Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 12
Influence of CYP2D6, CYP3A5, ABCB1, APOE polymorphisms and nongenetic factors on donepezil treatment in patients with Alzheimer’s disease and vascular dementia
Authors Yaowaluk T, Senanarong V, Limwongse C, Boonprasert R, Kijsanayotin P
Received 6 April 2019
Accepted for publication 16 July 2019
Published 4 September 2019 Volume 2019:12 Pages 209—224
DOI https://doi.org/10.2147/PGPM.S211259
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Thitipon Yaowaluk1, Vorapun Senanarong2, Chanin Limwongse3, Rasda Boonprasert4, Pornpimol Kijsanayotin1
1Department of Pharmacology and Physiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand; 2Division of Neurology, Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; 3Division of Medical Genetics, Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; 4Clinical Toxicology Laboratory, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Pornpimol Kijsanayotin
Department of Pharmacology and Physiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Phayathai Road., Pathumwan, Bangkok 10330, Thailand
Tel +66 2 218 8322
Fax +66 2 218 8324
Email [email protected]
Purpose: This study aims to evaluate the influence of genetic polymorphisms of CYP2D6, CYP3A5, ABCB1, and APOE genes and nongenetic factors on steady-state plasma concentrations (Cpss) of donepezil and therapeutic outcomes in Thai patients with Alzheimer’s disease (AD) and vascular dementia (VAD).
Patients and methods: Eighty-five dementia patients who received donepezil for at least six months were recruited. CYP2D6, CYP3A5, ABCB1, and APOE polymorphisms were genotyped. Cpss of donepezil was measured. Association of genetic and non-genetic factors with Cpss and clinical outcomes of donepezil (cognitive function as measured by the Thai Mental State Examination score; TMSE) were determined by using univariate and multivariate analysis.
Results: Both univariate and multiple linear regression analysis indicated that only CYP2D6*10 allele was associated with higher Cpss (p-value =0.029 and B =0.478, p-value =0.032, respectively) that might influence the clinical outcomes of donepezil. ie, TMSE (p-value =0.010 and B =4.527, p-value =0.001) and ΔTMSE (p-value =0.023 and B =4.107, p-value =0.002), especially in patients with AD. Interestingly, concomitant use of memantine was found to be associated with increased Cpss of donepezil (p-value =0.007 and B =0.511, p-value =0.014). Whereas, co-medication with antidepressant drugs attenuated clinical responses in patients with AD (TMSE: B =−2.719, p-value =0.013 and ΔTMSE: B =−2.348, p-value =0.028). Age was a significant predictor of donepezil response in VAD patients. No significant association of CYP3A5*3, ABCB1 3435C>T or ABCB1 1236C>T, and APOE ϵ4 genotypes with Cpss or clinical outcomes of donepezil was found in this study.
Conclusion: Our results suggests that CYP2D6*10 strongly influences Cpss and there is a trend toward better outcomes of donepezil in patients with AD. Nongenetic factors including concomitant drugs treatment might alter Cpss of donepezil or clinical outcomes.
Keywords: donepezil, CYP2D6 polymorphisms, concomitant drugs treatment, Alzheimer’s disease, vascular dementia
Introduction
Dementia is a neurodegenerative disorder, characterized by progressive cognitive decline.1 Dementia is a chronic illness that diminishes the quality of life and causes an increased burden on caregivers.2 Moreover, all burdens associated with dementia lead to an increase in family expenses and ultimately resulting in economic losses to the society as a whole.
At present, the main goal of pharmacological treatment of dementia is enhancing or modulating neurotransmitters, especially acetylcholine, with the ultimate goal of slowing or halting disease progression. Unfortunately, at the moment, such treatment has varying response, depending on interindividual factors. One such treatment is donepezil hydrochloride, a specific piperidine-based reversible inhibitor of acetylcholinesterase (AChE). Donepezil is widely used as first-line drug for treatment of certain dementia-related illnesses including Alzheimer’s disease (AD) and vascular dementia (VAD).3,4 Donepezil’s major metabolic pathway is through the CYP2D6, an enzyme with genetic polymorphisms, which may account for the tremendous interindividual variation in a success rate of 20–60%.5–10 In addition, donepezil has been shown to play a pivotal role in slowing amyloid plaque formation.11 However, due to elimination via efflux transporter namely P-glycoproteins(P-gp) which is encoded by ABCB1, polymorphisms of ABCB1 might have an influence on the steady-state plasma concentration of donepezil (Cpss) and clinical response.12
CYP2D6 phenotypes of metabolizers can be classified as poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs), and ultra-rapid metabolizers (UMs). The metabolic rates in PMs and UMs are distinguished from EMs by 5 to 15 folds.13 Some studies report the association between CYP2D6 polymorphisms and donepezil response.14,15 While others report no such association.16,17 In Thai population, where CYP2D6*10 allele frequency is found to be as high as 45%,18 this polymorphism is likely to explain interindividual variability of donepezil response and Cpss.
In addition, studies exploring innate susceptibility in the development of AD have suggested the association between apolipoprotein E and the risk of AD. Most of these studies concluded that APOE ε4 alleles increase the risk of AD in a gene dose-dependent manner.19 However, the effects of APOE polymorphisms on the clinical response of donepezil are still inconclusive.
Donepezil is the most frequently prescribed AChE drug in Thailand. Previous study on the Thai population shows that cognitive function response to AChE inhibitor (AChEI) is variable.20 Thus, it seems that innate factors may play a role in drug response. In addition, a study on the effect of a single gene on clinical drug response is unlikely to explain therapeutic outcomes being observed. Moreover, nongenetic factors such as age, gender, education level, comorbidities, and drug–drug interaction can influence pharmacokinetic profiles and drug responses. Therefore, the main objectives of this study are to evaluate the relationships between genetic polymorphisms of genes involved in metabolic pathways and steady-state plasma concentration of donepezil and to investigate the associations of genetic variations including pathogenic gene (APOE), drug metabolizing enzyme genes (CYP2D6, CYP3A5), and transporter gene (ABCB1), and nongenetic factors with therapeutic outcomes of donepezil in Thai patients with dementia using both univariate and multivariate analysis.
Patients and methods
Study populations and study design
In this retrospective cohort study, participants were Thai patients who were diagnosed with dementia and who received 10-mg donepezil for treatment for at least six months. The study was conducted at the Memory Clinic, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, from February to October 2017, and the study enrolled 85 eligible patients.
The study was conducted according to the Declaration of Helsinki 1975 and was approved by the Institutional Review Board of the Faculty of Medicine, Siriraj Hospital, Mahidol University (EC: 818/2016). Written informed consents were obtained from all participants.
Inclusion and exclusion criteria
The inclusion criteria for participants were Thai patients diagnosed with dementia according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association Work Group criteria for Alzheimer’s disease or NINDS – AIREN criteria for VAD and taking a 10-mg maintenance dose of donepezil for their dementia with no prior or concomitant treatment with other AChEIs.
Patients were excluded if they were diagnosed with early onset dementia or familial Alzheimer’s disease, or if they have unstable psychiatric illnesses including schizophrenia, depression, and other neurological disorders such as Parkinson’s disease, seizure, and stroke. Patients who were noncompliant to donepezil were also excluded. Noncompliance was defined as being unable to take donepezil due to side effects, irregular administration, out of drug supply before the next visit, and loss of drug supply. Patients or caregivers who refused or were reluctant to participate in the study were also excluded.
Data collection and cognitive evaluation
Data of cognitive function test of all eligible patients were collected. Cognitive function was evaluated at the initial treatment and every visit using the Thai Mental State Examination (TMSE) score.21 Test results from all visits since the initial treatment were included. TMSE score were considered the clinical outcome of the study. Drug treatment was expected to maintain or slow the decline in cognitive function, ie, to prevent a decrease in TMSE score. Of the 30 TMSE points, the cutoff of 23 points indicated dementia.21 Concomitant drugs data from patients who take concomitant drug for at least three months were collected.
Blood samplings
Venous blood samples were collected from all patients by the clinical research nurse. For each patient, 3 mL of blood samples were kept in the EDTA tube for genotyping procedure and 5-mL samples were kept in a heparinized tube for determining Cpss of donepezil.
DNA extraction and genotyping procedures
Genomic DNA was extracted from the whole blood, using the Gentra Puregene Blood Kit (QIAGEN®, Germany), and kept at −80 °C until genotyping.
CYP2D6 and ABCB1 polymorphisms were determined by TaqMan® SNP Genotyping Assay Kits using Applied Biosystem 7500 Real-time PCR system: ABI 7500, according to the manufacturer’s instruction. The TaqMan® SNP genotyping was performed to identify specific alleles, namely, CYP2D6*2 (rs1135840, C__27102414_10), CYP2D6*10 (rs1065852, C___11484460_40), CYP3A5*3 (rs 776746, C__26201809_30), and ABCB1 3435C>T (rs1045642, C___7586657_20) and ABCB1 1236C>T (rs112850, C___758662_10).
APOE polymorphisms were detected by Restriction Fragment Length Polymorphism technique. Genomic DNA extracts were subject to PCR with oligonucleotide primers specific to APOE gene consisting of a sense “5ʹ GCACGGCTGTCCAAGGAGCTG CAGGC 3ʹ” and its antisense “5ʹ GGCGCTCGCGGATGGCGCTGAG 3ʹ”. In brief, PCR mixture was composed of 0.5 µM of each primer, 1 µL of genomic DNA, 10 mM of each dNTP, 10× PCR buffer, and 10% DMSO in a final volume of 25 μL. Each 8 μL of PCR products was digested with 1 µL of Hha1 enzyme according to the supplier’s recommended procedure (Biolabs, New England, USA). The resultant fragments were separated on 8% polyacrylamide gel and stained with ethidium bromide. Bands were compared with 10-bp DNA marker and the different individual genotypes were separated and categorized based on the following band length criteria: ε2/ε2: 91, 83, 61; ε3/ε3: 91, 61, 48, 35; ε4/ε4: 72, 61, 48, 35; ε2/ε3: 91, 83, 61, 48, 35; ε2/ε4: 91, 83, 72, 61, 48, 35 and ε3/ε4: 91, 72, 61, 48, 35.22 Subjects who had at least one of the APOE ε4 alleles were classified as APOE ε4 carriers and those who had APOE ε2 or APOE ε3 alleles as APOE ε4 were noncarriers.
Determination of Cpss of donepezil
In our study, we included only patients who took 10 mg of donepezil for at least 6 months. This period covered the time to reach steady-state plasma concentration of donepezil. The steady-state plasma concentration of donepezil was determined by using reversed-phase ultra performance liquid chromatography with photo diode array (UPLC-PDA) detection with a minor modification.23 Diphenhydramine was used as an internal standard.24 Method validation had been performed according to US FDA guidance for bioanalytical method validation.25 The lower limit of quantification was 10 ng/mL. The average recovery of drug (%) was in a range of 85.14–85.57%. Quality control (QC) intra-day precision ranged from 1.22% to 3.90% while the inter-day precision range was set at 1.59–3.69%.
Samples were prepared by solid-phase extraction (SPE) (OASIS®) and hydrophilic-lipophilic-balanced reversed-phase sorbent (Waters Corporation, Milford, MA, USA). A 20-µL diphenhydramine solution with a concentration of 10,000 ng/mL was added to 1 mL of the QC sample and standard spiked Sample. The mixture’s pH was adjusted with 200 µL orthophosphoric acid. Each 1000-µL sample was loaded in SPE which was preconditioned by methanol and equilibrated by deionized water (Milli Q Water). A 1-mL solution of 2% ammonia solution in 5% methanol and a 1-mL solution of 2% ammonia in 20% methanol were used for washing the samples. The samples were eluted with 500 µL of 2% acetic acid in methanol. The samples were then diluted with 200 µL of 0.05% trifluoroacetic acid. Each 10-µL final sample solution was injected into the UPLC-PDA.26
Statistical procedures
All data analyses were performed using the IBM SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA) with a statistical significance set at a type I error of less than 5% (p<0.05). For univariate analysis, the associations of gene polymorphisms with Cpss and therapeutic outcomes (ie, TMSE score) of donepezil were determined by independent t-test or one-way ANOVA for variables normally distributed, and Mann–Whitney U test or Kruskal–Wallis test for variables not normally distributed. Multiple linear regression analysis was performed to assess the association of Cpss of donepezil and TMSE score with genetic and nongenetic factors. Chi-square was used to test for the deviation from Hardy–Weinberg equilibrium.
Results
Of 85 patients who met the eligible criteria, the average age was 78.42 years, and the majority of participants were in 75 years or older. The majority were diagnosed with AD (60.00%), followed by VAD (37.64%). AD dementia of frontal lobe type and dementia with Lewy body were found in negligible proportions. Their initial or baseline TMSE score before treatment was 20.01±6.03 points by average. The average years of educations were 8.56±5.48 years.
Evaluation of factors affecting Cpss of donepezil
Associations of CYP2D6, CYP3A5, and ABCB1 polymorphisms with Cpss of donepezil
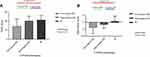
At 10-mg maintenance dose of donepezil, homozygous CYP2D6*10/*10 (ie, IMs), was found to be associated with the highest Cpss of donepezil. On the other hand, those with heterozygous EMs (CYP2D6 *1/*10) and homozygous EMs (CYP2D6*1/*1/CYP2D6*1/*2/CYP2D6*2/*2) were associated with lower Cpss of donepezil, respectively (Table S1). The Cpss of donepezil among these three phenotypic groups was significantly different (p-value =0.029). Cpss of the IM group was significantly higher than that of the homozygous EM, as shown in Figure 1.
No significant association between CYP3A5*3, ABCB1 3435C>T or ABCB1 1236C>T polymorphisms and Cpss of donepezil was found (p-value ≥0.05) (Table S1).
Association of the nongenetic factors and Cpss of donepezil
Nongenetic factors that might have an influence on interindividual variability of Cpss of donepezil were determined. Our results demonstrated that there was no statistically significant difference in Cpss of donepezil among gender. However, male patients trend to have lower median (IQR) of Cpss compared with female (71.13 (36.31–110.48) vs 99.16 (52.53–137.31); p-value =0.081). No significant association between concomitant CYP3A4, CYP2D6, or P-glycoprotein inhibitors and Cpss of donepezil was also observed (Table 3).
 |
Table 1 Baseline demographic and clinical characteristic of 85 Thai patients with dementia |
 |
Table 2 Genotype distribution and allele frequencies of the candidate gene in the study patients |
 |
Table 3 Association of the non-genetic factors and Cpss of donepezil at the 10 mg maintenance dose |
There was a strong association between concomitant memantine use and Cpss of donepezil. Patients who received concomitant memantine had higher Cpss of donepezil than those who were memantine nonusers (102.77 (75.50–161.27) vs 69.09 (37.83–123.78); p-value =0.007) as shown in Table 3. We further explored the effect of memantine doses on Cpss of donepezil. The results showed that Cpss of donepezil was directly proportional to the administered dose of memantine. The Cpss of donepezil in patients who did not take memantine and who took 10 or 20 mg memantine were 69.09, 93.79, and 173.37 ng/mL, respectively. The Cpss of donepezil corresponding to the three groups were significantly different (p-value =0.012).
Our finding also demonstrated a trend toward a combined effect of CYP2D6*10 carriers and concomitant memantine treatment on Cpss of donepezil. The patients who were CYP2D6*10 carriers and concurrent memantine users showed the highest Cpss of donepezil when compared with the rest as shown in Figure 2.
No significant association between Cpss of donepezil and BMI or body weight was observed.
Combined association of genetic and nongenetic factors with Cpss of donepezil
The results from multivariate analysis are shown in Table 4. The stepwise multiple linear regression analysis included CYP2D6 phenotypes, CYP3A5 phenotypes, time from drug intake, age, and gender as covariates. The final model revealed that CYP2D6 phenotypes and concomitant memantine use were significantly associated with Cpss of donepezil. These predictive variables could explain approximately 13% of variability in Cpss of donepezil (R2=0.133, p-value =0.003).
 |
Table 4 The final model of multiple linear regression analysis of explanatory variables for Cpss of donepezil at the 10 mg maintenance dose |
Evaluation of factors affecting cognitive function
In this study, two patients who were frontotemporal lobe dementia and mild cognitive impairment were excluded, because type of dementia might affect cognitive evaluation. Furthermore, we could not draw any conclusion due to negligible proportions of those patients. We also excluded 1 patient because of missing TMSE score. Therefore, a total of 82 patients were included in our data analysis. The 82 patients were categorized into two groups according to the types of dementia as AD and VAD.
Univariate analysis
When cognitive functions of AD patients were tested, IM group also showed a tendency toward a better therapeutic outcomes with the highest TMSE score (21.10±5.12 points) when compared with those heterozygous EM (20.20±5.30 points) and homozygous EM (14.30±8.10 points) groups (Table S1). In line with that, the decline of cognitive function was the least obvious in the IM group and the most obvious in the homozygous EM group. There was a statistically significant difference of TMSE score and ΔTMSE between IM and homozygous EM groups as shown in Figure 3.
In patients with VAD, the decline in cognitive function was high in homozygous EMs, while
Both patients with AD and VAD who were receiving antidepressant drugs had poorer cognitive function compared to those who were not receiving the antidepressant drugs, especially in AD as shown in Table S2.
Regarding univariate analysis, there was no significant association between CYP3A5, ABCB1, APOE genetic polymorphisms, concomitant memantine use, age, gender, education level, and TMSE score in both patients with AD and VAD as shown in Table S1.
Multivariate analysis
Covariates were selected from the result of univariate analysis (Table S1) by setting significant level for entry (SLE) at p-value of 0.25 or lower and were introduced into each multivariate model. The final models are shown in Table 5.
At the 10-mg maintenance dose of donepezil, stepwise multiple linear regression models using TMSE score at steady state or ΔTMSE as the dependent variables were constructed to determine the association of genetic and nongenetic factors associated with donepezil response of AD and VAD patients as shown in Table 5. The results revealed that in AD patients, CYP2D6 phenotype was the only genetic factor influencing TMSE score at steady state and ΔTMSE. On the contrary, AD patients who were treated with antidepressant drugs were significantly associated with worsened steady-state TMSE score after adjusting for covariates listed in Table 5. These two covariates could explain 74% of the variability in TMSE score at steady state (R2=0.747, p-value <0.001). The result also revealed that the only significant predictor of ΔTMSE was CYP2D6 phenotypes which could explain 32% of the variability (R2=0.321, p-value =0.002).
In VAD, the final stepwise multiple linear regression model demonstrated that increasing age was significantly associated with a more negative TMSE score at steady state and ΔTMSE. The magnitude of explanation for the variability in the models was 71% for TMSE score (R2=0.714, p-value <0.001) and 21% for ΔTMSE (R2=0.210, p-value =0.008).
Discussion
Our study suggests that CYP2D6 polymorphism are associated with Cpss. We saw a trend toward an influence on cognitive outcomes of donepezil as measured by the TMSE score in both univariate and multivariate analysis. Patients carrying a mutant allele of CYP2D6 (CYP2D6*10) have a higher Cpss of donepezil when compared with those noncarriers. The impact of CYP2D6*10 is consistent with previous studies in the Asian population.7,27
In the present study, CYP2D6*10 allele frequency in Thais was found to be the highest variant allele which is consistent with previous studies in Thais and is comparable with those of other Asian populations such as Chinese and Japanese.18,27–29 However, it is higher than those found in Europeans.30 This fact emphasizes the impact of CYP2D6*10 to Donepezil treatment.
Contrary to previous studies which have demonstrated that CYP2D6 inhibitors might increase Cpss of donepezil, the present study found no significant effects of CYP2D6 inhibitors on Cpss of donepezil. This can be due to the disparate strength of CYP2D6 inhibitors in our study including sertraline, venlafaxine, escitalopram, and desvenlafaxine which are relatively weak compared to other studies that used paroxetine.31 Moreover, evidence has been found that the coadministration with sertraline could decrease Cpss of donepezil. The suggested possible explanation was that sertraline has a slightly stronger affinity for CYP2D6 than donepezil. Thus, at a low plasma level, sertraline could be metabolized competitively with donepezil. Consequently, an increase in donepezil level could be expected. On the contrary, at a higher plasma concentration particularly at steady state, donepezil level was not changed. This can also explain the phenomenon whereby CYP2D6 exerted less influence at higher plasma concentration due to a shift of donepezil biotransformation to CYP3A4 since the capacity of CYP2D6 was limited by sertraline.32
Interestingly, we observed a significant higher level of Cpss of donepezil in patients who use concomitant memantine than that of nonusers as shown in Table 3. This phenomenon could be possibly due to the fact that memantine can inhibit CYP2D6 enzyme as described by Micuda et al.33 Our study serves as the first association study to illustrate the effect of concomitant memantine use on Cpss of donepezil. The result from the multivariate analysis is concordant with univariate analysis. The result emphasized that homozygous of CYP2D6*10 and concomitant memantine use toward strongly positive associated with Cpss of donepezil. These covariates could explain the interindividual variability of Cpss for approximately 13%. The remaining unexplained interindividual variability may derive from other contributing factors such as race, concomitant use of P-glycoprotein or CYP3A4 inhibitors, gene–environment interaction and some physiological function that cannot assuredly be excluded in our cohorts. Moreover, the comorbid condition in elderly deteriorating physiological function may attribute to altered drug concentration in the blood and brain and so it is difficult to predict precise Cpss of donepezil. Physiological function especially creatinine clearance may have greater influence in the elderly. However, our result indicates that no association was found between Cpss of donepezil and creatinine clearance. In relation to cognitive function, homozygous of CYP2D6*10 (IM) shows the highest TMSE score when compared with the rest. Possible association of the genetic polymorphisms of CYP2D6 in susceptibility to donepezil outcome might be described by the following reasons. Donepezil is predominantly metabolized by CYP2D6, and human CYP2D6 in the brain was prominently localized in the pyramidal cell of the cortex and hippocampus which a certain region that account for cognitive function. Penas Liam Zaidel shows that donepezil accumulates in the frontal cortex, one of the regions which affected the neuropathology of AD.34 Consequently, CYP2D6*10 carriers might increase donepezil and greaterly inhibit AChE in frontal cortex resulting in an improvement in cognitive function as measured by TMSE in AD. Furthermore, Darreh founded that CSF donepezil concentration appears to be approximately tenfold lower compared with plasma levels but exhibits a similar dose-proportional pattern. These implied that CYP2D6*10 carriers might have a higher donepezil level in CSF and could be expected to provide more achievement in clinical responses.35
In contrast to AD, in VAD patients, CYP2D6 variants did not affect the cognitive response of donepezil. This may be a reflection of the fact that frontal cortex and hippocampus which abundant of CYP2D6 have a less responsible in the neuropathological process in VAD when compared with AD. In VAD, the region of the brain which plays a role in the pathological process is the small vessels in the subcortical area. Jellinger KA found that older ages may contribute to small vessel disorder. Moreover, advanced age is an addition predisposing factor which could aggravate clinical response of AChEI treatment.36 This is consistent with our findings.
Another possible explanation is that CYP2D6 might play a role in the biotransformation of several endogenous substances or xenobiotics in the brain. CYP2D6 phenotypes also have an influence on neurocognition as described by Peñas-LLedó et al.34 For these reasons, it may imply that genetic variations of CYP2D6 could mediate the progression of the disease and therapeutic outcomes of donepezil. Furthermore, Kirchheiner et al suggested that IM of CYP2D6 has higher brain perfusion in the hippocampus compared with EM.37
Moreover, homozygous EM of CYP2D6 tend to have lower TMSE score at baseline in AD group when compared to the rest (Table S1). So, this could possibly explain why the homozygous EM group is more deteriorated at follow-up.
In contrast to our results, a prospective study reported by Miranda et al showed that good response pattern was associated with concentration of donepezil, not by CYP2D6 and APOE genotypes.38 The recent meta-analysis indicated that normal function of CYP2D6 alleles may have a better response to donepezil treatment and there was no association of APOE on donepezil outcome.39 The discrepancy results from our study may arise from the differences in study design, evaluation score, duration of study, and genotyping data. Moreover, most of the studies included in the meta-analysis were conducted in Caucasian population. It should be acknowledged that larger cohort study in Asian populations are required.
In this study, no significant effect of CYP3A5 and ABCB1 polymorphisms on Cpss of donepezil and cognitive score was found. These results were concordant with studies of Magliulo et al12 and Noetzli et al.40 This phenomenon could be possibly due to the fact that donepezil prominently underwent CYP2D6 as its main metabolic pathway. Whereas, CYP3A5 and ABCB1 might play a minor role in donepezil disposition.
Some studies had attempted to explore the association of APOE ε4 alleles with AChEinhibitors response in AD.5 The rationales whereby APOE ε4 plays a role in contributing pathogenesis of AD such as abnormal cholesterol transportation and the augmentation of amyloid plaque and neurofibrillary tangles might have a negative impact on drug treatment. Some observations found that APOE ε4 carriers may worsen the TMSE score of donepezil treatment outcome. But no significant association between APOE ε4 carriers and TMSE score was found in this study. The effects of APOE ε4 on clinical response of donepezil were not homogeneous (32–34). Therefore, larger and well-designed study are required to confirm these association.
One of our findings was that concomitant use of antidepressant drugs which were weak CYP2D6 inhibitors (including sertraline, venlafaxine, escitalopram, desvenlafaxine) was negatively associated with TMSE score in AD and VAD. This phenomenon was astonishing because one previous study showed that CYP2D6 inhibitors could have increased the Cpss of donepezil31 and could be expected to provide more achievement in therapeutic responses. The association of declined TMSE score was more obvious among patients with moderate AD as indicated by lower baseline TMSE score compared to those with mild AD. When controlling the effect of severity of dementia on TMSE score by introducing baseline TMSE score into multiple linear regression model, the result confirmed the significant negative correlation of the drugs on TMSE score or ΔTMSE score. This finding emphasized the negative impact of antidepressant drugs on cognitive function. It is possible that concomitant use of antidepressant drugs such as selective serotonin reuptake inhibitors (SSRIs) may influence cognitive function.41 These results were in agreement with the findings of Wattmo et al that donepezil treatment outcomes diminished faster in patients with depression treated with antidepressants including SSRIs.42 The possible explanation is that depression condition can deteriorate neurocognitive function which goes beyond the pharmacological effect of antidepression treatment. Another possibility could be due to anticholinergic effect of some antidepressant drugs that might diminish the cognitive function of the patients.43 On the other hand, no significant relationship was found in VAD since depression condition was not commonly found in VAD.
Duration of use is positively associated with clinical response. This finding suggests that long-term use of donepezil could be beneficial in improving cognitive function which is supported by the fact that donepezil might modify the underlying mechanism of disease progression in in vivo study.11,44 The different results observed in previous association studies may be accounted for assessment score, different inclusion or exclusion criteria, or duration of treatment. Our study recruited patients in all stages of dementia and so we included baseline severity as determined by baseline TMSE score as a covariate for multivariate analysis. Moreover, we evaluate ΔTMSE as well as TMSE at steady state to increase the reliability of our results. All patients enrolled in our study were treated for at least 6 months with the same dose of donepezil. We explore the duration of treatment as an additional covariate in the multivariate model.
Since this study was a retrospective cohort design, there were some unrecord data especially in the aspects of adverse drug events. So, we could not explore the association between some side effects including nausea, vomit, anorexia, and genetic factors. However, no association was found between CYP2D6 genotypes and systolic or diastolic blood pressure or pulse rate in this cohort.
Our study has some strengths. First, this study examined simultaneously several genes including drug metabolizing enzyme genes (CYP2D6, CYP3A5), transporter gene (ABCB1), pathological gene (APOE), and certain nongenetic factors that could have an influence on Cpss and therapeutic outcomes of donepezil by using multivariate analysis. The use of multiple linear regression analysis could identify covariates that could better predict clinical response than univariate analysis. Second, we did not restrict the inclusion criteria because we intended to perform the study in a real-life clinical setting. Several factors especially age, gender, and concomitant drugs which were not proven in the previous study were allowed and tested as nongenetic covariates in the multivariate analysis. These factors could contribute to a more reliable prediction and the result could be more applicable to routine clinical practice.
However, its retrospective cohort design presents a limitation, making the temporal relationship between the dose of donepezil and corresponding Cpss difficult to establish. In addition, a long-term follow-up cannot be done. Further prospective study, especially randomized controlled trials with stratification on doses of donepezil according to individual genotypes, should be conducted to determine practically important predictive variables. Notably, genetic variation of pharmacodynamic gene such as AChE which might have an influence on clinical response of AChEIs was not identified in the present study. In addition, it should be acknowledged that association study does not provide a causal relationship. Therefore, further functional studies to ascertain any findings from pharmacogenetic association studies should be performed.
Conclusion
Patients with AD or VAD carrying CYP2D6*10 allele were associated with higher Cpss of donepezil and tendency of better therapeutic outcome in AD. Nongenetic factors including concomitant memantine use was also significantly associated with increased Cpss of donepezil. Whereas, concomitant antidepressant treatment and age may attenuate clinical responses in AD and VAD, respectively. The negative impact of concomitant antidepressant treatment on donepezil outcomes should be further investigated. Determination of genetic factors, ie, CYP2D6*10 genotypes together with nongenetic factors including individual demographics and concomitant drug exposure could be useful for tailoring of donepezil treatment in the forthcoming personalized medicine
Acknowledgments
This work was supported by the 90th Anniversary of Chulalongkorn University Fund (GCUGR1125613040D), Chulalongkorn University. The authors would like to thank Assoc. Prof. Chulathida Chomchai, MD, Dean of International College Mahidol University and Assist. Prof. Charoen Treesak, Department of Clinical Pharmacy, Faculty of Pharmacy, Srinakharinwirot University, for constructive comments and manuscript revision as well as Assist. Prof. Chulaluk Komoltri, Department of Health Research and Development, Siriraj Hospital, Mahidol University, for statistical consultation. Finally, we are grateful to all patients for participating in this study.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest with respect to this work.
References
1. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi:10.1038/nrdp.2015.56
2. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi:10.1016/S0140-6736(05)67889-0
3. Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2018;6:1–2.
4. Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev. 2004;1:1–2.
5. Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2013;52(4):225–241. doi:10.1007/s40262-013-0038-9
6. Cacabelos R. Donepezil in Alzheimer’s disease: from conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3(3):303–333.
7. Lu J, Wan L, Zhong Y, et al. Stereoselective metabolism of donepezil and steady-state plasma concentrations of S-donepezil based on CYP2D6 polymorphisms in the therapeutic responses of Han Chinese patients with Alzheimer’s disease. J Pharmacol Sci. 2015;129(3):188–195. doi:10.1016/j.jphs.2015.10.010
8. Saumier D, Murtha S, Bergman H, Phillips N, Whitehead V, Chertkow H. Cognitive predictors of donepezil therapy response in Alzheimer disease. Dement Geriatr Cogn Disord. 2007;24(1):28–35. doi:10.1159/000102569
9. Cacabelos R, Torrellas C, Carrera I. Opportunities in pharmacogenomics for the treatment of Alzheimer’s disease. Future Neurol. 2015;10(3):229–252. doi:10.2217/fnl.15.12
10. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41(10):719–739. doi:10.2165/00003088-200241100-00003
11. Kim HG, Moon M, Choi JG, et al. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology. 2014;40:23–32. doi:10.1016/j.neuro.2013.10.004
12. Magliulo L, Dahl ML, Lombardi G, et al. Do CYP3A and ABCB1 genotypes influence the plasma concentration and clinical outcome of donepezil treatment? Eur J Clin Pharmacol. 2011;67(1):47–54. doi:10.1007/s00228-010-0883-5
13. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 2009;48(11):689–723. doi:10.2165/11318030-000000000-00000
14. Pilotto A, Franceschi M, D’Onofrio G, et al. Effect of a CYP2D6 polymorphism on the efficacy of donepezil in patients with Alzheimer disease. Neurology. 2009;73(10):761–767. doi:10.1212/WNL.0b013e3181b6bbe3
15. Varsaldi F, Miglio G, Scordo MG, et al. Impact of the CYP2D6 polymorphism on steady-state plasma concentrations and clinical outcome of donepezil in Alzheimer’s disease patients. Eur J Clin Pharmacol. 2006;62(9):721–726. doi:10.1007/s00228-006-0168-1
16. Klimkowicz-Mrowiec A, Wolkow P, Sado M, et al. Influence of rs1080985 single nucleotide polymorphism of the CYP2D6 gene on response to treatment with donepezil in patients with alzheimer’s disease. Neuropsychiatr Dis Treat. 2013;9:1029–1033. doi:10.2147/NDT.S46689
17. Liu M, Zhang Y, Huo YR, et al. Influence of the rs1080985 single nucleotide polymorphism of the CYP2D6 gene and APOE polymorphism on the response to donepezil treatment in patients with Alzheimer’s disease in China. Dement Geriatr Cogn Dis Extra. 2014;4(3):450–456. doi:10.1159/000367596
18. Suwannasri P, Thongnoppakhun W, Pramyothin P, Assawamakin A, Limwongse C. Combination of multiplex PCR and DHPLC-based strategy for CYP2D6 genotyping scheme in Thais. Clin Biochem. 2011;44(13):1144–1152. doi:10.1016/j.clinbiochem.2011.06.985
19. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi:10.1038/nrneurol.2012.263
20. Rungsanpanya T, Muangpaisan W, Praditsuwan R. Clinical practice with antidementia drugs in a geriatric clinic. J Med Assoc Thai. 2012;95(8):1081–1089.
21. Train The Brain Forum Committee TTBFC. Thai mental state examination (TMSE). Siriraj Medical Journal. 2017;45(6):16.
22. Chaudhary R, Likidlilid A, Peerapatdit T, et al. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovasc Diabetol. 2012;11:36. doi:10.1186/1475-2840-11-36
23. Ponnayyan Sulochana S, Sharma K, Mullangi R, Sukumaran SK. Review of the validated HPLC and LC-MS/MS methods for determination of drugs used in clinical practice for Alzheimer’s disease. Biomed Chromatogr. 2014;28(11):1431–1490. doi:10.1002/bmc.3116
24. Xie Z, Liao Q, Xu X, Yao M, Wan J, Liu D. Rapid and sensitive determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry: application to a pharmacokinetic study. Rapid Commun Mass Spectrom. 2006;20(21):3193–3198. doi:10.1002/rcm.2718
25. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Rockville, MD : US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; 2001.
26. Koeber R, Kluenemann -H-H, Waimer R, et al. Implementation of a cost-effective HPLC/UV-approach for medical routine quantification of donepezil in human serum. J Chromatogr B. 2012;881–882:1–11. doi:10.1016/j.jchromb.2011.10.027
27. Zhong Y, Zheng X, Miao Y, Yan H, Wang B, Wan L. Effect of CYP2D6*10 and APOE polymorphisms on the efficacy of donepezil in patients with Alzheimer’s disease. Am J Med Sci. 2013;345(3):222–226. doi:10.1097/MAJ.0b013e318255a8f9
28. Chamnanphon M, Gaedigk A, Vanwong N, et al. CYP2D6 genotype analysis of a Thai population: platform comparison. Pharmacogenomics. 2018;19(12):947–960. doi:10.2217/pgs-2018-0075
29. Ota T, Kamada Y, Hayashida M, Iwao-Koizumi K, Murata S, Kinoshita K. Combination analysis in genetic polymorphisms of drug-metabolizing enzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A5 in the Japanese population. Int J Med Sci. 2015;12(1):78–82. doi:10.7150/ijms.10263
30. Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci. 2007;96(9):2224–2231. doi:10.1002/jps.20892
31. Coin A, Pamio MV, Alexopoulos C, et al. Donepezil plasma concentrations, CYP2D6 and CYP3A4 phenotypes, and cognitive outcome in Alzheimer’s disease. Eur J Clin Pharmacol. 2016;72(6):711–717. doi:10.1007/s00228-016-2033-1
32. Nagy CF, Kumar D, Perdomo CA, Wason S, Cullen EI, Pratt RD. Concurrent administration of donepezil HCl and sertraline HCl in healthy volunteers: assessment of pharmacokinetic changes and safety following single and multiple oral doses. Br J Clin Pharmacol. 2004;58(Suppl 1):25–33. doi:10.1111/j.1365-2125.2004.01801.x
33. Micuda S, Mundlova L, Anzenbacherova E, et al. Inhibitory effects of memantine on human cytochrome P450 activities: prediction of in vivo drug interactions. Eur J Clin Pharmacol. 2004;60(8):583–589. doi:10.1007/s00228-004-0825-1
34. Peñas-Lledó EM, Llerena A. CYP2D6 variation, behaviour and psychopathology: implications for pharmacogenomics-guided clinical trials. Br J Clin Pharmacol. 2014;77(4):673–683. doi:10.1111/bcp.12227
35. Darreh-Shori T, Meurling L, Pettersson T, et al. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer’s disease following chronic donepezil treatment. J Neural Transm (Vienna). 2006;113(11):1791–1801. doi:10.1007/s00702-006-0526-2
36. Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi:10.3389/fnagi.2013.00017
37. Kirchheiner J, Seeringer A, Godoy AL, et al. CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry. 2010;16:333. doi:10.1038/mp.2010.42
38. Miranda LF, Gomes KB, Tito PA, et al. Clinical response to donepezil in mild and moderate dementia: relationship to drug plasma concentration and CYP2D6 and APOE genetic polymorphisms. J Alzheimers Dis. 2017;55(2):539–549. doi:10.3233/JAD-160164
39. Xiao T, Jiao B, Zhang W, Tang B, Shen L. Effect of the CYP2D6 and APOE polymorphisms on the efficacy of donepezil in patients with Alzheimer’s disease: a systematic review and meta-analysis. CNS Drugs. 2016;30(10):899–907. doi:10.1007/s40263-016-0356-1
40. Noetzli M, Guidi M, Ebbing K, et al. Population pharmacokinetic approach to evaluate the effect of CYP2D6, CYP3A, ABCB1, POR and NR1I2 genotypes on donepezil clearance. Br J Clin Pharmacol. 2014;78(1):135–144. doi:10.1111/bcp.12325
41. Mokhber N, Abdollahian E, Soltanifar A, et al. Comparison of sertraline, venlafaxine and desipramine effects on depression, cognition and the daily living activities in Alzheimer patients.Pharmacopsychiatry. 2014;47:131–140.
42. Wattmo C, Wallin ÅK, Minthon L. Functional response to cholinesterase inhibitor therapy in a naturalistic Alzheimer’s disease cohort. BMC Neurol. 2012;12(1):134. doi:10.1186/1471-2377-12-134
43. Pasqualetti G, Tognini S, Calsolaro V, Polini A, Monzani F. Potential drug-drug interactions in Alzheimer patients with behavioral symptoms. Clin Interv Aging. 2015;10:1457–1466. doi:10.2147/CIA.S87466
44. Ye CY, Lei Y, Tang XC, Zhang HY. Donepezil attenuates Abeta-associated mitochondrial dysfunction and reduces mitochondrial Abeta accumulation in vivo and in vitro. Neuropharmacology. 2015;95:29–36. doi:10.1016/j.neuropharm.2015.02.020
45. Hu P, Qin YH, Jing CX, Lu L, Hu B, Du PF. Does the geographical gradient of ApoE4 allele exist in China? A systemic comparison among multiple Chinese populations. Mol Biol Rep. 2011;38(1):489–494. doi:10.1007/s11033-010-0132-0
46. Kobayashi S, Tateno M, Park TW, et al. Apolipoprotein E4 frequencies in a Japanese population with Alzheimer’s disease and dementia with Lewy bodies. PLoS One. 2011;6(4):e18569–e18569. doi:10.1371/journal.pone.0018569
Supplementary materials
 |
Table S1 Cpss of donepezil and TMSE score in association with CYP2D6, CYP3A5, ABCB1, and APOE genotypes at the 10 mg maintenance dose |
 |
Table S2 Association of non-genetic factor and TMSE score of donepezil at 10-mg maintenance dose |
 |
Table S3 Bivariate analysis: Association of non-genetic continuous variable and TMSE score |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.