Back to Journals » OncoTargets and Therapy » Volume 11
Influence of different ex vivo cell culture methods on the proliferation and anti-tumor activity of cytokine-induced killer cells from gastric cancer patients
Authors Shi B, Sun AX, Zhang XR
Received 11 January 2018
Accepted for publication 23 March 2018
Published 8 May 2018 Volume 2018:11 Pages 2657—2672
DOI https://doi.org/10.2147/OTT.S162281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Bin Shi,1 Aixia Sun,2 Xiaorui Zhang3
1Department of Gastrointestinal Surgery, Liaocheng People’s Hospital of Taishan Medical University, Liaocheng, Shandong Province, China; 2Department of Clinical Laboratory, Liaocheng People’s Hospital, Liaocheng Clinical School of Taishan Medical University, Liaocheng, Shandong Province, China; 3Department of Health, Liaocheng People’s Hospital of Taishan Medical University, Liaocheng, Shandong Province, China
Purpose: In cytokine-induced killer (CIK) cell therapy, the phenotypes and the numbers of CIK cells have a great influence on the therapeutic effects. This study aimed to investigate the effects of different ex vivo cell culture methods on the proliferation and cytotoxicity of CIK cells that were obtained from gastric cancer patients.
Patients and methods: CIK precursor (Pre-CIK) cells were collected by either hydroxyethyl starch (HES) sedimentation (HES method, unpurified group) or Ficoll-Hypaque density gradient centrifugation (Ficoll method, purified group). Cell number, collection time, and morphology of Pre-CIK cells in the two groups were determined. The proliferation ability, cytokines, phenotypes, and cytotoxicity of CIK cells in the two groups were evaluated ex vivo and in vivo.
Results: In this study, the number of Pre-CIK cells in the unpurified group was significantly higher than that in the purified group (P<0.05). Numbers of erythrocytes, platelets, and granulocytes were reduced significantly following the purification step (P<0.05). Compared to CIK cells in the purified group, those in the unpurified group showed more active proliferation, accompanied by higher percentages of CD8+, CD3-CD56+, and CD3+CD56+ cells, which were responsible for cytotoxicity of CIK cells (P<0.05). This research also showed that the levels of interferon-γ, interleukin-2, and tumor necrosis factor-α, which can enhance the proliferation and cytotoxicity of CIK cells, were significantly increased in the unpurified group (P<0.05). Furthermore, CIK cells in the unpurified group also showed stronger anti-tumor effects against gastric cancer cells than those in the purified group, both ex vivo and in vivo (P<0.05).
Conclusion: The removal of Ficoll-Hypaque purification step reduces the time and cost of the Pre-CIK separation and provides more CIK cells with higher cytotoxicity, which is of great importance in the clinical application of CIK cell therapy.
Keywords: red blood cells, cytokine-induced killer cells, CIK precursor cells, gastric cancer
Introduction
Gastric cancer caused 723,000 deaths worldwide in 2012 and was reported to be the third leading cause of cancer death, according to World Cancer Report, 2014.1,2 Early gastric cancer is prone to be misdiagnosed due to the lack of clinical manifestation, and the 5-year survival rate of gastric cancer patients at advanced stage is <20%.2,3 At present, surgery, radiotherapy, and chemotherapy are the three most widely used therapeutic methods for gastric cancer.2–4 It has been widely reported that the efficacy of these therapeutic approaches was not satisfied for malignant tumor patients, as they were not able to completely eradicate small lesions and metastatic cells, which most likely cause cancer reccurence.2,4 Moreover, drug resistance and severe adverse reactions limited the application of these treatment approaches.2,3,5 Therefore, it is imperative to develop a more effective and safer therapeutic approach.
In the past few years, cellular immunotherapy using cytokine-induced killer (CIK) cells,5 tumor-infiltrating lymphocytes,6 and other immune cells7,8 for cancer treatment has been increased rapidly.2,3,5,9 Compared with other immune cells, CIK cells exhibit a greater proliferation capability, broader anti-tumor spectrum, and stronger anti-tumor ability.2,3,10,11 CIK cells primarily consist of CD3+CD56+ subset and are induced by interferon (IFN)-γ, anti-CD3 monoclonal antibodies, and interleukin (IL)-2 ex vivo.2,3,5,10 CIK cells can migrate to the tumor site, where they directly contact the tumor cells and induce tumor cell apoptosis through FasL- and perforin-mediated pathways.12 On the other hand, they also secrete cytokines such as IL-2, tumor necrosis factor (TNF)-α, and IFN-γ to enhance their tumor cell-killing effects.12 It has been found that the cytotoxicity of CIK cells was not affected by immune inhibitors such as CsA and FK506.2,11,13 The anti-tumor activity of CIK cells is mostly major histocompatibility complex-unrestricted, through activating NK-cell receptors such as DNAX accessory molecule-1, NKp46, NKG2D, and NKp30.14,15 A number of clinical studies have reported considerable curative effects of CIK cells in multiple solid tumors, including gastric, colon, and other cancers, without causing severe adverse reactions.2,6,9,11 However, the therapeutic effects among different studies varies a lot, which may be affected by cell number, phenotypes, and secreted cytokines and other factors.16,17 After ex vivo culture and proliferation, the numbers and phenotypes of CIK cells varied among individuals and among studies using different culture methods. Therefore, suitable ex vivo cell culture methods are critical to obtain a greater number of CIK cells with higher cytotoxicity, by which to promote the clinical application of CIK cell therapy.
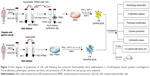
At present, Ficoll-Hypaque density gradient centrifugation is the most common method of isolation of CIK precursor (Pre-CIK) cells.17 In this method, Ficoll-Hypaque purification step was designed to remove red blood cell (RBC), platelet (PLT), and neutrophil (NEUT), because it was generally believed that purified mononuclear cells would give the most effective reinfusion product of CIK cells. However, the exact effects of removed cells, such as RBC, on the proliferation and anti-tumor ability of CIK cells have not been verified in previous work. Several research studies have reported that the presence of human, mouse, or sheep RBC in cultures of peripheral blood lymphocytes influenced T- and B-cell activities, such as lymphocyte (LYM) proliferation, secretion of cytokines such as IL-2, TNF-α, and IFN-γ, B-cell cloning efficiency, antibody synthesis, and activity of natural killer cells by direct contact.18–22 In addition, Yannelli et al’s research showed that autologous RBC enhanced the generation and cytotoxicity of lymphokine-activated killer (LAK) cell in IL-2-induced culture.23 Shau et al also found that the addition of RBC at the initiation of cytotoxicity assays significantly potentiate NK cell-mediated cytotoxicity24 (Figure 1). Similarly, RBC may also promote the proliferation and anti-tumor effect of CIK cells. If these assumptions are confirmed, it will not be necessary to remove RBC, and probably the Ficoll-Hypaque purification step can be omitted; correspondingly, time-consuming and expenses for the preparation of CIK cells would be reduced. In other words, greater numbers of CIK cells with higher cytotoxicity could be obtained from peripheral blood without Ficoll-Hypaque purification. To address this issue, we explored the effects of RBC on the biological characteristics of CIK cells derived from Pre-CIK cells of gastric cancer patients with or without Ficoll-Hypaque purification, including the proliferation capacity, cell phenotype, cytokine secretion, and cytotoxicity against gastric cancer cells ex vivo and in vivo (Figure 2).
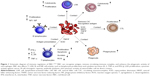  | Figure 1 Schematic diagram of immune regulation of RBC.16–22 RBC can recognize antigen, remove circulating immune complex, and enhance the phagocytic activity of macrophages. RBC also affects T, LAK, B, and NK cell activity, such as lymphocyte proliferation and cytokine secretion (IL-2, TNF-α, and IFN-γ), LAK proliferation, cytotoxic activity against cancer, B-cell clonogenic capacity, antibody synthesis, and cytotoxic activity of natural killer cell via direct contact. |
Patients and methods
Patients
Patients with advanced gastric cancer were recruited for this study in Liaocheng People’s Hospital from May 2012 to June 2015. Patients were enrolled in this study following the listed criteria: 1) patients were diagnosed and confirmed as stages III or IV gastric cancer by histopathological examination, according to the American Joint Committee on Cancer tumor node metastasis Staging Classification (Figure S1); 2) participants were all primary gastric cancer patients without previous tumor history; 3) patients did not have serious heart, lung, liver, and kidney dysfunction; 4) patients were not in pregnancy and lactation; 5) expected survival time was over 6 months; 6) white blood cell counts were over 3×109/L; and 7) patients did not have a serious autoimmune disease. Informed consent in accordance with the Declaration of Helsinki were obtained from all patients, and this study was approved by the Ethics Committee of Liaocheng People’s Hospital. A total of 46 patients were included in the study and were randomly distributed into two groups after chemotherapy (Table 1).
Peripheral blood samples were collected from all subjects, and Pre-CIK cells were isolated through Ficoll-Hypaque density gradient centrifugation for 25 patients (Ficoll method, purified group) and through hydroxyethyl starch (HES) sedimentation for the remaining 21 patients (HES method, unpurified group).
Pre-CIK cell collection
Pre-CIK cell collection using HES method (unpurified group)
Peripheral blood sample was collected 2 weeks after chemotherapy and diluted by equal volume of 0.9% NaCl. About 50 mL of 6% HES (Voluven; Fresenius Kabi, Bad Homburg, Germany) was added and allowed to stand for 30 minutes at room temperature; then the liquid supernatant was transferred into a 50 mL centrifuge tube and centrifuged at 2,500 rpm for 5 minutes. Cells from the bottom of centrifuge tube were collected and washed twice with 0.9% saline.
Pre-CIK cell collection using Ficoll method (purified group)
Cells that were collected by using HES method were further applied to Ficoll-Hypaque (GE Healthcare Bioscience, Uppsala, Sweden) purification procedure to further remove RBC, PLT, and granulocytes. The cell suspension was slowly and carefully transferred into a tube containing 25 mL Ficoll paque (1.077 g/mL) with a pipette tip against the inner wall of tube. After centrifuged for 30 minutes at 1,700 rpm at 20°C, the clouding layer containing peripheral blood mononuclear cells (PBMCs) at the middle level was collected and washed twice with 0.9% saline.
Culture of CIK cells
Both unpurified and purified Pre-CIK cells were cultured in serum free aim v medium (AIM-V) complete medium (Thermo Fisher Scientific, Waltham, MA, USA) at a concentration of 5×106 cells/mL. Cells were incubated for 2 hours at 37°C with 5% CO2. Non-adherent cells were collected and cultured at a concentration of 3×106 cells/mL in AIM-V complete medium containing 1,000 U/mL rhIFN-γ and 5% inactivated human serum with 5% CO2 at 37°C. After 24 hours of incubation, monoclonal antibody against CD3 (OKT-3, 50 ng/mL; PeproTech Inc., Rocky Hill, NJ, USA), IL-1α (100 U/mL; PeproTech Inc.), and rhIL-2 (300 IU/mL; PeproTech Inc.) were added. Medium with IL-2 was changed every 2–3 days.
Preparation of RBC, PLT, and NEUT
RBC, PLT, and NEUT were isolated by using Ficoll-Hypaque density gradient centrifugation. A centrifuge tube containing 5 mL of Ficoll and 10 mL of 0.9% saline diluted peripheral blood (volume ratio 1:2) was centrifuged at 1,300 rpm for 20 minutes at 20°C. The granulocyte layer was collected from the RBC pellet by aspiration and then washed twice by centrifugation at 1,300 rpm for 7 minutes. The residual erythrocytes were then removed by lysis in 0.6 M KCl aqueous solution (NEUT preparation).25 RBC fraction was also isolated by aspiration method and washed thrice in PBS for subsequent test (RBC preparation).23 The upper layer containing PLT was isolated and washed twice in PBS for the follow-up study (PLT preparation).
Culture of gastric cancer cells
Human gastric cancer cell lines (SGC7901 and BGC823) were purchased from the Shanghai Cell Research Institute (Shanghai, China) and cultured in Central Laboratory of Liaocheng People’s Hospital. DMEM (Thermo Fisher Scientific) was used for gastric cancer cell culture.
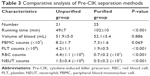
Comparative evaluation of Pre-CIK cell collection
To evaluate the efficiency of different methods for Pre-CIK cell separation, cell numbers of RBC, PLT, PBMC, and NEUT, volume of blood, and separation time were determined and compared between the two groups.
Proliferation and phenotype analysis of CIK cells
After ex vivo culture, proliferative ability of CIK cells was evaluated by staining with trypan blue and counting; morphology of Pre-CIK and CIK cells separated following two protocols were observed in a light field under microscope; phenotype of CIK cells was also analyzed by antibody staining for cell surface markers. About 5×105 CIK cells were collected and resuspended in PBS and stained with monoclonal antibodies against CD3, CD4, CD8, CD56, and CD25 (BD Biosciences, Franklin Lakes, NJ, USA) at 4°C for 30 minutes. Cell subset populations were counted and analyzed by using flow cytometry (BD Biosciences).
Cytokine production of CIK cells
Same number of CIK cells from two groups was cultured in a complete medium at a density of 2×106 cells/mL for 48 hours. The supernatant was collected and analyzed for IFN-γ, IL-2, TNF-α, and IL-10 by using enzyme-linked immunosorbent assay kit (R&D Systems, Inc., Minneapolis, MN, USA).
Cytotoxicity assay of CIK cells
The target cells, SGC7901 and BGC823 cells, were seeded into 96-well plate (100 μL/well) at a density of 1×105/mL, and 100 μL of CIK cells was co-cultured in each well as effector cells (effector cells:tumor cells =5:1, 10:1, 20:1). Simplex effector cells or target cells were cultured separately as controls. Each group consisted of five parallel wells as repeats. After cultured for 12 and 24 hours, 10 μL cell counting kit-8 (CCK-8) (Beyotime, Shanghai, China) was added to treat cells for 2 hours, and absorbance (A) was measured at the wave length of 450 nm by an automatic microplate reader. Cytotoxicity rate (CR) was calculated as follows: CR =[1−(A experiment−A effect)/A target]×100%.
Effect of RBC, PLT, and NEUT on the biological activity of CIK cells
In this study, the proliferative activity, cytokine secretion, and cytotoxicity of CIK cells were found to be significantly improved, and RBC, PLT, and NEUT counts were significantly decreased when the purification step was used. In order to further investigate the underlying mechanism of the enhancement effects on the generation and cytotoxicity of CIK cells, the removed types of cells, which included RBC, PLT, and NEUT, were respectively co-cultured with PBMCs before induction. PBMCs at a concentration of 2×106 cells/mL were cultured in sic-well plates in AIM-V complete medium containing 1,000 U/mL rhIFN-γ. RBC, PLT, and NEUT were respectively mixed with PBMCs in each well at multiple concentration ratios (RBC:PBMC =10:1, 20:1, 50:1; PLT:PBMC =10:1, 20:1, 50:1; NEUT:PBMC =1:2, 1:1, 2:1). Cells obtained in unpurified group were set as positive control with same culture conditions and same number of PBMCs. The proliferative capacity, phenotypes, cytokine production, and cytotoxicity of CIK cells in each group were analyzed. All cells involved in these experiments were derived from the same donor, in order to exclude individual differences.
Establishment of nude mouse xenograft tumor model
Female BALB/C nu/nu nude mice (n=60, 4–6 weeks, 17–20 g) were housed under specific pathogen-free condition and reared at room temperature (23°C±2°C) with day-night cycle of 12 hours light and 12 hours dark. All animal experiment processes were performed following the regulations of the Animal Ethics Committee of Liaocheng People’s Hospital, Liaocheng, Shandong. Nude mice were subcutaneously injected with 1×107 BGC823 cells at logarithmic growth phase on the right side. Solid tumor growth was monitored every other day for 1 week, and a nude mouse gastric cancer xenograft model was successfully established with tumor formation rate at l00%.
In vivo inhibition of tumor growth by CIK cells
A total of 60 mice were randomly assigned to six groups with 10 in each group and were treated with corresponding cells: group 1 (control group), PBS; group 2, CIK cells from purified group; group 3, CIK cells co-cultured with RBC (RBC:PBMC =10:1); group 4, CIK cells co-cultured with RBC (RBC:PBMC =20:1); group 5, CIK cells co-cultured with RBC (RBC:PBMC =50:1); group 6, CIK cells from unpurified group. When tumor size reached 70 mm3, mice were intravenously injected with 0.1 mL of cell suspension at a concentration of 1×l08/mL for each injection thrice every other day.
The tumor size was measured every 5 days with a vernier caliper. The animals were sacrificed 4 weeks after final injection, and tumors were eviscerated and weighed. Tumor volume (TV) was calculated as follows: TV (mm3) = width2 × length/2. Implantation effects were evaluated by inhibition ratio (%): inhibition ratio = ([average tumor weight of control group – tumor weight of experimental group]/average tumor weight of control group)×100%.
Statistical analysis
Student’s t-test or χ2 test was applied to analyze the gathered data. Statistical results are shown as percentages in the form of mean ± standard deviation. All analyses were performed by SPSS 21.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered statistically significant.
Results
Patient characteristics
Clinical characteristics (age, sex, tumor site, tumor size, histological differentiation, tumor stage, and chemotherapy regimen) and blood routine indexes (white blood cell [WBC], RBC, PLT, NEUT, monocyte [MNC], and LYM) of gastric cancer patients in two groups are presented in Tables 1 and 2. Patients in the two groups did not show difference in clinical characteristics and blood routine indexes (P>0.05).
As shown in Table 3, more PBMCs were obtained by HES method (unpurified group) than Ficoll method (purified group), but the difference was not significant (P>0.05); RBC, PLT, and NEUT numbers were significantly reduced after the purification step (P<0.05); compared to purified group, cell processing time was significantly decreased in the unpurified group (P<0.05). These results indicate that the elimination of Ficoll-Hypaque purification step could save the separation time of Pre-CIK as well as reduce cell loss.
Proliferation analysis of CIK cells
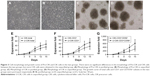
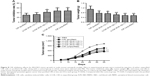
The proliferative capacity of CIK cells were determined by expansion folds. Pre-CIK cells were evenly seeded and proliferated rapidly in clonal manner (Figure 3A and B). Images showed that the morphology of Pre-CIK and CIK cells in the two groups was similar, while the number of CIK cells in the unpurified group was greater than that in the purified group (Figure 3C and D). The amplification folds of all cells, CD3+ subset, and CD3+CD56+ subset in unpurified group was significantly higher than that in purified group (Figure 3E–G; Table 4), which indicates that skipping Ficoll-Hypaque purification step may promote CIK cell proliferation.
  | Table 4 Cell expansion obtained from the two groups |
Phenotypic analysis of CIK cells
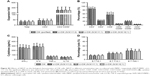
In ex vivo culture, CIK cell phenotypes were evaluated before and after induction. No significant differences were observed between the two groups before induction (Figure 4A, P>0.05). After induction, the percentages of CD3+, CD8+, and CD3+CD56+ subsets were significantly increased, while the percentages of CD4+, CD3−CD56+, and CD4+CD25+ subsets were significantly decreased in both the groups (Figure 4B–D, P<0.05). In addition, after induction, the percentages of CD8+, CD3−CD56+ and CD3+CD56+ cells, which were identified as the main subsets responsible for CIK cells cytotoxicity, were significantly higher in the unpurified group than those in the purified group (Figure 4B, P<0.05).
Cytokine production of CIK cells
In this study, the secretion of IL-2, IFN-γ, and TNF-α in unpurified group was significantly increased compared with purified group (P<0.05), whereas no significant difference in IL-10 was observed between the two groups (P>0.05, Figure 5). These results suggest that CIK cells in the purified group have stronger cytokine secretion ability, which may enhance the anti-tumor effect of CIK cells.
Cytotoxicity of CIK cells against gastric cancer cells
To assess the anti-tumor activity of CIK cells derived from Pre-CIK cells obtained following two different protocols, cytotoxicity of CIK cells against gastric cancer cells (SGC7901 and BGC823) was tested by using CCK-8 kit (Promega Corporation, Fitchburg, WI, USA). Results showed that CIK cells in both the groups exhibited strong cytotoxic activity against SGC7901 and BGC823 cells, and cytotoxicity was improved as the effector: target cell ratio and co-culture duration increased (P<0.05). Moreover, the cytotoxicity against SGC7901 and BGC823 cells in unpurified group was more significantly enhanced compared with purified group (P<0.05), which suggests that the tumor-cytotoxicity of CIK cells was significantly enhanced when Ficoll-Hypaque purification step is omitted (Figure 6).
Effect of RBC, PLT, or NEUT on the biological activity of CIK cells
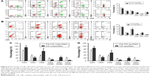
In order to explore the underlying mechanism of the enhancement effects of purification-removed cells on CIK cell generation and cytotoxicity, RBC, PLT, or NEUT were respectively co-cultured with PBMCs in different proportions. Compared to purified group, when PBMCs were co-cultured with PLT or NEUT, no obvious difference was found in CIK cell proliferation, phenotypes (CD3+, CD4+, CD8+, CD3−CD56+, CD3+CD56+, and CD4+CD25+), cytokine secretion (IFN-γ, IL-2, TNF-α, and IL-10), and tumor cytotoxicity (Figures S2 and S3, P>0.05), while PBMCs co-cultured with RBC (RBC:PBMC =20:1 and RBC:PBMC =50:1) produced higher percentages of CD8+, CD3−CD56+, and CD3+CD56+ cells (Figure 7A, P<0.05). Moreover, RBCs were also able to enhance tumor cytotoxicity (RBC:PBMC =20:1 and RBC:PBMC =50:1, P<0.05) and secretion of IFN-γ, IL-2, and TNF-α in CIK cells in a dose-dependent manner (Figure 7B–D, IFN-γ and IL-2, RBC:PBMC =10:1, P>0.05, RBC:PBMC =20:1 and RBC:PBMC =50:1, P<0.05; TNF-α, RBC:PBMC =10:1 and RBC:PBMC =20:1, P>0.05, RBC:PBMC =50:1, P<0.05).
We further investigated whether cell to cell direct contact was necessary for the enhancement of CIK cell generation and cytotoxicity by RBC. RBC and PBMC in costar transwell chambers can either be separated by a membrane or directly contacted during cell culture (RBC:PBMC =50:1). Our research showed that when PBMCs and RBCs were directly contacted, the proliferation, the percentages of CD8+ and CD3+CD56+ subsets, cytokine secretion (IFN-γ, IL-2, and TNF-α), and cytotoxicity of CIK cells were significantly improved (Figure 7E–H, P<0.05), which verified the promotion effect of RBC in enhancing cytotoxicity of CIK cells, which may require direct contact between RBC and PBMC.
Inhibition of tumor growth by CIK therapy in nude mouse xenograft models
Gastric cancer mouse models were treated with CIK cells, and those in the control group were injected with PBS instead. Tumor weight in mice treated by purified or unpurified CIK cells was significantly lower compared with the control group (Figure 8A, P<0.05). Compared with mice treated by purified cells (group 3), those treated by co-cultured cells (group 4) and unpurified cells (group 5) showed higher tumor inhibition rates (Figure 8B, group 3, P>0.05; groups 4 and 5, P<0.05). After treatment, tumor in the control group was found to be grown rapidly, while in groups 2–6, tumor growth rate was slow; the TV showed a tendency toward better efficacy in the co-culture group and unpurified group compared with purified group (Figure 8C, group 3, P>0.05; groups 4–6, P<0.05). In general, CIK cells in co-culture groups and unpurified CIK cells showed better therapeutic potential against gastric cancer than purified CIK cells in vivo.
Discussion
In tumor immunotherapy, CIK cells have been widely used to treat malignant tumor.2,5,26–31 In a clinical study of hepatocellular carcinoma (HCC), routine treatment combined with CIK cell immunotherapy significantly increased the recurrence-free and overall survival rate of HCC patients.2,27 It was also reported in malignancy patients that adoptive CIK cell immunotherapy significantly improved patients’ immune function.2,26 Other studies also showed that autologous, allogeneic, or umbilical cord blood-derived CIK cells were safe and effective for patients with hematologic tumors.2,28–31 All the above studies indicate that CIK cell immunotherapy is a promising approach for malignant tumors.
The anti-tumor efficiency of CIK cells is regulated by multiple key factors.13,16,31 First, to ensure the anti-tumor effect of CIK cells, sufficient number of cells is required.17 Second, as CD8+ and CD3+CD56+ subsets are mainly responsible for tumor cytotoxicity of CIK cells and were also found closely related to cancer patients’ survival, their proportion among obtained cells are of great importance.16 Moreover, the levels of secreted IL-2, IFN-γ, and TNF-α are also responsible for tumor cytotoxicity of CIK cells.32 All these factors could be affected by the process of obtaining CIK cells, which makes cell isolation and cultural condition optimization necessary in CIK cells immunotherapy.17 At present, Ficoll-Hypaque density gradient centrifugation is the most commonly used method for Pre-CIK cell collection. This purification step was designed mainly to eliminate RBC, PLT, and NEUT.23 RBCs are the major component of peripheral blood, and RBC number is 1,000 times the number of WBCs.24 In cell immunology research studies, people revealed that RBCs also possess immunomodulatory effects, such as antigen recognition, removing circulating immune complex, and enhancing the phagocytic activity of macrophages.18,19 In a human lymphocyte ex vivo transformation study, T-cell mitogen phytohemagglutinin-induced proliferation was increased with the presence of autologous RBC.20,33 Lymphocyte death analysis revealed that the improved T-cell proliferation induced by RBC was the result of a reduction in activation-induced cell death or apoptosis.34 RBCs can enhance T-cell proliferation presumably through CD2 and CD58 interaction;35 it can also inhibit activated T-cell apoptosis and facilitate their entrance into cell cycle possibly through reducing the production of reactive oxygen species and acrolein formation, which were produced upon T-cell activation and may enhance T-cell death.20,35
This research aimed to investigate the effects of different Pre-CIK cell isolation methods and optimize CIK cell culture condition. We evaluated and compared the volume of collected blood, the total amount of RBC, PLT, PBMC, and NEUT and time consumption for Pre-CIK cell collection following two protocols. More Pre-CIK cells were obtained by using HES method than Ficoll in our study. Omitting Ficoll-Hypaque purification step was advantageous in time and cost-saving, and no detrimental effect was observed. Numbers of RBC, PLT, and NEUT were reduced significantly after Ficoll-Hypaque purification. In this study, unpurified CIK cells showed better multiplication compared to those obtained in the purified group. To explore which subgroup of cells was responsible for the enhancement of CIK cell proliferation and tumor cytotoxicity, RBC, PLT, and NEUT were respectively co-cultured with PBMC before induction. Compared with the purified group, the proliferation and cytotoxicity of CIK cells were significantly improved in the RBC and PBMC co-cultured group, and this enhancement depended on the direct contact of RBCs and PBMCs.
RBC has been reported to promote CD8+ T-cell expansion ex vivo.20,36 CD8+ T cells activated in the presence of RBC showed more restrained apoptosis and more active proliferation than CD4+ T cells.37 In addition, RBC significantly enhances NK cell-mediated cytotoxicity, which may be related to NK cell enhancement factor presented at RBC cytoplasm.24 In this study, the proportions of both CD8+ and CD3+CD56+ subsets were increased significantly in both the groups after ex vivo culture (P<0.05), and those in the unpurified group were higher than that in the purified group (P<0.05). Previous studies suggested that CD4+ and CD3−CD56+ subsets might not be crucial for tumor cytotoxicity of CIK cells.16,17 In our study, the percentages of CD4+ and CD3−CD56+ subsets were decreased in both the groups (P<0.05), while the percentage of CD3−CD56+ subset in the unpurified group was higher than the purified group (P<0.05). Furthermore, CD4+CD25+ subset (regulatory T cells, Tregs) had a negative impact on CIK anti-tumor activity;38 in our study, the percentage of Tregs in both the groups was also significantly reduced (P<0.05). However, we did not find obvious differences between the two groups.
Cytokines such as IFN-γ, IL-2, and TNF-α play important role in the anti-tumor process of CIK cells.32 IFN-γ produced by T and NK cells is essential in the immune regulation, such as the activation and differentiation of B cells, NK cells, T cells, and macrophages.39 IL-2 is an important immunomodulatory cytokine identified as “T-cell growth factor,” and it has different stimulatory effects on NK, CIK, and B cells.39,40 It was also shown that IL-2 signaling has an important role in CIK cell survival and differentiation, and CIK cell phenotype could be varied depending on the IL-2 level.17,41 TNF-α is a pleiotropic cytokine secreted by activated lymphocytes and MNCs and is pivotal in inflammation, immune regulation, and cell apoptosis.41 Research suggested that TNF-α can induce tumor cell apoptosis and enhance the anti-tumor ability of CIK cells.32,39,42 Furthermore, accumulating evidences indicated that IL-10 has a negative effect on immune responses against tumors, which suggests that a high level of IL-10 may suppress tumor-cytotoxicity of CIK cells.32 In this study, the levels of IFN-γ, IL-2, and TNF-α were increased with the presence of autologous RBCs in unpurified group, whereas no significant difference was found in IL-10, which was consistent with the research of Yannelli et al, Kalechman et al, and Bate and Kwiatkowski.23,35,43 They found that when lymphocytes were co-cultured with autologous RBC, they expressed more IL-2 receptor but released less lymphokine including IFN-γ, IL-2, and TNF-α.23,35,43 All these results suggest that RBC may enhance the generation and cytotoxicity of CIK cells by promoting IFN-γ, IL-2, and TNF-α secretion. Although CIK cells obtained from both the groups exhibited potent cytotoxic activities against gastric cancer cells, both ex vivo and in vivo, CIK cells in unpurified group showed higher anti-tumor activity, which may be associated with the higher percentages of the CD8+, CD3−CD56+, and CD3+CD56+ subsets.
Limitations
The enhancement effect mechanism of RBC on the generation and cytotoxicity of CIK cells need to be further investigated, which will be valuable for CIK immunotherapy application in the future. Moreover, regardless of the impressive clinical outcomes of adoptive CIK cell immunotherapy in the treatment of malignant tumors, its clinical application was limited by low specificity. Recently, novel immunotherapy strategies have been developed. One approach is using antibody to block immune checkpoints such as programmed death 1, lymphocyte activation gene 3, and T-cell immunoglobulin domain and mucin domain-3.44–46 These immune checkpoints are responsible for tumor immune escape in many malignancies, thus they are designed as targets for cancer immune therapies.46 Clinical work has proved the effectiveness of immune checkpoint blockade, and research studies also have demonstrated an improved antitumor activity of CIK cells with inhibitory receptor blockade.44,45 Therefore, the combination of CIK cells with checkpoint inhibitors could be a novel immunotherapy strategy for cancer treatment.
Conclusion
Different techniques for the collection of Pre-CIK cells influence the biological activities of the obtained CIK cells. In this research, Pre-CIK cells were partially lost following Ficoll-Hypaque purification step, while unpurified method was more effective in improving the proliferation and anti-tumor efficacy of CIK cells, which suggests that the purification step maybe neither required nor advised. Elimination of this step not only reduces the time and cost of Pre-CIK cell separation but also provides more CIK cells with higher cytotoxicity after activation. In addition, our data indicated that RBC may promote proliferation, cytokine secretion, and cytotoxicity of CIK cells in a dose-dependent manner by having direct contact with PBMC during CIK cell culture. Therefore, we speculate that these enhancement effects of HES protocol (unpurified method) on the generation and cytotoxicity of CIK cells may relate to the direct contact between RBC and PBMC. Underlying mechanism will be further investigated in the future, which may contribute to the clinical application protocol of CIK cell immunotherapy.
Author contributions
All the authors contributed toward data analysis, drafting, and critically revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–419. | ||
Mu Y, Wang WH, Xie JP, Zhang YX, Yang YP, Zhou CH. Efficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized phase II study. Onco Targets Ther. 2016;9:4617–4627. | ||
Mu Y, Zhou CH, Chen SF, et al. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysis. Cytotherapy. 2016;18(9):1162–1177. | ||
Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108958. | ||
Zhang Z, Wang L, Luo Z, et al. Efficacy and safety of cord blood-derived cytokine-induced killer cells in treatment of patients with malignancies. Cytotherapy. 2015;17(8):1130–1138. | ||
Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. | ||
Wang Y, Xu Z, Zhou F, et al. The combination of dendritic cells-cytotoxic T lymphocytes/cytokine-induced killer (DC-CTL/CIK) therapy exerts immune and clinical responses in patients with malignant tumors. Exp Hematol Oncol. 2015;4:32. | ||
Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–2259. | ||
Zhao L, Lv M, Sayimu W, et al. Therapeutic effect of lymphokine-activated killer cells treated with low-dose ionizing radiation on osteosarcoma. Oncol Lett. 2015;10(2):879–882. | ||
Lin T, Song C, Chuo DY, Zhang H, Zhao J. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol. 2016;37(4):4367–4372. | ||
Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86(9):3493–3499. | ||
Wang Y, Lv B, Li K, Zhang A, Liu H. Adjuvant immunotherapy of dendritic cells and cytokine-induced killer cells is safe and enhances chemotherapy efficacy for multiple myeloma in China: a meta-analysis of clinical trials. Drug Des Devel Ther. 2017;11:3245–3256. | ||
Li GX, Zhao SS, Zhang XG, et al. Comparison of the proliferation, cytotoxic activity and cytokine secretion function of cascade primed immune cells and cytokine-induced killer cells in vitro. Mol Med Rep. 2015;12(2):2629–2635. | ||
Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer. 2015;34(3):99–107. | ||
Gao X, Mi Y, Guo N, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774. | ||
Pan K, Wang QJ, Liu Q, et al. The phenotype of ex vivo generated cytokine-induced killer cells is associated with overall survival in patients with cancer. Tumour Biol. 2014;35(1):701–707. | ||
Liu H, Li J, Wang F, et al. Comparative study of different procedures for the separation of peripheral blood mononuclear cells in cytokine-induced killer cell immunotherapy for hepatocarcinoma. Tumour Biol. 2015;36(4):2299–2307. | ||
Siegel I, Liu TL, Gleicher N. The red-cell immune system. Lancet. 1981;2(8246):556–559. | ||
Silva A, Lopez-Botet M, Alvarez J, de Landazuri MO. Enhancement of the functional activities of human T cells after their interaction with SRBC. J Immunol. 1981;126(2):393–397. | ||
Arosa FA, Pereira CF, Fonseca AM. Red blood cells as modulators of T cell growth and survival. Curr Pharm Des. 2004;10(2):191–201. | ||
Jyonouchi H, Kincade PW, Misra HP. Analysis of the effects of erythrocytes on mitogen-dependent clonal proliferation of murine B lymphocytes. Cell Immunol. 1984;83(1):189–198. | ||
Rugeles MT, La Via M, Goust JM, Kilpatrick JM, Hyman B, Virella G. Autologous red blood cells potentiate antibody synthesis by unfractionated human mononuclear cell cultures. Scand J Immunol. 1987;26(2):119–127. | ||
Yannelli JR, Thurman GB, Mrowca-Bastin A, Pennington CS, West WH, Oldham RK. Enhancement of human lymphokine-activated killer cell cytolysis and a method for increasing lymphokine-activated killer cell yields to cancer patients. Cancer Res. 1988;48(20):5696–5700. | ||
Shau H, Gupta RK, Golub SH. Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunol. 1993;147(1):1–11. | ||
Mosca T, Forte WC. Comparative efficiency and impact on the activity of blood neutrophils isolated by percoll, Ficoll and spontaneous sedimentation methods. Immunol Invest. 2016;45(1):29–37. | ||
Olioso P, Giancola R, Di Riti M, Contento A, Accorsi P, Iacone A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27(3):130–139. | ||
Lee JH, Lim YS, Yeon JE, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391. | ||
Linn YC, Niam M, Chu S, et al. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012;47(7):957–966. | ||
Introna M, Pievani A, Borleri G, et al. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(11):1603–1607. | ||
Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. | ||
Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11(3):181–187. | ||
Zhang Z, Zhao X, Zhang T, et al. Phenotypic characterization and anti-tumor effects of cytokine-induced killer cells derived from cord blood. Cytotherapy. 2015;17(1):86–97. | ||
Tarnvik A. Red cells erythroagglutinating activity of phytohaemagglutinin, and lymphocyte stimulation. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):635–640. | ||
Fonseca AM, Porto G, Uchida K, Arosa FA. Red blood cells inhibit activation-induced cell death and oxidative stress in human peripheral blood T lymphocytes. Blood. 2001;97(10):3152–3160. | ||
Kalechman Y, Herman S, Gafter U, Sredni B. Enhancing effects of autologous erythrocytes on human or mouse cytokine secretion and IL-2R expression. Cell Immunol. 1993;148(1):114–129. | ||
Hamann D, Roos MT, van Lier RA. Faces and phases of human CD8 T-cell development. Immunol Today. 1999;20(4):177–180. | ||
Fonseca AM, Pereira CF, Porto G, Arosa FA. Red blood cells promote survival and cell cycle progression of human peripheral blood T cells independently of CD58/LFA-3 and heme compounds. Cell Immunol. 2003;224(1):17–28. | ||
Tao Q, Wang H, Zhai Z. Targeting regulatory T cells in cytokine-induced killer cell cultures (review). Biomed Rep. 2014;2(3):317–320. | ||
Tovey MG, Lallemand C. Adjuvant activity of cytokines. Methods Mol Biol. 2010;626:287–309. | ||
Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):377–390. | ||
Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21(16):3127–3132. | ||
Zhang Q, Wang L, Luo C, et al. Phenotypic and functional characterization of cytokine-induced killer cells derived from preterm and term infant cord blood. Oncol Rep. 2014;32(5):2244–2252. | ||
Bate CA, Kwiatkowski DP. Stimulators of tumour necrosis factor production released by damaged erythrocytes. Immunology. 1994;83(2):256–261. | ||
Dai C, Lin F, Geng R, et al. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget. 2016;7(9):10332–10344. | ||
Liu LW, Yang MY, Zhou M, Li JJ, Liu B, Pan YY. Improvement of cytotoxicity of autologous CIKs from patients with breast cancer to MCF-7 cells by suppressed PD-1 expression. Cancer Biomark. 2017;20(4):609–615. | ||
Poh SL, Linn YC. Immune checkpoint inhibitors enhance cytotoxicity of cytokine-induced killer cells against human myeloid leukaemic blasts. Cancer Immunol Immunother. 2016;65(5):525–536. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.