Back to Journals » Clinical Interventions in Aging » Volume 14
Influence of diabetes mellitus on anterior segment of the eye
Authors Han SB , Yang HK , Hyon JY
Received 12 October 2018
Accepted for publication 7 December 2018
Published 27 December 2018 Volume 2019:14 Pages 53—63
DOI https://doi.org/10.2147/CIA.S190713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Sang Beom Han,1 Hee Kyung Yang,2 Joon Young Hyon2
1Department of Ophthalmology, School of Medicine, Kangwon National University, Kangwon National University Hospital, Chuncheon, Korea; 2Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
Abstract: Diabetes mellitus (DM) has been emerging as one of the most serious health problems worldwide. Ocular complications of DM are currently one of the major causes of blindness in developed countries, among which diabetic retinopathy is relatively well studied and understood. However, although ocular surface complications of DM are common, diabetic complications of anterior segment of the eye, such as, cornea, conjunctiva, and lacrimal glands, are often overlooked. DM is associated with progressive damage to corneal nerves and epithelial cells, which increases the risk of anterior segment disorders including dry eye disease, corneal erosion, persistent epithelial defects, and even sight-threatening corneal ulcer. In this review, the authors will discuss the association of DM with disorders of anterior segment of the eye. Studies indicating the value of corneal nerve assessment as a sensitive, noninvasive, and repeatable biomarker for diabetic neuropathy will also be introduced. In addition, treatment modalities of anterior segment disorders associated with DM is discussed. The studies introduced in this review suggest that early and periodic screening of the anterior segment of the eye, as well as the retina, is important for the optimal treatment of DM.
Keywords: anterior segment disease, corneal neuropathy, diabetes mellitus, dry eye disease, ocular surface disease, keratopathy
Introduction
Diabetes mellitus (DM), defined as “a chronic disease that occurs when the pancreas does not produce enough insulin, or when the body cannot effectively use the insulin it produces”,1 is a major global public health problem.2 It is one of the most prevalent systemic diseases in the world with increasing prevalence.3 DM was reported to affect 366 million people worldwide in 2011 and estimated to affect >555 and 640 million people by 2030 and 2040, respectively.3
DM has also been increasingly prevalent in Korea, with an age-standardized prevalence among adults aged ≥30 years showing 8.6% in 2001, 9.6% in 2007, and 11.1% in 2013, according to the Korean National Health and Nutrition Examination Survey (KNHANES) data.4 Data from the National Health Insurance Service also showed a rising trend in the prevalence of type 2 DM and impaired fasting glucose from 5.6% and 21.5% in 2006, to 8.0% and 25% in 2013, respectively.4 As the prevalence of DM increases with age, the KNHANES data demonstrated a high prevalence of DM in age groups of ≥70 years old and 60–69 years of 27.6% and 25.2%, respectively, while the prevalence in age groups of 30–39 years and 40–49 years were only 2.5% and 7.3%, respectively.4
DM leads to complications such as neuropathy, retinopathy, nephropathy, and cardiovascular disorders, in which hyperglycemia plays a major role.3 Ophthalmologic complications have emerged as the leading cause of blindness in developed countries, of which retinopathy is the major manifestation that has been relatively well understood by health care providers.3,5
On the contrary, anterior segment complications associated with DM, including the cornea, conjunctiva, and lacrimal glands, are not well recognized, although up to two-thirds of patients are reported to experience diabetic keratopathy during the course of DM.5,6 Patients with DM demonstrate progressive decrease in corneal nerve density and reduction in corneal sensitivity,7,8 which subsequently result in the impairment of corneal epithelial wound healing process and increased susceptibility to persistent epithelial defects and corneal infections.9–11 These complications can potentially lead to blindness, which underscores the importance of understanding the impact of DM on anterior segment diorders.12
In this review, we aimed to provide an overview of the association between DM and anterior segment diseases and discuss the underlying pathophysiologic mechanisms and treatment methods for anterior segment disorders associated with DM, as summarized in Figure 1.
  | Figure 1 Graphical overview of this review. |
Diabetic corneal neuropathy
Diabetic peripheral neuropathy is the most common neuropathic presentation in DM.13 Approximately half of the patients were reported to have diabetic peripheral neuropathy after a 25-year follow-up of DM.14
Pathogenesis
Chronic hyperglycemia is the core causative mechanism underlying the pathogenesis of diabetic neuropathy as well as other systemic complications.15 It induces pathological pathways, such as generation of reactive oxidative stress (ROS), advanced glycation end (AGE) products, sorbitol–aldose reductase pathway, and protein kinase C activation.16,17
First, chronic hyperglycemia leads to excessive influx of glucose into the mitochondria, which promotes the production of ROS due to accelerated oxidative metabolism of glucose.17 These ROS induce disruption in the mitochondrial electron transport chain, which results in mitochondrial injury.17 Nerve fibers are more prone to mitochondrial damage due to their greater mitochondrial volume that subsequently leads to demyelination and conduction dysfunction.16 Mitochondrial injury is also associated with decreased neurotrophic factors including the nerve growth factor (NGF).18 An experimental study demonstrated that a marker of oxidative stress, 8-hydroxydeoxyguanosine, was increased in the diabetic rat cornea, suggesting the possible role of oxidative stress in the apoptosis of corneal cells in DM.19
Second, AGE products generated by glycation may also play an important role in the pathogenesis of peripheral neuropathy.20,21 Glycation is the nonenzymatic bonding of sugar molecules including glucose or fructose to a protein or lipoprotein, which leads to the formation of AGE products with altered structure and function.17 DM induces glycation of myelin proteins and deposition of AGE products in perineurial collagen and in the axoplasm of Schwann cells and neurons, which leads to microvascular dysfunction, damage to the peripheral nerves, and axonal degeneration.21,22 Moreover, the accumulation of AGE in the corneal epithelium promotes proapoptotic and antiproliferative local cell signaling pathways.19
Third, elevated intracellular glucose level caused by chronic hyperglycemia leads to decreased Na+/K+ ATPase activity on cell membrane, which reduces nerve conduction velocity and inhibits nerve regeneration.23,24 Saturation of normal glycolytic pathway caused by hyperglycemia in nerve cells results in shunting of the surplus glucose into the sorbitol–aldose reductase pathway, in which the glucose is converted to fructose and sorbitol by the enzymes sorbitol dehydrogenase and aldose reductase.25 In the shunted pathway, aldose reductase causes depletions of NADPH.24 Moreover, accumulation of fructose and sorbitol results in decreased free nerve myoinositol, which causes reduced membrane Na+/K+ ATPase activity.24,26 Increased level of intracellular glucose is also associated with activation of protein kinase C pathway, which can inhibit the activity of membrane Na+/K+ ATPase (Figure 2).23,27
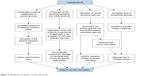  | Figure 2 Pathogenesis of diabetic corneal neuropathy. |
An experimental study using a diabetic mouse model revealed a high concentration of antigen-presenting cells including Langerhans cells and dendritic cells in the cornea and aggregation of the cells around corneal nerve fibers.28 The number of dendritic cells had a negative correlation with corneal nerve fiber density, suggesting the possible role of inflammation in the development of corneal neuropathy in DM.28
Corneal nerve in DM
Changes in corneal nerve parameters, such as a reduction in corneal subbasal nerve fiber density, length, and branch density, have been reported in both type 1 and type 2 DM,29,30 which show correlation with diabetic peripheral and autonomic neuropathy.31–34 Edwards et al32 showed that patients with diabetic peripheral neuropathy had significantly decreased corneal subbasal nerve fiber length and branch density compared with individuals without DM and those with DM but without diabetic peripheral neuropathy. Pritchard et al34 demonstrated that reduction in corneal nerve fiber length measured with corneal confocal microscopy (CCM) can predict the development of diabetic peripheral neuropathy. Misra et al31 revealed that corneal subbasal nerve density changes assessed using CCM precede other functional changes detected by clinical and electrophysiology tests of neuropathy and that corneal sensitivity has a negative correlation with autonomic nerve analysis, suggesting that CCM and corneal sensitivity testing can be surrogate markers for the assessment of diabetic peripheral and autonomic neuropathy.31 Tavakoli et al35 demonstrated that parameters assessed using CCM had a significant correlation with the composite autonomic symptom scale and showed a high sensitivity and specificity for the diagnosis of diabetic autonomic neuropathy, indicating that CCM can be a rapid, noninvasive, and reliable diagnostic test for subclinical diabetic autonomic neuropathy.35 Maddaloni et al36 also suggested that CCM could be a noninvasive tool for the evaluation of cardiac autonomic neuropathy in patients with type 1 DM. A recent study on patients with type 1 DM revealed that among seven measures of diabetic peripheral neuropathy, including CCM, peroneal nerve conduction velocity, cold, warm and vibration perception, monofilament testing, and neuropathy disability score, corneal subbasal nerve morphology evaluated using CCM demonstrated the earliest and most consistent changes in neuropathy status during a 4-year follow-up period.37 A meta-analysis reported in 2016 concluded that corneal nerve fiber density, length, and branch density assessed using CCM were significantly reduced in people with diabetic peripheral neuropathy, and CCM can be useful for early detection of nerve damage in diabetic peripheral neuropathy.38
Changes in corneal cells and nerve fibers were also shown to predict the development of diabetic retinopathy.39–41 Alterations of the corneal subbasal nerve plexus have shown to progress in parallel with diabetic retinopathy and peripheral neuropathy.39 In patients with type 1 DM, CCM demonstrated corneal cellular changes including decreased epithelial and endothelial cell densities and higher keratocyte cell density, as well as small nerve fiber changes, such as lower corneal nerve fiber density and length, lower nerve branch density, and greater nerve fiber width in patients without retinopathy, which worsens with the progression of diabetic retinopathy.40 Bitirgen et al41 revealed that changes in corneal nerve parameters including reduction of nerve fiber density, length, and branch density were observed in patients without diabetic retinopathy and aggravated with the progression of retinopathy.
Corneal nerve changes as a window to diabetic neuropathy
Although diabetic peripheral neuropathy involves both large and small nerve fibers, small nerve fibers can be more sensitive indicators of peripheral neuropathy as they are affected earlier.42 Vibration perception using biothesiometry has been the gold standard for the diagnosis of diabetic peripheral neuropathy.43 However, the method has its limitation based on the fact that only large nerve fiber functions are measured rather than small nerve fibers.3 Visualization of small nerve fiber damage is possible using skin punch biopsy.44 However, biopsy is invasive and nonrepeatable and is also associated with an increased risk of wound complications in patients with DM.3 By contrast, CCM can allow noninvasive, direct visualization of subtle corneal nerve changes in vivo.30 It can also detect diabetic nerve fiber damage earlier than vibration perception or corneal sensation testing.30 Changes in corneal nerve fibers may be detected earlier compared with diabetic peripheral neuropathy in other parts of the body, ie, lower limb, due to high density of small nerve fibers in the cornea.30
Alterations in the corneal subbasal nerve plexus assessed using CCM have close correlation with changes in the peripheral nerves.11 These findings indicate the potential of CCM as a sensitive, noninvasive, and reliable surrogate marker for the evaluation of diabetic peripheral neuropathy,11 which can enable early detection of diabetic peripheral neuropathy and prevention of serious neuropathic complications including diabetic foot (Table 1).2 As retinal examination can be a window to systemic vascular changes in DM, visualization of corneal nerves using CCM can provide a window to systemic peripheral and autonomic nerve changes associated with DM.
  | Table 1 Studies suggesting corneal nerve assessment can be a reliable biomarker for peripheral and autonomic nerve damage in diabetes mellitus (DM) |
Corneal nerve changes with diabetic control
Studies showed that diabetic neuropathy can be improved after interventions for diabetic control,45 although it may not be completely reversed.46 Smith et al47 reported that significant improvements in intraepidermal nerve fiber density were detected using skin biopsy after 1 year of strict glycemic control and lifestyle modification. Improvement in risk factors for diabetic neuropathy, such as hyperglycemia, dyslipidemia, and hypertension, was associated with regeneration of corneal nerve fibers, which was confirmed using in vivo CCM.45 In this study, improvement in HbA1c had significant correlation with an increase in corneal nerve fiber density.45 In vivo CCM also demonstrated significant improvement in corneal nerve fiber length 1 year after simultaneous pancreas and kidney transplantation in patients with type 1 DM.48 These results suggest that the evaluation of corneal nerve changes using CCM can be a viable option for monitoring the efficacy of therapy for diabetic control.
Ocular surface abnormalities in DM
DM can also cause alterations in the corneal epithelial basal cells and basement membrane, leading to corneal epitheliopathy and adhesion disorders.49 In addition, loss of corneal nerves in DM leads to reduced neurotrophic support, resulting in accelerated loss and reduced proliferation of epithelial cells.50,51 DM also causes production of abnormal basal lamina and inadequate adhesion of epithelial cells to an abnormal basement membrane.6,10,52
An experimental study demonstrated delayed corneal wound healing with decreased activation of endothelial growth factor receptor in a diabetic rat model.53 Diminished expression of the tight junction proteins including β-catenin and zonula occludens-1 was also observed, indicating the disruption of cell to cell junctions in diabetic cornea.53 Other animal studies showed detrimental effects of hyperglycemia on the corneal epithelium basement membrane complex and demonstrated findings suggesting compromise of corneal epithelial function, such as increase in corneal thickness, disruption of tight junctions, and loss of basal epithelial cells.19,54 The accumulation of AGE in the corneal epithelium basement membrane complex promotes proapoptotic and antiproliferative pathways, which results in corneal epithelial damage.19 Di et al55 demonstrated an excessive inflammatory response manifested by the accumulation of polymorphonuclear cells in mice diabetic corneas, resulting in increased levels of proinflammatory cytokines and delayed wound healing.
DM is consequently associated with damaged epithelial barrier function and impaired epithelial healing, which increases the risk of ocular surface diseases, such as dry eye disease (DED), superficial punctate keratitis, recurrent corneal erosion, persistent epithelial defects, and neurotrophic corneal ulcer.6,56,57
Ocular surgery in DM
Corneal refractive surgery induces destruction and reconstruction of corneal basal nerves, and DM might conceivably impair the corneal epithelial wound healing process. Indeed, the Food and Drug Administration declared DM to be a relative contraindication to laser-assisted in situ keratomileusis (LASIK) in 2000.58 Patients with DM had a substantially higher risk of corneal complications including erosions and persistent epithelial defects and worse visual outcome after LASIK compared with those without DM (47% vs 6.9%).59
However, other studies revealed that LASIK did not increase the risk of corneal complications in individuals with well-controlled DM,60 and there was no significant difference in anatomic and visual outcomes between the patients with DM and those without.61 Although data regarding the safety and efficacy of LASIK in DM patients are limited, there are only few reports of significant corneal complications despite the large number of refractive surgery performed annually.62 These findings indicate that LASIK can be performed effectively and safely at least in selected patients with well-controlled DM.61,62
Therefore, in 2005, the American Academy of Ophthalmology recommended that LASIK can be safely performed in a selected group of DM patients despite the risk of delayed corneal wound healing, although informed consent and close postoperative monitoring are mandatory.62 The candidates eligible for LASIK should have stable and well-controlled fasting glucose with HbA1c <9 and no evidence of systemic DM complications including nephropathy or peripheral neuropathy.62 Although those with mild DM retinopathy can be considered for LASIK on a case-by-case basis, individuals with significant DM retinopathy or a history of diabetic ocular complications should be excluded.62
Because DM is associated with an increased risk of cataract even in the population younger than 65 years, increasing number of cataract surgery has been performed in patients with DM.3 In addition to a higher risk of postoperative complications of the retina including cystoid macular edema and exacerbation of diabetic retinopathy, DM is also associated with an increased risk of corneal complications, such as persistent corneal edema, corneal abrasion, and delayed epithelial wound healing.63 Cataract surgery can worsen the subbasal nerve damage in patients with reduced tear production or an impaired corneal epithelial integrity associated with DM.64
DM is a significant risk factor for corneal complications following other procedures, such as trabeculectomy, vitrectomy, and laser photocoagulation.65–67 Dogru et al65 demonstrated that patients with DM had increased corneal staining score, decreased corneal sensitivity, and decreased tear film breakup time after argon laser photocoagulation using coupling fluid and retinal laser lens compared with those without DM.65 DM is also a risk factor for developing DED after intraocular surgery.68
These findings indicate that special attention should be paid for corneal complications in patients with DM after any kind of ocular surgery, including even minor procedures.69
DM and DED
Diabetic peripheral neuropathy can theoretically increase the risk of DED,70 and half of patients with DM experience dry eye symptoms.71 Misra et al72 reported impairment of lipid layer quality and tear film stability in DM patients. Hyperglycemia can lead to microvascular damage to the lacrimal gland, and diabetic autonomic neuropathy is associated with impairment of lacrimal innervation, which both contribute to diminished tear production.8 Diabetic peripheral neuropathy leads to decreased corneal nerve fiber density, which results in impaired corneal sensitivity and diminished reflex tearing.71,73 Corneal hypoesthesia can cause decreased mucin production by goblet cells, which leads to reduced tear film stability.8 A higher tear osmolarity probably caused by reduced tear production or increased tear evaporation was also observed in patients with DM.33
The severity of dry eye signs has close correlation with the degree of peripheral neuropathy and severity of diabetic retinopathy.10,71,74 However, with prolonged disease duration, patients are often asymptomatic even in the presence of serious ocular surface damage due to reduced corneal sensitivity, which reflects the progression of diabetic peripheral neuropathy.75
The presence of DED in DM patients further increases the risk of damage to the corneal epithelium.51 Therefore, periodic screening of ocular surface damage and dry eye symptoms in addition to retinal examination would be necessary for patients with DM.70,75
Tear film abnormality in DM
Substance P plays a role in the maintenance of healthy ocular surface by providing neurotrophic support and promoting proliferation and migration of corneal epithelial cells.76 Reduction in substance P level may result in impairment of corneal epithelial homeostasis, potentially leading to exacerbation of corneal complications associated with DM.77 The concentration of substance P in the tear film is correlated with corneal nerve fiber density,78 and corneal hypoesthesia is associated with diminished substance P level.79 The tear substance P level was also shown to be related to the duration of DM and severity of diabetic retinopathy.77
Insulin is found in the tear film, and insulin receptors are identified on the ocular surface.80 Insulin is thought to provide neurotrophic support to the ocular surface and promote the metabolism and growth of the lacrimal gland81: thus, decrease in insulin activity is postulated to be associated with damage to the corneal nerves in DM.82 Upregulation of insulin-like growth factor binding protein 3 and activation of insulin-like growth factor receptor are observed in type 2 DM, which may cause corneal epithelial damage by the disruption of corneal epithelial cell–basement membrane complex.83 Experimental studies showed that topical insulin can facilitate improvement of corneal epithelial integrity and sensitivity in animal models of diabetic keratopathy.84–86
DM and meibomian gland dysfunction
An experimental study revealed that insulin stimulated the proliferation of immortalized human meibomian gland epithelial cells in a dose-dependent manner, while excess glucose resulted in progressive cell loss, suggesting that insulin deficiency and hyperglycemia may be toxic for the meibomian gland epithelial cells and increase the risk of meibomian gland disorders.81
Contact lens (CL) use in DM
CL wear is associated with theoretical risks as follows10: 1) exacerbated corneal epithelial fragility increases the risk of corneal damage; 2) tear film instability could deteriorate DED; 3) impairment of corneal hydration control could lead to corneal edema; and 4) CL-induced endothelial polymegathism can cause corneal endothelial decompensation after intraocular surgery. These factors, together with reduced corneal sensitivity and the vulnerability to infection in DM, may increase the risk of corneal complications including infectious keratitis in the diabetic CL wearer.10 Reports of ocular complications in diabetic CL wears do exist, mostly occurred in patients with advanced diabetic ocular complications or those using extended wear CL.87
However, prospective studies suggest that CL wear might not be contraindicated in patients with DM.88,89 O’Donnell et al89 demonstrated that there was no significant difference in conjunctival injection, corneal staining score, corneal thickness, or sensitivity after 1 year of CL wear between patients with DM and those without.89 Another prospective study revealed that DM had no significant influence on corneal hydration and recovery, indicating that CL wear can be recommended in DM patients.88 However, given the adverse impact of DM on the ocular surface, detailed advice to the patients and close monitoring for corneal complications are mandatory.3
Based on the findings that tear glucose concentrations correlate with blood glucose levels,3 tear glucose sensing devices for noninvasive monitoring of glycemic control using CL has been developed and may be widely used in the near future.
Treatment of anterior segment complications
As in other diabetic complications, strict glycemic control is essential for the treatment and prevention of anterior segment disorders associated with DM.2 Topical artificial tears can be helpful for the maintenance of healthy ocular surface and clear visual axis. Topical anti-inflammatory medications, such as NSAIDs, steroids, and cyclosporine A, are also useful for alleviating ocular surface inflammation and encouraging re-epithelialization.90 Bandage CL can be helpful for an irregular ocular surface, recurrent corneal erosion, and persistent epithelial defects.2
Autologous serum is theoretically beneficial for facilitating corneal wound healing and nerve regeneration as it contains high amounts of growth factors.2,91 Topical autologous serum was shown to accelerate corneal epithelial healing after vitrectomy in DM patients.92 Topical application of umbilical cord serum and platelet-derived plasma was also suggested to be effective in facilitating corneal nerve regeneration and epithelial healing.91
Substance P can attenuate apoptosis of corneal epithelial cells induced by hyperglycemia and accelerate the epithelial healing process via the neurokinin-1 receptor signaling pathway.37,93 Insulin-like growth factor 1 (IGF-1) was also shown to promote regeneration of corneal nerves and ocular surface homeostasis.94 Topical application of substance P and IGF-1 derivatives promote proliferation and migration of corneal epithelial cells in neurotrophic keratopathy including diabetic corneal neuropathy.5,76 A prospective clinical study demonstrated that topical administration of substance P and IGF-1 combination eye drops was effective in the prevention of postoperative superficial punctate keratopathy.95
NGF can stimulate regeneration of damaged neurons and mucin production by goblet cells.96 Topical NGF was shown to improve ocular surface integrity and corneal sensitivity in corneal neuropathy.97 Park et al98 reported that topical NGF can alleviate inflammation and hyperglycemia-induced apoptosis of epithelial cells in diabetic corneas. Kim et al99 revealed that oral nicergoline promoted corneal wound healing in diabetic rat corneas, which was conceivably related to increased NGF in the cornea and lacrimal gland.99
Aldose reductase inhibitor can theoretically reduce nerve damage and promote corneal epithelial regeneration by attenuating activation of the sorbitol–aldose reductase pathway.3 Oral aldose reductase inhibitor was effective in improving corneal epithelial damage and corneal sensitivity after cataract surgery in DM patients.69 Topical aldose reductase inhibitor has also shown to promote corneal epithelial wound healing in DM.100
Naltrexone, an opioid antagonist, is useful for corneal wound healing by accelerating DNA synthesis.101 DM is associated with the production of excessive opioid growth factors, which leads to inhibition of cell proliferation. Naltrexone is expected to promote wound healing in diabetic corneas as it blocks the opioid growth factor and its receptor pathway. Its topical application was shown to improve corneal regeneration and tear production in a type 1 diabetic rat model.102 Topical naltrexone also promoted corneal epithelial repair in a type 2 diabetic mouse model.103
Resolvin D, a docosahexaenoic acid-derived anti-inflammatory mediator, is also expected to be effective for the treatment of diabetic anterior segment disorders.104 An in vitro study revealed that resolvin D attenuated the synthesis of inflammatory cytokines in the corneal epithelium.105 An experimental study using a diabetic rat model also reported that oral administration of resolvin D alleviated corneal and peripheral nerve degeneration.106
Antioxidants, such as carnosine and β-carotene, were also suggested to be beneficial for the prevention of DM-related corneal changes.106,107 Experimental treatment modalities including gene therapy, molecular, and stem cell therapies have been developed.12,55 Di et al55 recently showed that bone marrow-derived mesenchymal stem cells could attenuate excessive inflammatory responses, activate corneal progenitor cells, and enhance would healing of diabetic cornea. However, these experimental methods still have to overcome myriad of barriers to be translated into clinical practice.2 The effects of medications for anterior segment complications associated with DM are summarized in Table 2.
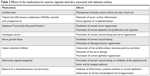  | Table 2 Effects of the medications for anterior segment disorders associated with diabetes mellitus |
Conclusion
DM has an adverse impact on ocular surface integrity, corneal sensitivity, corneal epithelial regeneration, and tear production.3 Diabetic keratopathy is common, and its severity can vary from mild DED to sight-threatening corneal ulcer. The severity of diabetic corneal neuropathy correlates with diabetic peripheral and autonomic neuropathy of other organs. Subtle diabetic neuropathy in the cornea often precedes retinopathy and neuropathy of other parts of the body. Therefore, examination of the corneal nerve plexus using CCM can be a viable biomarker for the assessment of diabetic neuropathies. Although several medications were introduced to be effective for the treatment and prevention of anterior segment disorders associated with DM, further researches are still necessary for the development of better treatment methods.
The studies introduced in this review highlight the need for periodic screening of anterior segment diseases as well as retinal examination for patients with DM. Guidelines for screening ocular surface pathologies should also be established. An enhanced understanding in both patients and medical practitioners of the impact of DM on the anterior segment of the eye would be important for the optimal management of DM.2
Acknowledgment
This study was supported by 2018 Research Grant from Kangwon National University (No.520180051).
Disclosure
The authors report no conflicts of interest in this work.
References
Alberti KG, Zimmet PZ, Definition ZPZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. | ||
Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7(3):e251. | ||
Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018;16(1):45–57. | ||
Noh J. The diabetes epidemic in Korea. Endocrinol Metab (Seoul). 2016;31(3):349–353. | ||
Abdelkader H, Patel DV, McGhee CNJ, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Exp Ophthalmol. 2011;39(3):259–270. | ||
Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. BioMed Res Int. 2016;2016(3):1–14. | ||
Pritchard N, Edwards K, Russell AW, et al. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38(4):671–5. | ||
Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3):586–592. | ||
Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18(2):168–174. | ||
O’Donnell C, Efron N. Diabetes and contact lens wear. Clin Exp Optom. 2012;95(3):328–337. | ||
Pritchard N, Edwards K, Shahidi AM, et al. Corneal markers of diabetic neuropathy. Ocul Surf. 2011;9(1):17–28. | ||
Bikbova G, Oshitari T, Baba T, et al. Diabetic corneal neuropathy: clinical perspectives. Clin Ophthalmol. 2018;12:981–987. | ||
Smith HS, Argoff CE. Pharmacological treatment of diabetic neuropathic pain. Drugs. 2011;71(5):557–589. | ||
Pirart J. [Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part)]. Diabete Metab. 1977;3(4):245–256. French. | ||
Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. | ||
Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011;2(1):18–32. | ||
Babizhayev MA, Strokov IA, Nosikov VV, et al. The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem Biophys. 2015;71(3):1425–1443. | ||
Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46(Suppl 2):S43–S49. | ||
Kim J, Kim CS, Sohn E, Jeong IH, Kim H, Kim JS. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):529–536. | ||
Aubert CE, Michel PL, Gillery P, et al. Association of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30(8):679–685. | ||
Ryle C, Donaghy M. Non-enzymatic glycation of peripheral nerve proteins in human diabetics. J Neurol Sci. 1995;129(1):62–68. | ||
Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14(10):953–961. | ||
Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. | ||
Greene DA, Sima AA, Stevens MJ, Feldman EL, Lattimer SA. Complications: neuropathy, pathogenetic considerations. Diabetes Care. 1992;15(12):1902–1925. | ||
Stavniichuk R, Shevalye H, Hirooka H, Nadler JL, Obrosova IG. Interplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mitogen-activated protein kinases in the pathogenesis of diabetic peripheral neuropathy. Biochem Pharmacol. 2012;83(7):932–940. | ||
Chung SSM, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14(8 Suppl 3):233S–236S. | ||
Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. | ||
Leppin K, Behrendt AK, Reichard M, et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014;55(6):3603–3615. | ||
Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–2463. | ||
Messmer EM, Schmid-Tannwald C, Zapp D, Kampik A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1307–1312. | ||
Misra SL, Craig JP, Patel DV, et al. In vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2015;56(9):5060–5065. | ||
Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95(3):348–354. | ||
Demill DL, Hussain M, Pop-Busui R, Shtein RM. Ocular surface disease in patients with diabetic peripheral neuropathy. Br J Ophthalmol. 2016;100(7):924–928. | ||
Pritchard N, Edwards K, Dehghani C, et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104(2):248–256. | ||
Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2015;52(3):363–370. | ||
Maddaloni E, Sabatino F, Del Toro R, et al. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. 2015;32(2):262–266. | ||
Edwards K, Pritchard N, Dehghani C. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. Diabetes. 2017;31:1325–1327. | ||
Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol. 2016;100(1):9–14. | ||
Nitoda E, Kallinikos P, Pallikaris A, et al. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37(10):898–906. | ||
Szalai E, Deák E, Módis L, et al. Early corneal cellular and nerve fiber pathology in young patients with type 1 diabetes mellitus identified using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2016;57(3):853–858. | ||
Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014;31(4):431–438. | ||
Malik RA, Veves A, Tesfaye S, et al. Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27(7):678–684. | ||
Albers JW, Brown MB, Sima AA, Greene DA. Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat study group for the early diabetes intervention trial. Neurology. 1996;46(1):85–91. | ||
Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–2154. | ||
Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28(10):1261–1267. | ||
Yorek MS, Obrosov A, Shevalye H, et al. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst. 2015;20(1):24–31. | ||
Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–1299. | ||
Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62(1):254–260. | ||
Holland EJ, Mannis MJ, Lee WB. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. London: Elsevier/Saunders; 2013. | ||
Leong YY, Tong L. Barrier function in the ocular surface: from conventional paradigms to new opportunities. Ocul Surf. 2015;13(2):103–109. | ||
Cai D, Zhu M, Petroll WM, Koppaka V, Robertson DM. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am J Pathol. 2014;184(10):2662–2670. | ||
Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69(1):196–201. | ||
Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(9):6589–6596. | ||
Kim EC, Kim DJ, Lee SS, Kim MS. Ultrastructural changes of cornea after ethanol ingestion in Otsuka Long-Evans Tokushima fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) rats. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1457–1466. | ||
Di G, Du X, Qi X, et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6–dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci. 2017;58(10):4344–4354. | ||
Göbbels M, Spitznas M, Oldendoerp J. Impairment of corneal epithelial barrier function in diabetics. Graefes Arch Clin Exp Ophthalmol. 1989;227(2):142–144. | ||
Gekka M, Miyata K, Nagai Y, et al. Corneal epithelial barrier function in diabetic patients. Cornea. 2004;23(1):35–37. | ||
US Food and Drug Administration. [homepage on the Internet] When is LASIK not for me? Available from: https://www.fda.gov/medicaldevices/productsandmedicalprocedures/surgeryandlifesupport/lasik/ucm061366.htm. Accessed April 20, 2018. | ||
Fraunfelder FW, Rich LF. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. 2002;21(3):246–248. | ||
Halkiadakis I, Belfair N, Gimbel HV. Laser in situ keratomileusis in patients with diabetes. J Cataract Refract Surg. 2005;31(10):1895–1898. | ||
Cobo-Soriano R, Beltrán J, Baviera J. LASIK outcomes in patients with underlying systemic contraindications. Ophthalmology. 2006;113(7):1118.e1–1118.e8. | ||
Moshirfar M. LASIK in patients with diabetes mellitus. Available from: http://eyewiki.aao.org/LASIK_in_Patients_With_Diabetes_Mellitus. Accessed April 20, 2018. | ||
Pershing S, Morrison DE, Hernandez-Boussard T. Cataract surgery complications and revisit rates among three states. Am J Ophthalmol. 2016;171:130–138. | ||
Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84(1):19–21. | ||
Dogru M, Kaderli B, Gelisken O, et al. Ocular surface changes with applanation contact lens and coupling fluid use after argon laser photocoagulation in noninsulin-dependent diabetes mellitus. Am J Ophthalmol. 2004;138(3):381–388. | ||
Ono T, Yuki K, Ozeki N, Shiba D, Tsubota K. Ocular surface complications after trabeculectomy: incidence, risk factors, time course and prognosis. Ophthalmologica. 2013;230(2):93–99. | ||
Friberg TR, Ohji M, Scherer JJ, Tano Y. Frequency of epithelial debridement during diabetic vitrectomy. Am J Ophthalmol. 2003;135(4):553–554. | ||
Jiang D, Xiao X, Fu T, Mashaghi A, Liu Q, Hong J. Transient tear film dysfunction after cataract surgery in diabetic patients. PLoS One. 2016;11(1):e0146752. | ||
Fujishima H, Tsubota K. Improvement of corneal fluorescein staining in post cataract surgery of diabetic patients by an oral aldose reductase inhibitor, ONO-2235. Br J Ophthalmol. 2002;86(8):860–863. | ||
Achtsidis V, Eleftheriadou I, Kozanidou E, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37(10):e210–e211. | ||
Lv H, Li A, Zhang X, et al. Meta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patients. Acta Ophthalmol. 2014;92(2):e96–e104. | ||
Misra SL, Patel DV, McGhee CNJ, et al. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res. 2014;2014:848659. | ||
Dehghani C, Pritchard N, Edwards K, et al. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2014;55(12):7982–7990. | ||
Creuzot-Garcher C, Lafontaine PO, Gualino O, D’Athis P, Petit JM, Bron A. [Study of ocular surface involvement in diabetic patients]. J Fr Ophtalmol. 2005;28(6):583–588. French. | ||
Demill DL, Hussain M, Pop-Busui R, Shtein RM. Ocular surface disease in patients with diabetic peripheral neuropathy. Br J Ophthalmol. 2016;100(7):924–928. | ||
Nakamura M, Ofuji K, Chikama T, Nishida T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res. 1997;16(3):275–278. | ||
Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P in human tears. Cornea. 2003;22(7 Suppl):S48–S54. | ||
Markoulli M, You J, Kim J, et al. Corneal nerve morphology and tear film substance P in diabetes. Optom Vis Sci. 2017;94(7):726–731. | ||
Yamada M, Ogata M, Kawai M, Mashima Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am J Ophthalmol. 2000;129(5):671–672. | ||
Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJ, Velloso LA. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest Ophthalmol Vis Sci. 2002;43(4):963–967. | ||
Ding J, Liu Y, Sullivan DA. Effects of insulin and high glucose on human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2015;56(13):7814–7820. | ||
Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst. 2013;18(4):306–315. | ||
Wu YC, Buckner BR, Zhu M, Cavanagh HD, Robertson DM. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul Surf. 2012;10(2):100–107. | ||
Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125(8):1082–1088. | ||
Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes. 2006;55(4):1141–1147. | ||
Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type I diabetic rats. Exp Eye Res. 2009;89(5):686–692. | ||
Spoor TC, Hartel WC, Wynn P, Spoor DK. Complications of continuous-wear soft contact lenses in a nonreferral population. Arch Ophthalmol. 1984;102(9):1312–1313. | ||
O’Donnell C, Efron N. Corneal hydration control in contact lens wearers with diabetes mellitus. Optom Vis Sci. 2006;83(1):22–26. | ||
O’Donnell C, Efron N, Boulton AJ. A prospective study of contact lens wear in diabetes mellitus. Ophthalmic Physiol Opt. 2001;21(2):127–138. | ||
Han SB, Yang HK, Hyon JY, Wee WR. Association of dry eye disease with psychiatric or neurological disorders in elderly patients. Clin Interv Aging. 2017;12:785–792. | ||
Goyal S, Hamrah P. Understanding neuropathic corneal pain – gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31(1–2):59–70. | ||
Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142(2):207–211. | ||
Thai LC, Tomlinson A, Ridder WH. Contact lens drying and visual performance: the vision cycle with contact lenses. Optom Vis Sci. 2002;79(6):381–388. | ||
Wang C, Peng Y, Pan S, Li L. Effect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett. 2014;558:169–174. | ||
Chikamoto N, Chikama T, Yamada N, Nishida T, Ishimitsu T, Kamiya A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn J Ophthalmol. 2009;53(5):464–469. | ||
Priyadarsini S, Rowsey TG, Ma JX, Karamichos D. Unravelling the stromal-nerve interactions in the human diabetic cornea. Exp Eye Res. 2017;164:22–30. | ||
Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338(17):1174–1180. | ||
Park JH, Kang S-S, Kim JY, Tchah H. Nerve growth factor attenuates apoptosis and inflammation in the diabetic cornea. Invest Ophthalmol Vis Sci. 2016;57(15):6767–6775. | ||
Kim SY, Choi JS, Joo CK. Effects of nicergoline on corneal epithelial wound healing in rat eyes. Invest Ophthalmol Vis Sci. 2009;50(2):621–625. | ||
Nakahara M, Miyata K, Otani S, et al. A randomised, placebo controlled clinical trial of the aldose reductase inhibitor CT-112 as management of corneal epithelial disorders in diabetic patients. Br J Ophthalmol. 2005;89(3):266–268. | ||
Zagon IS, Sassani JW, McLaughlin PJ. Reepithelialization of the human cornea is regulated by endogenous opioids. Invest Ophthalmol Vis Sci. 2000;41(1):73–81. | ||
Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Dry eye reversal and corneal sensation restoration with topical naltrexone in diabetes mellitus. Arch Ophthalmol. 2009;127(11):1468–1473. | ||
Zagon IS, Sassani JW, Immonen JA, McLaughlin PJ. Ocular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexone. Clin Exp Ophthalmol. 2014;42(2):159–168. | ||
Hampel U, Krüger M, Kunnen C, Garreis F, Willcox M, Paulsen F. In vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cells. Exp Eye Res. 2015;140:139–148. | ||
Erdinest N, Ovadia H, Kormas R, Solomon A. Anti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro study. J Inflamm. 2014;11(1):6. | ||
Shevalye H, Yorek MS, Coppey LJ, et al. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol. 2015;114(1):199–208. | ||
Abdul-Hamid M, Moustafa N. Amelioration of alloxan-induced diabetic keratopathy by beta-carotene. Exp Toxicol Pathol. 2014;66(1):49–59. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
