered 60 Gy radiotherapy. Besides, it should be noted that not all the 186 patients underwent 6 cycles of temozolomide adjuvant treatment, the median temozolomide administration cycle was 3 with the range of 1–6 cycles actually.
 |
Table 2 Baseline Characteristics of 186 Patients with HGG According to the Genotype Status 313A>G |
Regarding the polymorphism analysis, 313A>G polymorphism was of clinical significance in the association analysis. The prevalence of 313A>G among the 186 patients with HGG was: AA genotype: 126 cases (67.7%), AG genotype: 54 cases (%), GG genotype: 6 cases (3.2%), minor allele frequency of 313A>G was 0.18. Distribution of three genotypes was in accordance with Hardy-Weinberg Equilibrium (P=0.942). Given that patients with GG genotype were relatively rare, patients with GG and AG genotypes were merged in a pattern of dominant inheritance to explore the potential clinical significance. Interestingly, as exhibited in Table 2, the baseline characteristics of patients with AA and AG/GG genotypes were balanced and comparable.
Prognostic Data of the 186 Patients with HGG Who Received Temozolomide Plus Radiotherapy
All the 186 patients with HGG receiving temozolomide combined with radiotherapy were available for the prognostic assessment. The last follow-up date of the present study was June 2021, and the median follow-up duration for all patients from enrollment to the last follow-up date was 14.5 months (follow-up range: 0.6–45 months). Accordingly, the prognostic data of the 186 patients exhibited that the median PFS of the 186 patients with HGG was 8.5 months [95% confidence interval (CI): 5.95–11.05], and the median OS of the 186 patients with HGG was 15.5 months (95% CI: 11.49–19.51).
Implication of 313A>G Polymorphism on the Prognosis of 186 Patients with HGG
Furthermore, clinical significance of GSTP-1 polymorphism was established to identify the potential relevance to the prognosis of 186 patients with HGG. As exhibited in Figure 2, the relevance of 313A>G polymorphism to PFS of the 186 patients with HGG suggested that the median PFS of patients with AA and AG/GG genotype was 11.2 months (95% CI: 8.69–13.71) and 5.0 months (95% CI: 3.99–6.01), respectively. And the difference was statistically significant (χ2=11.17, P=0.001). Furthermore, in order to identify the clinical significance of GSTP-1 313A>G independently, a multivariate Cox regression analysis was introduced for PFS analysis. Univariate analysis between baseline characteristics and PFS was implemented firstly. As illustrated in Table 3, ECOG PS score, WHO grade, extent of surgery, cycles of temozolomide adjuvant treatment and genotype status 313A>G had significant association with PFS in the univariate analysis. Therefore, these characteristics were included in the multivariate Cox regression analysis accordingly. And the multivariate analysis results were also presented in Table 3, genotype status 313A>G polymorphism was an independent factor for PFS [HR=0.48 (95% CI: 0.27–0.81), P=0.006] after multivariate adjustment. Moreover, other statistically significant characteristics in the multivariate Cox analysis were ECOG PS score (HR=0.74, P=0.008), WHO grade (HR=0.52, P=0.001), extent of surgery (HR=1.51, P=0.009) and cycles of temozolomide adjuvant treatment (HR=1.34, P=0.015), which suggested that patients with ECOG PS 0 score had superior PFS compared with that of patients with 1–2 score (median PFS: 9.4 vs 6.9 months), patients with grade III had better PFS than patients with grade IV (median PFS: 10.1 vs 5.2 months), patients who underwent total resection were correlated with superior PFS compared to that of patients with biopsy or partial resection (median PFS: 9.8 vs 6.5 months). Patients who underwent >3 cycles of temozolomide adjuvant treatment were associated with longer PFS than that of patients with ≤3 cycles of temozolomide therapy (median PFS: 9.4 vs 7.1 months).
 |
Table 3 PFS of the 186 Patients with HGG According to Baseline Characteristics and Polymorphism in Univariate Analysis and Multivariate Cox Analysis |
 |
Figure 2 The progression-free survival of the 186 patients with high-grade glioma according to genotype status GSTP-1 313A>G. |
Given that the follow-up duration was long enough, OS was also assessed in our study simultaneously. The OS survival curve according to 313A>G genotype status was exhibited in Figure 3. Accordingly, the median OS of patients with AA genotype and AG/GG genotype was 18.9 months (95% CI: 14.82–22.98) and 10.5 months (95% CI: 7.34–13.66), respectively. And the difference was statistically significant (χ2=12.684, P<0.001).
 |
Figure 3 The overall survival of the 186 patients with high-grade glioma according to genotype status GSTP-1 313A>G. |
Association Analysis Between 313A>G Genotype Status and mRNA Expression of GSTP-1
Eventually, a total of 65 PBMC specimens were available for GSTP-1 mRNA expression analysis. And the relevance analysis between the genotype status 313A>G and mRNA expression was analyzed. Firstly, the prevalence of 313A>G polymorphism among the 65 PBMC specimens were: AA genotype: 43 cases (66.2%), AG genotype: 19 cases (29.2%), and GG genotype: 3 cases (4.6%). The MAF was 0.19 and distribution frequency of the three genotypes was in accordance with the Hardy-Weinberg equilibrium (P=0.634) as well and were comparable with that of the 186 patients. Similarly, AG and GG genotypes were merged in the subsequent analysis. As illustrated in Figure 4, the relative expression of GSTP-1 mRNA in PBMC of patients with AG/GG genotype was significantly higher than that of patients with AA genotype of 313A>G polymorphism (3.99±0.603 vs 2.87±0.873), which was statistically significant (t=5.351, P<0.001).
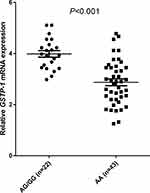 |
Figure 4 Relative expression levels of GSTP-1 mRNA in 65 PBMC specimens according to genotype status GSTP-1 313A>G. |
Additionally, GSTP-1 mRNA expression status was divided into GSTP-1 high expression (GSTP1-H) and GSTP-1 low expression (GSTP1-L) according to the median GSTP-1 mRNA expression level to identify the potential association with OS. Patients with GSTP1-H and GSTP1-L were observed in 33 cases and 32 cases, respectively. As exhibited in Figure 5, patients with GSTP1-H showed a trend of worse OS compared with those with GSTP1-L [median OS: 9.2 (95% CI: 3.01–15.39) months vs 16.3 (95% CI: 12.42–20.18) months], although the difference was not statistically significant (χ2=3.247, P=0.072).
 |
Figure 5 The overall survival of the 65 patients with high-grade glioma according to mRNA expression status of GSTP-1 gene. |
Discussion
In our opinion, the present retrospective study highlights the real-world evidence regarding the prognosis of 186 patients with HGG who received temozolomide plus radiotherapy adjuvant treatment. Simultaneously, polymorphism analysis in this study suggested that patients with AG/GG genotype of 313A>G were associated with worse PFS and OS. GSTP-1 polymorphism 313A>G might be used as a potential biomarker to predict the prognosis of patients with HGG who received temozolomide plus radiotherapy adjuvant treatment.
As a highly heterogeneous solid malignancy, HGG has been more sensitive to temozolomide plus radiotherapy adjuvant regimen for the past several decades.23 Despite the superior response to temozolomide plus radiotherapy regimen, patients with HGG almost always relapse inevitably.24 To our knowledge, the 2018 Nobel Prize in Medicine was awarded to James Allison and Tasuku Honjo for their discovery of the cancer therapy approach involving cancer immunotherapy in considerable solid tumors. However, striking clinically significant research progress regarding immunotherapy for glioma has been relatively limited.25 Therefore, the traditional surgical resection and postoperative temozolomide plus radiotherapy still play an essential role in the therapy of patients with HGG.26 Interestingly, considerable biomarker studies regarding pharmacogenomic exploration of glioma were reported recently. Panciani et al explored, in 2021, the fact that involvement of TP53 polymorphisms was associated with the PFS of adjuvant treatment.27 Guo et al reported in 2020 that genetic variation in MIR3142HG gene had positive relevance to the predisposition of patients with glioma and was associated with prognosis of glioma in Chinese Han population.28 Zhou et al observed in 2019 that GOLGA7 polymorphism might play a role in the prognosis of glioma and TYMS polymorphism was associated with susceptibility of glioma.29 Collectively, these data suggested the potential significance of pharmacogenomics to predict the clinical outcomes of patients with glioma.
To our knowledge, the present study was the first study focusing on the association between the prognosis of patients with HGG who received temozolomide plus radiotherapy adjuvant regimen and GSTP-1 gene polymorphism analysis in a Chinese population. And our study concluded that 313A>G was associated with worse PFS and OS. Interestingly, the MAF value of 313A>G polymorphism in our study was 0.18, which was consistent with the prevalence of the polymorphism among Chinese population in NCBI database. However, the frequency of this polymorphism was strikingly different from that in the western population,30 which demonstrated the individual differences in genetic variation of GSTP-1 gene to some extent. Besides, the recently reported study initiated by Hajdinák et al investigated the association between GSTP-1 polymorphism and clinical outcomes in 33 patients with various autoimmune diseases who were treated with cyclophosphamide. And the conclusion highlighted that patients with AG genotype of rs1695 polymorphism were associated with a higher response rate to cyclophosphamide. The clinical outcome of this study was in line with that of our study to some extent in spite of the fact that they failed to perform the prognosis analysis.31 And another retrospective study initiated by Yuan et al investigated the association of GSTP-1 and clinical outcome of gemcitabine-cisplatin chemotherapy in Chinese patients with NSCLC.32 And the results suggested that A genotype carriers were significantly higher in sensitive group than in non-sensitive group, which was consistent with our study as well. However, the previous study initiated by Pasqualetti et al in a Caucasian population included a total of 50 patients with wild-type IDH1/2 glioma who received surgical resection and concurrent chemoradiotherapy regimen,20 and the result indicated that patients with AA genotype of rs1695 polymorphism were correlated with worse prognosis. Additionally, the previous study initiated by Lv et al explored the clinical implication of genetic polymorphism of GSTP-1 and ERCC1 in patients with NSCLC who received platinum-based chemotherapy.33 The results exhibited that patients with AG/GG genotype of GSTP-1 were associated with superior clinical outcome, which contradicted the conclusion in our study. Hypothetically, we speculated that this discrepancy could be attributed to the heterogeneity of the subjects and the treatment regimens. Besides, it should be noted that both studies failed to investigate the association between the genotype status of the polymorphism and GSTP-1 gene mRNA expression.
Interestingly, mRNA expression of GSTP-1 was carried out among 65 PBMC specimens in our study and the results disclosed that patients with AG/GG genotype of 313A>G were correlated with higher GSTP-1 mRNA expression. Previous research showed that GSTP-1 gene might contribute to the function of bio-interpretation or bio-transformation by binding to reduced glutathione appropriately.34 We speculated that the reason why higher GSTP-1 mRNA expression predicted worse prognosis could be because higher GSTP-1 mRNA expression might reduce the level of chemotherapy drug or the dosage of radiotherapy in tumor cells, thus compromising the efficacy of concurrent temozolomide plus radiotherapy regimen directly. Additionally, located at the coding area of GSTP-1 gene, 313A>G polymorphism resulted in the alteration of amino acid from Isoleucine to Valine, which could attenuate the expression and function of GSTP-1 gene to some extent.35 At present, the association between GSTP-1 gene expression level and prognosis of patients is still controversial. Previously, Yang et al performed research among patients with primary epithelial ovarian cancer (EOC) to investigate the prognostic significance of GSTP-1 gene expression.36 The results concluded that higher expression of GSTP-1 predicted worse prognosis among patients with EOC, which was consistent with the mRNA expression results in our study. Another exploratory study reported in 2017 initiated by Liu et al identified the association between GSTP-1 expression and prognosis of patients with hepatocellular carcinoma (HCC).37 The results indicated that higher GSTP-1 levels could provide a superior PFS and OS through the suppression of tumorigenesis in HCC, which contradicted the results in our study and the discrepancy could be related to the different clinical significance of GSTP-1 gene expression in various cancers. Additionally, it should be noted that another previous study initiated by Tjiong et al investigated the expression profile during disease progression and under systemic therapy,38 they concluded that no significant difference in GSTP-1 gene mRNA expression was observed before and after chemotherapeutical treatment, which indicated that chemotherapy had no influence on the GSTP-1 gene mRNA expression. Collectively, whether patients with higher expression of GSTP-1 have worse prognosis should be validated in large-scale prospective trials subsequently. Furthermore, this study also observed that ECOG PS score, WHO grade, extent of surgery, and cycles of temozolomide adjuvant treatment all had an independent influence on PFS, which was basically in agreement with the results of a previous study.39
Limitations were observed in our investigation, inevitably. Firstly, the sample size included in our study was small and it is necessary to explore this polymorphism in a larger sample size. Secondly, it is obvious that some biases could not be avoided in retrospective analysis. Consequently, the conclusion of our study should be validated in large-scale prospective trials subsequently. Collectively, our study adequately established the prognostic significance of 313A>G polymorphism and revealed part of the reasons for the prognostic difference caused by this polymorphism from the expression of GSTP-1 mRNA, which could be of clinical guiding significance for the prognostic evaluation regarding patients with HGG who received temozolomide plus radiotherapy adjuvant treatment.
Acknowledgments
The authors would like to express sincere gratitude to Bai-Man Chen for participating in this study. We would like to thank all the staff who took part in this study.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Xu S, Tang L, Li X, Fan F, Liu Z. Immunotherapy for glioma: current management and future application. Cancer Lett. 2020;476:1–12. doi:10.1016/j.canlet.2020.02.002
2. Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. doi:10.1093/neuonc/noy131
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
5. Ruff M, Kizilbash S, Buckner J. Further understanding of glioma mechanisms of pathogenesis: implications for therapeutic development. Expert Rev Anticancer Ther. 2020;20(5):355–363. doi:10.1080/14737140.2020.1757440
6. Oberoi RK, Parrish KE, Sio TT, et al. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro Oncol. 2016;18(1):27–36. doi:10.1093/neuonc/nov164
7. Ontario Health. 5-Aminolevulinic Acid Hydrochloride (5-ALA)-guided surgical resection of high-grade gliomas: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(9):1–92.
8. Raucher D. Tumor targeting peptides: novel therapeutic strategies in glioblastoma. Curr Opin Pharmacol. 2019;47:14–19. doi:10.1016/j.coph.2019.01.006
9. Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108(1):11–27. doi:10.1007/s11060-011-0793-0
10. Chen X, Yan Y, Zhou J, et al. Clinical prognostic value of isocitrate dehydrogenase mutation, O-6-methylguanine-DNA methyltransferase promoter methylation, and 1p19q co-deletion in glioma patients. Ann Transl Med. 2019;7(20):541. doi:10.21037/atm.2019.09.126
11. Caragher S, Chalmers AJ, Gomez-Roman N. Glioblastoma’s next top model: novel culture systems for brain cancer radiotherapy research. Cancers. 2019;11(1):44. doi:10.3390/cancers11010044
12. Gupta K, Burns TC. Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front Oncol. 2018;8:503. doi:10.3389/fonc.2018.00503
13. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. doi:10.1038/s41571-020-00447-z
14. Huang L, Wang D, Feng Z, et al. Inhibition of intermedin (Adrenomedullin 2) suppresses the growth of glioblastoma and increases the antitumor activity of temozolomide. Mol Cancer Ther. 2021;20(2):284–295. doi:10.1158/1535-7163.mct-20-0619
15. Singh RR, Reindl KM. Glutathione S-Transferases in Cancer. Antioxidants. 2021;10(5). doi:10.3390/antiox10050701
16. Farmohammadi A, Arab-Yarmohammadi V, Ramzanpour R. Association analysis of rs1695 and rs1138272 variations in GSTP1 gene and breast cancer susceptibility. Asian Pac J Cancer Prev. 2020;21(4):1167–1172. doi:10.31557/apjcp.2020.21.4.1167
17. Cui J, Li G, Yin J, et al. GSTP1 and cancer: expression, methylation, polymorphisms and signaling (Review). Int J Oncol. 2020;56(4):867–878. doi:10.3892/ijo.2020.4979
18. Pourkeramati A, Zare Mehrjardi E, Dehghan Tezerjani M, Seifati SM. Association of GSTP1, GSTT1 and GSTM1 gene variants with coronary artery disease in Iranian population: a Case-Control Study. Int J Gen Med. 2020;13:249–259. doi:10.2147/ijgm.s252552
19. Bushra MU, Rivu SF, Sifat AE, et al. Genetic polymorphisms of GSTP1, XRCC1, XPC and ERCC1: prediction of clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer patients of Bangladesh. Mol Biol Rep. 2020;47(9):7073–7082. doi:10.1007/s11033-020-05771-2
20. Pasqualetti F, Gonnelli A, Cantarella M, et al. Association of Glutathione S-Transferase P-1 (GSTP-1) rs1695 polymorphism with overall survival in glioblastoma patients treated with combined radio-chemotherapy. Invest New Drugs. 2018;36(2):340–345. doi:10.1007/s10637-017-0516-2
21. Liu JY, Liu QM, Li LR. Association of GSTP1 and XRCC1 gene polymorphisms with clinical outcomes of patients with advanced non-small cell lung cancer. Genet Mol Res. 2015;14(3):10331–10337. doi:10.4238/2015.August.28.19
22. Bai M, Li ZG, Ba Y. Influence of KDR genetic variation on the efficacy and safety of patients with chemotherapy refractory metastatic CRC who received apatinib treatment. Int J Gen Med. 2021;14:1041–1055. doi:10.2147/ijgm.s300968
23. Wang LM, Banu MA, Canoll P, Bruce JN. Rationale and clinical implications of fluorescein-guided supramarginal resection in newly diagnosed high-grade glioma. Front Oncol. 2021;11:666734. doi:10.3389/fonc.2021.666734
24. Mudassar F, Shen H, O’Neill G, Hau E. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J Exp Clin Cancer Res. 2020;39(1):208. doi:10.1186/s13046-020-01724-6
25. Ballas ZK. The 2018 Nobel prize in physiology or medicine: an exemplar of bench to bedside in immunology. J Allergy Clin Immunol. 2018;142(6):1752–1753. doi:10.1016/j.jaci.2018.10.021
26. Serventi J, Behr J. Surgery and evidence-based treatments in patients with newly diagnosed high-grade glioma. Semin Oncol Nurs. 2018;34(5):443–453. doi:10.1016/j.soncn.2018.10.009
27. Panciani PP, Giordana MT, Gallone S, et al. Blood-tissue analysis of TP53 polymorphisms and survival of patients with glioma. J Neurosurg Sci. 2021;65(1):8–13. doi:10.23736/s0390-5616.18.04284-4
28. Guo X, Zhang M, Li Q, et al. Evaluation of genetic variants in MIR3142HG in susceptibility to and prognosis of glioma. Am J Clin Oncol. 2020;43(1):1–8. doi:10.1097/coc.0000000000000587
29. Zhou L, Dong S, Deng Y, et al. GOLGA7 rs11337, a polymorphism at the microRNA binding site, is associated with glioma prognosis. Mol Ther Nucleic Acids. 2019;18:56–65. doi:10.1016/j.omtn.2019.08.006
30. Rezaei M, Saadat M. Association between GSTP1 Ile105Val genetic polymorphism and dependency to heroin and opium. Biochem Genet. 2018;57:214–221. doi:10.1007/s10528-018-9885-2
31. Hajdinák P, Szabó M, Kiss E, et al. Genetic polymorphism of GSTP-1 affects cyclophosphamide treatment of autoimmune diseases. Molecules. 2020;25(7):1542. doi:10.3390/molecules25071542
32. Yuan ZJ, Zhou WW, Liu W, et al. Association of GSTP1 and RRM1 polymorphisms with the response and toxicity of gemcitabine-cisplatin combination chemotherapy in Chinese patients with non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16(10):4347–4351. doi:10.7314/apjcp.2015.16.10.4347
33. Lv H, Han T, Shi X, et al. Genetic polymorphism of GSTP1 and ERCC1 correlated with response to platinum-based chemotherapy in non-small cell lung cancer. Med Oncol. 2014;31(8):86. doi:10.1007/s12032-014-0086-5
34. Dong X, Sun R, Wang J, et al. Glutathione S-transferases P1-mediated interleukin-6 in tumor-associated macrophages augments drug-resistance in MCF-7 breast cancer. Biochem Pharmacol. 2020;182:114289. doi:10.1016/j.bcp.2020.114289
35. Ferracini AC, Lopes-Aguiar L, Lourenço GJ, et al. GSTP1 and ABCB1 polymorphisms predicting toxicities and clinical management on carboplatin and paclitaxel-based chemotherapy in ovarian cancer. Clin Transl Sci. 2021;14(2):720–728. doi:10.1111/cts.12937
36. Yang F, Gao B, Chen W, et al. Expression of resistance gene and prognosis of chemotherapy in primary epithelial ovarian cancer. Medicine. 2018;97(41):e12364. doi:10.1097/md.0000000000012364
37. Liu X, Tan N, Liao H, et al. High GSTP1 inhibits cell proliferation by reducing Akt phosphorylation and is associated with a better prognosis in hepatocellular carcinoma. Oncotarget. 2018;9(10):8957–8971. doi:10.18632/oncotarget.23420
38. Tjiong R, Stavrinou P, Röhn G, et al. Heterogeneity of human gliomas: Glutathione-S-Transferase expression profile during disease progression and under systemic therapy. Anticancer Res. 2019;39(4):1795–1805. doi:10.21873/anticanres.13286
39. Liu LQ, Feng LF, Nan CR, Zhao ZM. CREB3L1 and PTN expressions correlate with prognosis of brain glioma patients. Biosci Rep. 2018;38(3). doi:10.1042/bsr20170100
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
