Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Influence of Carotid Intima-Media Thickness Levels at Bifurcation on Short-Term Functional Outcomes Among Non-Cardiogenic Ischemic Stroke Patients with and without Type 2 Diabetes Mellitus
Authors Guo XJ, Wu M , Pei SF, Xie P, Wu MY
Received 14 December 2021
Accepted for publication 10 March 2022
Published 23 March 2022 Volume 2022:15 Pages 897—906
DOI https://doi.org/10.2147/DMSO.S351679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Xiao-Jing Guo,1,* Mian Wu,2,* Shao-Fang Pei,1 Ping Xie,3 Min-Ya Wu1
1Department of Neurology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 2Department of Endocrinology and Metabolism, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 3Department of Ultrasonography, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min-Ya Wu, Department of Neurology, The Affiliated Suzhou Hospital of Nanjing Medical University, 242 Guangji Road, Suzhou, Jiangsu, People’s Republic of China, Tel +86 15151429862, Email [email protected]
Purpose: The intima-media thickness (IMT) is broadly reported to have relationships with non-cardiogenic ischemic stroke and with diabetes. But how does IMT affect the short-term prognosis of stroke seems unknown yet. We investigated the influence of the intima-media thickness at carotid bifurcation (IMTbif) on short-term functional outcomes among non-cardiogenic ischemic stroke patients with and without type 2 diabetes mellitus (T2DM).
Patients and Methods: A total of 314 patients with non-cardiogenic ischemic stroke (122 with T2DM and 192 without diabetes) were included in this retrospective study. Poor functional outcome was defined as a modified Rankin Scale (mRS) > 2 at 3 months after stroke onset. Group comparisons were done in favorable and poor outcome groups. Linear regression analysis was utilized to verify the associations between IMTbif and mRS in subgroups with and without diabetes, respectively.
Results: The median IMTbif of total patients was 1.40mm. Patients with poor outcomes were significantly older, had higher National Institutes of Health Stroke Scale (NIHSS) scores, lower haemoglobin, higher fasting glucose and higher systolic blood pressure values. Their IMTbif levels were also markedly higher. Among 122 included stroke patients with T2DM, IMTbif levels and NIHSS were independently associated with functional outcomes at 3 months, whereas there was no significant association between IMTbif levels and short-term functional outcomes among patients without diabetes.
Conclusion: The IMTbif levels were significantly associated with 3-month functional outcomes in non-cardiogenic ischemic stroke patients with T2DM. The ultrasound detection of the IMTbif therefore suggests a prognostic value among patients with stroke and T2DM.
Keywords: cerebrovascular disease, type 2 diabetes, prognosis, carotid bifurcation
Introduction
Acute Ischemic Stroke is a leading cause of death in Chinese population,1,2 with 75% of survivors being left with various degrees of disability or limb dysfunction.3 For all acute stroke patients, noninvasive imaging of the cervical vessels was recommended as part of the routine evaluation for stroke pathogenesis and future management, according to the AHA/ASA guidelines.4 The intima-media thickness (IMT) is broadly used as a surrogate marker to evaluate cervical vessel evolution and can be easily assessed by carotid B-mode ultrasound.5,6 The initial IMT occurs mostly at carotid bifurcation, where hemodynamic force, shear stress and cyclic strain give rise to accumulative damage to endothelial cells.7,8 Endothelial dysfunction further gathers lipid to localize within foam cells, stimulates thick fatty streaks formation and then thicken intima-media wall, thus restricting local blood flow over time.9 Accordingly, the ultrasound measurement of intima-media thickness at carotid bifurcation (IMTbif) provides a detailed view of baseline vascular condition in stroke patients.
Diabetes Mellitus (DM) is an established modifiable risk factor for acute non-cardiogenic ischemic stroke (NC-AIS).10 Vascular endothelial dysfunction also occurs in type 2 diabetes mellitus (T2DM) at early stage and triggers consecutive systemic inflammation.11 These subtle pathological changes gradually contribute to vascular complications such as IMT, atherosclerosis and increase the risk of poor prognosis in stroke.12,13
Hence, IMT and diabetes in stroke share some similar pathophysiology through endothelium dysfunction and interlinked. To the best of our knowledge, previous studies tried to verify whether IMT could predict occurrence of stroke with diabetes.14,15 Nevertheless, studies regarding the influence of IMT on stroke outcomes with diabetes are still scarce. In order to evaluate the prognostic value of IMTbif of patients with non-cardiogenic stroke, particularly among those patients with or without diabetes, we thus investigated the association of IMTbif with 3-month functional outcomes after stroke in the present study.
Patients and Methods
Study Population and Ethics
Medical records were extracted from the stroke unit of Suzhou Municipal Hospital, from January 2018 to June 2020. Data collection for registry was performed by chart abstractors with neurological expertise. Ethical approval was obtained from the local ethics review board. This was a retrospective study, only collecting clinical data without interfering with patients’ medication. The study complied with the Declaration of Helsinki, which meant the authors protected patients’ information privacy while did not bring physiological risks to them. So the informed consent requirement was waived.
The inclusion criteria were: (a) patients diagnosed with acute ischemic stroke, confirmed by clinical symptoms and neuroimages, according to the recommendations by the World Health Organization (WHO);16 (b) those admitted within 72 hours of the acute onset of ischemic stroke and (c) individuals who received a carotid ultrasound measure within 72 hours of admission. On the other hand, the exclusion criteria were: (a) individuals in which atrial fibrillation or other relevant cardiac arrhythmias or heart diseases were found in the past or during hospitalization; (b) those with thrombolysis or undergoing intravascular interventional therapy; (c) carotid ultrasound examination indicated that the internal carotid or common carotid artery stenosis was ≥50% or vulnerable plaques were detected; (d) individuals incapable of self-care before stroke; (e) those complicated with liver or renal dysfunction and (f) individuals complicated with severe infection. Additionally, T2DM was defined according to the new American Diabetes Association (ADA) criteria.17
Data Sources
The following information was obtained by reviewing medical records for each patient: age, sex, height, weight, risk factors for vascular disease and venous blood sample values collected between 07:00 and 08:00 after over 8-hour fasting within one day of admission, including haemoglobin, fasting blood glucose, glycosylated haemoglobin (HbA1c), total cholesterol, triglyceride, high density lipoprotein (HDL-C), low density lipoprotein (LDL-C), uric acid and homocysteine. The Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure was recorded based on the maximum systolic blood pressure (SBP) and its corresponding diastolic blood pressure (DBP) between 0:00 and 6:00 of the next day of admission. Blood samples were measured in this stroke unit on a Hitachi 7600 analyser using an enzymatic assay (Hitachi Inc., Tokyo, Japan). In addition, the records of initial functional status as determined by the National Institutes of Health Stroke Scale (NIHSS) in the first 6 hours on admission, and the modified Rankin Scale (mRS) scores at 3 months through telephone follow-up by a trained neurologist were extracted. Poor functional outcome was defined as the mRS of 3–6, while 6 stands for death.18
Of note, those individuals who underwent a carotid ultrasound detection within 72 hours of admission were included in the study. The ultrasound measurement was carried out and validated by two professional sonographers using the high-resolution Doppler ultrasound diagnostic instrument (Xario SSA-660A, Toshiba Medical Systems Corporation, Tochigi, Japan) in the hospital, with the Image Software Package (National Institute of Health, Baltimore, Md). The linear array probe was 8.0MHz. The maximum value of the posterior intima-media thickness at carotid bifurcation was measured and recorded as the IMTbif level for this study. If a stable plaque was present, it was included in the IMT measurement, as shown in Figure 1.
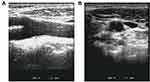 |
Figure 1 (A) Sagittal scan of carotid ultrasonogram. (B) Longitudinal scan of carotid ultrasonogram. Measurement of IMT (*) at posterior wall of carotid artery bifurcation is shown. |
Validation by duplicate chart abstraction showed excellent agreement for key variables in the stroke unit registry and ultrasound records.
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 package (IBM Inc., Chicago, IL, USA). Data on normally distributed variables was expressed as the mean ± SD, while the non-normally distributed variables were presented as medians (quartiles 25% and 75%). Unpaired Student’s t-tests were used to compare characteristics between poor and favorable outcome groups (Table 1) as well as between diabetic and non-diabetic groups (Table 2). In order to elucidate the independent relationships between prognosis and clinical parameters, the mRS score was selected as the dependent variable while the other clinical parameters were taken as independent variables to build multiple linear regression equations, for diabetic (Table 3) and non-diabetic subgroups (Table 4). Only variables that were significantly (P <0.05) related to mRS from univariate linear regression analysis were included into the multivariate models. Missing values were not artificially added and a P-value < 0.05 was considered to be significant in all the statistical tests.
 |
Table 1 Characteristics of All Study Groups Presented Separately in Groups with Poor and Favorable Outcomes |
 |
Table 2 Characteristics of Study Groups Presented Separately in Patients with DM (-) and T2DM (+) |
 |
Table 3 Linear Regression Analysis of Relationships Between Short-Term Functional Outcomes and Variables in Non-Cardiogenic Stroke Patients with T2DM (+) |
 |
Table 4 Linear Regression Analysis of Relationships Between Short-Term Functional Outcomes and Variables in Non-Cardiogenic Stroke Patients with DM (-) |
Results
Baseline Characteristics of the Study Population
A total of 314 adult patients (214 males and 100 females) with available data were included in the analysis. The mean age of the patients was 67.98±11.71 years, and the median IMTbif level was 1.40mm (1.10–1.80mm, 25–75% quartile). In addition, the median NIHSS was 3 and the median mRS at 3 months was 2. And 7 patients died during the follow-up. Their deaths were also included and presented as mRS= 6. All the patients were divided into two groups based on functional outcomes and the prevalence of poor outcomes in patients was 35.7%. The findings in Table 1 showed that patients with poor outcomes were significantly older, had higher NIHSS, fasting glucose, SBP and lower haemoglobin values. Moreover, the IMTbif levels of patients with poor outcomes were markedly higher than those with favorable outcomes. As shown in Table 2, no significant differences were found in NIHSS or mRS values between type 2 diabetic and non-diabetic subgroups.
Associations Between the Stratified IMTbif Levels and the mRS Scores
The study divided the included patients into 3 groups according to 3 segmentation points of IMTbif levels, namely: (a) the high IMTbif level (IMTbif >1.6mm); (b) the moderate IMTbif level (IMTbif 1.2–1.6mm) and (c) the low IMTbif level (IMTbif <1.2mm). Moderate IMTbif level was observed in 138 (43.9%) patients and 91 (29.0%) patients had high IMTbif levels. The distribution of functional outcomes at 3 months, according to this stratification method, is shown in Figure 2. The results revealed that a significant difference exists between the three groups, with regard to short-term functional outcomes in post hoc analysis (P =0.001).
 |
Figure 2 Functional outcomes at 3 months stratified for IMT levels at carotid bifurcation. |
Linear Regression Analysis of Relationships Between the mRS Scores and Variables in Patients with and without Type 2 Diabetes
The study population included 122 type 2 diabetic patients and 192 non-diabetic patients. In those patients with T2DM, mRS and NIHSS scores were significantly associated with IMTbif levels after adjusting for homocysteine, haemoglobin and SBP values in the multivariate regression equation (Table 3). Meanwhile, the fasting glucose, haemoglobin values and NIHSS scores were independently associated with mRS scores in non-diabetic group (Table 4).
Discussion
The present study showed that the IMTbif level was independently associated with short-term functional outcome in non-cardiogenic stroke patients with T2DM. After adjusting for confounding factors including NIHSS scores, homocysteine, haemoglobin and SBP values, the linkage of elevated IMTbif with poor outcomes still persisted in those patients with T2DM.
Numerous studies have shown that IMT evolution is linked with the occurrence of cerebrovascular diseases.19 We thus hypothesized that IMTbif was significantly associated with functional outcomes in non-cardiogenic stroke patients, regardless of the complication of T2DM or not. The hypothesis resembles what Lehmann et al have found, although their data were processed using machine learning method.20 In their study, IMT and NIHSS are both strongly associated 3-month outcomes in patients with ischemic stroke. Nevertheless, in our study, the association is only valid in patients with T2DM, but no longer valid in those without diabetes.
We tried to explain this phenomenon. On one hand, large artery and small artery diseases constitute the majority part in non-cardiogenic ischemic stroke subtypes.21 Reflecting initial endothelial dysfunction and subsequent systemic vascular change, IMTbif affects the microcirculation and recanalization, which have an imperative impact on cerebral ischemia, hypoxic injury and neuronal repair.22 Meantime, T2DM is highly established to induce both microvascular and macrovascular abnormalities.23 The complication of T2DM might strengthen the underlying vascular change, which could be represent in IMTbif development in the first place. Thus, the influence of vascular condition has overwhelmed many other conventional risk factors to affect recovery duration and functional outcomes in those coupled with DM. The IMTbif still showed independent association with neurological outcomes in diabetic patients, even taking the widely acknowledged strong stroke severity predictor NIHSS into account as a confounding variable statistically.24
On the other hand, compared with non-diabetic patients, the statistical significance of IMTbif was attenuated upon adjusted for poststroke fasting glucose. Poststroke hyperglycemia is defined as a blood glucose level of 6.1–8.0 mmol/L, even 72 hours after the stroke episode.25 It releases pro-inflammatory mediators (tumor necrosis factor-α, interleukin-6) by stress reaction and alters blood-barrier permeability, leads to blood-barrier disruption, further aggravates brain edema formation and thus affects neural function repair ability.26 Accumulating evidence found that a relatively minor elevation in glucose level might produce an immediate and obvious effect on acute stroke patients’ prognosis without diabetes.27–30 Similar to their findings, our data indicated that metabolic risk factors may overwhelm the influence of underlying vascular changes IMTbif to predict functional disability in non-diabetic patients.
One strength of our study was that the specific posterior wall at carotid bifurcation was selected as the routine ultrasound detection spot to assess IMT. Previous conclusions on the relationships between IMT and vascular diseases are conflicting.4,31–33 We consider that it was likely resulted from the different positioning selection of IMT measurement using ultrasound: the bifurcation of carotid artery,33 the mean value of near and/or far wall of common/internal carotid artery,14 the maximum value of common carotid artery,32 or the combination of these locations. The alterations of detection spots contribute to inconsistent results. As for our research, IMT at carotid bifurcation was chosen as the IMT variable to predict outcomes, same as atherosclerotic burden in Shore’s research.15 This was mainly because: for one thing, autopsy studies have shown that initiation site of IMT in the carotid arteries is carotid bifurcation.34 For another, since low wall shear stress could trigger the development of atherosclerosis, as Strecker C uncovered in his research,7 wall shear stress is significantly lower at posterior wall compared to anterior wall of carotid bifurcation. Thus, we chose the posterior wall at bifurcation as ultrasound target place to represent the underlying vascular change. To note, in order to inhibit the effect of lumen stenosis on hemodynamic changes in arteries, we confine cases to those with carotid stenosis less than 50%.
Another strength of our study was the intention to provide prognostic information. A number of previous studies have tried to investigate relationships among IMT, DM and stroke. But their study mostly utilized IMT to predict the occurrence of vascular events complicated with DM, whereas they did not supply short-term functional outcomes of stroke. For instance, Shore’ team found that measures of IMT are associated with clinically manifest ischemic stroke in subjects with T2DM.15 Kota et al concluded that high IMT is a surrogate and reliable marker of higher risk of non-cardiogenic ischemic stroke amongst T2DM patients.14 However, our study evaluated the value of IMT with functional outcomes in stroke and DM.
Certain limitations exist for the present study. The sample size was small because this was a single-center study. We did not evaluate the influence of DM duration and blood glucose fluctuations during the follow-up. Medications were not included. Therefore, we address that a multi-center study with a large sample size need to be conducted and further verification, including more patients’ information and therapy, is also needed.
Conclusion
In the light of above, the study revealed that IMTbif contributed to the early estimation of short-term functional outcomes in non-cardiogenic ischemic stroke patients with T2DM. Therefore, the ultrasound detection of the IMTbif may be useful as a screening tool for evaluating prognosis of patients with stroke and diabetes.
Ethics Approval
Ethics Committee of Suzhou Municipal Hospital has approved this study, and the permit number is KL901127.
Acknowledgments
This study was financially supported by the Natural Science Foundation of Jiangsu Province, China [grant number BK20200211]. The authors also wish to thank Dr Qiang Du for the excellent methodology assistance.
Disclosure
Xiao-Jing Guo and Mian Wu are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
2. Han Y, Hu Y, Yu C, et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021;42(34):3374–3384. doi:10.1093/eurheartj/ehab413
3. Feigin V, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917
4. Yoon HJ, Kim KH, Park H, et al. Carotid plaque rather than intima-media thickness as a predictor of recurrent vascular events in patients with acute ischemic stroke. Cardiovasc Ultrasound. 2017;15(1):19. doi:10.1186/s12947-017-0110-y
5. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. doi:10.1159/000343145
6. Raggi P, Stein JH. Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: a recommended update to the editorial policy at atherosclerosis. Atherosclerosis. 2020;312:119–120. doi:10.1016/j.atherosclerosis.2020.09.015
7. Strecker C, Krafft AJ, Kaufhold L, et al. Carotid geometry is an independent predictor of wall thickness - a 3D cardiovascular magnetic resonance study in patients with high cardiovascular risk. J Cardiovasc Magn Reson. 2020;22(1):67. doi:10.1186/s12968-020-00657-5
8. Lee SW, Antiga L, Spence JD, Steinman DA. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke. 2008;39(8):2341–2347. doi:10.1161/STROKEAHA.107.510644
9. Jiang P, Chen Z, Hippe DS, et al. Association between carotid bifurcation geometry and atherosclerotic plaque vulnerability: a Chinese atherosclerosis risk evaluation study. Arterioscler Thromb Vasc Biol. 2020;40(5):1383–1391. doi:10.1161/ATVBAHA.119.313830
10. Siri SRA, Braaten T, Jacobsen BK, Melhus M, Eliassen BM. Distribution of risk factors for cardiovascular disease and the estimated 10-year risk of acute myocardial infarction or cerebral stroke in Sami and non-Sami populations: the SAMINOR 2 Clinical Survey. Scand J Public Health. 2018;46(6):638–646. doi:10.1177/1403494818773534
11. Muzurović E, Mikhailidis D. Diabetes mellitus and noncardiac atherosclerotic vascular disease-pathogenesis and pharmacological treatment options. J Cardiovasc Pharmacol Ther. 2021;26(1):25–39. doi:10.1177/1074248420941675
12. Zhu J, Yu X, Zheng Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8(3):192–205. doi:10.1016/S2213-8587(19)30422-X
13. American Diabetes A. 10. cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–S123. doi:10.2337/dc19-S010.
14. Kota S, Mahapatra G, Kota S, et al. Carotid intima media thickness in type 2 diabetes mellitus with ischemic stroke. Indian J Endocrinol Metab. 2013;17(4):716–722. doi:10.4103/2230-8210.113767
15. Shore A, Colhoun H, Natali A, et al. Measures of atherosclerotic burden are associated with clinically manifest cardiovascular disease in type 2 diabetes: a European cross-sectional study. J Intern Med. 2015;278(3):291–302. doi:10.1111/joim.12359
16. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
17. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi:10.2337/dc11-S062.
18. Choi KH, Kim JH, Kang KW, et al. Impact of microbleeds on outcome following recanalization in patients with acute ischemic stroke. Stroke. 2018:STROKEAHA118023084. doi:10.1161/STROKEAHA.118.023084.
19. Willeit P, Tschiderer L, Allara E, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020;142(7):621–642. doi:10.1161/circulationaha.120.046361
20. Lehmann A, Alfieri D, de Araújo M, et al. Carotid intima media thickness measurements coupled with stroke severity strongly predict short-term outcome in patients with acute ischemic stroke: a machine learning study. Metab Brain Dis. 2021;36(7):1747–1761. doi:10.1007/s11011-021-00784-7
21. Adams H, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.str.24.1.35
22. Cahill PA, Redmond EM. Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi:10.1016/j.atherosclerosis.2016.03.007
23. Barbu E, Popescu M, Popescu A, Balanescu S. Inflammation as A precursor of atherothrombosis, diabetes and early vascular aging. Int J Mol Sci. 2022;23(2):963. doi:10.3390/ijms23020963
24. Mistry E, Yeatts S, de Havenon A, et al. Predicting 90-day outcome after thrombectomy: baseline-adjusted 24-hour NIHSS is more powerful than NIHSS score change. Stroke. 2021;52(8):2547–2553. doi:10.1161/strokeaha.120.032487
25. Savopoulos C, Kaiafa G, Kanellos I, Fountouki A, Theofanidis D, Hatzitolios A. Is management of hyperglycaemia in acute phase stroke still a dilemma? J Endocrinol Invest. 2017;40(5):457–462. doi:10.1007/s40618-016-0584-8
26. Plummer M, Deane A. Dysglycemia and glucose control during sepsis. Clin Chest Med. 2016;37(2):309–319. doi:10.1016/j.ccm.2016.01.010
27. Yao M, Ni J, Zhou L, Peng B, Zhu Y, Cui L. Elevated fasting blood glucose is predictive of poor outcome in non-diabetic stroke patients: a sub-group analysis of SMART. PLoS One. 2016;11(8):e0160674. doi:10.1371/journal.pone.0160674
28. Xing L, Liu S, Tian Y, et al. C-R relationship between fasting plasma glucose and unfavorable outcomes in patients of ischemic stroke without Diabetes. J Stroke Cardiovasc Dis. 2019;28(5):1400–1408. doi:10.1016/j.jstrokecerebrovasdis.2019.02.009
29. Yu J, Zhang C, Zhang S, et al. Inordinate glucose variation poststroke is associated with poor neurological improvement in patients without history of diabetes. CNS Neurosci Ther. 2014;20(6):503–508. doi:10.1111/cns.12251
30. Roberts G, Sires J, Chen A, et al. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J Diabetes. 2021;13(12):1034–1042. doi:10.1111/1753-0407.13223
31. Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–2062. doi:10.1016/S0140-6736(12)60441-3
32. Kumar P, Sharma R, Misra S, et al. CIMT as a risk factor for stroke subtype: a systematic review. Eur J Clin Invest. 2020;50(11):e13348. doi:10.1111/eci.13348
33. Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30(4):841–850. doi:10.1161/01.str.30.4.841
34. Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38(10):2698–2705. doi:10.1161/STROKEAHA.107.486480
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
