Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
Influence of Age and Sex on Disease Course and Treatment in Rheumatoid Arthritis
Authors Nilsson J, Andersson MLE , Hafstrom I, Svensson B , Forslind K , Ajeganova S, Leu Agelii M , Gjertsson I
Received 12 February 2021
Accepted for publication 7 May 2021
Published 24 May 2021 Volume 2021:13 Pages 123—138
DOI https://doi.org/10.2147/OARRR.S306378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Jenny Nilsson,1 Maria LE Andersson,2,3 Ingiäld Hafström,4 Björn Svensson,2 Kristina Forslind,2,3 Sofia Ajeganova,4,5 Monica Leu Agelii,1,* Inger Gjertsson1,*
1Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden; 2Lund University, Faculty of Medicine, Department of Clinical Sciences Lund, Rheumatology, Lund, Sweden; 3Spenshult Research and Development Center, Halmstad, Sweden; 4Division of Gastroenterology and Rheumatology, Department of Medicine Huddinge, Karolinska Institutet, and Karolinska University Hospital, Stockholm, Sweden; 5Department of Clinical Sciences, Rheumatology Division. Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium
*These authors contributed equally to this work
Correspondence: Monica Leu Agelii
Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, Gothenburg University, Box 480, Gothenburg, 40530, Sweden
Tel +46- 31-342 4692
Email [email protected]
Objective: More than 50% of patients with rheumatoid arthritis (RA) are > 65 years at diagnosis. Age of onset and sex may influence the disease course, outcome and treatment. This study follows a large cohort of patients with early RA to assess contributions of age and sex to disease outcomes.
Methods: Patients from the BARFOT cohort, n=2837 (68% women), were followed for eight years at predefined time points to assess inflammation, function, joint destruction and treatment with disease modifying anti-rheumatic drugs (DMARDs) and glucocorticoids (GC). The patients were divided by sex and age at inclusion (< 40, 40– 54, 55– 69 and ≥ 70 years).
Results: For both sexes, disease activity, function and pain improved over time, significantly more in men than in women in all age groups. In men, those < 40 years displayed significantly lower DAS28 compared with all other groups. This group was also the least represented group in the study. The Sharp van der Heijde Score (SHS) increased over time in both sexes and all age groups. Women ≥ 70 years showed less improvement in disability and the highest progression of SHS mainly due to increased joint space narrowing. Patients < 40 years were more likely to receive biological DMARDs, while those ≥ 70 years more often received only GC treatment.
Conclusion: There were significant age- and sex-dependent differences in the medical treatment and in outcome of RA 8 years after diagnosis. The differences were most pronounced in men< 40 and women ≥ 70 years, but whether they are due to disease phenotype or treatment is unclear.
Keywords: rheumatoid arthritis, disease course, treatment, age- and sex-dependent differences
Plain Language Summary
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that often results in joint destruction and disability. It affects approximately 1% of the population and more often women than men. The demographics of the Western world are changing and today, more than 50% of RA patients are older than 65 years at diagnosis.
The aim of this study was to look at the influence of age and sex on the outcome and treatment of RA. To this end, we have examined a cohort of 2837 RA patients from disease onset and over the following 8 years.
The patients were divided into groups by sex and age at inclusion (<40, 40–54, 55–69 and ≥70 years). For all patients, disease activity and pain improved over time, but joint destruction increased. Generally, men were doing better compared to women. Women ≥70 years had a more destructive disease compared with all other patients. Biologic anti-rheumatic drugs were introduced during the collection of the cohort and were mainly prescribed to younger patients, preferentially men. Less than 20% of the patients were treated with glucocorticoids only, but the majority of these patients were ≥70 years.
We suggest that advanced age, especially for women, should be considered a risk factor for worse disease outcome and that specific treatment guidelines are desirable for patients ≥70 years at disease onset.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that often results in joint destruction and disability. The prevalence is 0.5–1% in European and North-American populations. In Sweden, the prevalence of RA is 0.77% and the overall incidence is 41 per 100,000 inhabitants, which peaks between 70–79 years. The prevalence of RA is 2–3 times higher in women than in men, but this ratio decreases with age at onset.1 Women generally have a worse outcome than men with respect to disease activity2,3 and disability,3 while joint destruction is the same for women and men.2,4 The demography in the Western world is changing and more than 50% of all patients with RA are over 65 years of age at diagnosis.1 Aging is associated with increased prevalence of cardiovascular disease and osteoporosis, both of which are overrepresented as comorbidities in patients with RA compared with the general population.4 They contribute, alongside RA per se, to the impairment of quality of life, and this combination of morbidities magnifies the socioeconomic costs of supporting patients with RA relative to healthy individuals.5
International treatment guidelines recommend that treatment with disease modifying anti-rheumatic drugs (DMARDs) at diagnosis, irrespective of sex and age and the presence of poor prognostic factors, should be pursued actively.6,7 Although the guidelines do not discriminate on the basis of sex or age, younger patients seem to receive more treatment with both conventional synthetic (cs) and biological (b) DMARDs,8–10 whereas elderly patients are more likely to receive glucocorticoids (GC) only11 and less likely to receive DMARD12 especially bDMARDs.11–14
Previous studies found that age at diagnosis and sex may influence the choice of treatment in RA. However, they were performed during different time periods, had varying lengths of follow-up and varying age stratifications, suggesting a need for further studies. Long-term follow-up studies are necessary to study the disease outcome. However a long-term study means that changes in treatment strategies as well as classification criteria may occur. In this study, the patients were grouped according to sex and age at inclusion and followed for eight years. The rationale for the age group division at disease onset was based on estimated sex hormone levels. Women have the highest incidence of RA around the age of menopause and for men there is an increase in incidence after 40 years of age.2 Sex hormones are believed to contribute to the pathogenesis of RA. The age groups were: < 40 years (no menopause; normal testosterone levels), 40–54 years (woman enters menopause; testosterone levels might start to slowly decrease), 55–69 years (all women have entered menopause; testosterone levels continue to slowly decrease) and ≥70 years (other factors such as comorbidities become important).2 In this paper we examined the impact of age and sex on the disease course as to disease activity, function, joint destruction and treatment in the BARFOT (Better Anti Rheumatic Pharmaco Therapy) long-term cohort that included patients with early RA.
Method
Patients
This study included 2837 BARFOT patients (1916 women and 921 men). BARFOT is a multicenter longitudinal observational study of RA patients in southern Sweden.15 In short, patients with newly diagnosed RA were consecutively included between 1992 and 2006; inclusion criteria were disease duration of 12 months or less and fulfilling the ACR 1987 classification criteria.16 The patients were assessed at inclusion, 3 and 6 months, at 1, 2, 5 and 8 years according to the same structured protocol, see “Clinical assessments” below. Assessments of the 15-year outcomes are ongoing. Smoking habits (current, previous or never smoker) and rheumatic factor (RF) according to respective hospital standards were assessed at inclusion. As antibodies to cyclic citrullinated peptides (anti-CCP) were not routinely collected at the standard time-points, for the majority of patients they were determined subsequently from serum samples taken at inclusion. Seropositive patients were defined as being RF positive, anti-CCP positive or positive for both these markers, whereas seronegative patients were those negative for both, or RF negative with anti-CCP status unknown.
The patients were grouped according to sex and age at inclusion. The age groups were: < 40 years, 40–54 years, 55–69 years and ≥70 years.
Clinical Assessments
Disease activity was assessed through the composite disease activity score (DAS28), calculated on swollen and tender 28-joint counts (SJC and TJC), erythrocyte sedimentation rate (ESR) and patient’s assessment of global health on a visual analogue scale (GH-VAS) range 0–100 mm (best to worst).17 Similarly, patients reported pain on a VAS (pain-VAS) and functional disability using the Swedish version of Stanford Health Assessment Questionnaire, HAQ, range 0–3.18 The radiographs of hands and feet were scored chronologically using the Sharp van der Heijde scoring method (SHS) by two experienced readers who were blinded to clinical data. The SHS provides separate scores for joint space narrowing (JSN) and erosions (erosion score, ES).19
Medical Treatment
Treatments were registered as: no DMARD, methotrexate only, sulfasalazine only, other csDMARD, combination of at least two csDMARDs or bDMARD alone or in combination. Whether or not the patient received GC was also recorded. At inclusion, the patients were treated with DMARDs in accordance with the recommended therapy strategy in Sweden at that time. This strategy specified in early 1990s initial DMARD monotherapy and early use of low-dose GC, and a “step-up” combination therapy reserved for those with a more severe disease, and from early 2000s recommended the possibility to initiate treatment with biologics when the first DMARD or combination of DMARDs failed.
Ethics Approval and Consent to Participate
The study complied with the Declaration of Helsinki and was approved by the Regional Ethical Review Boards at Gothenburg University (Ggb Ö 282-01), Lund University (398–01) and Karolinska Institute (02–075, T2016/297-31/1). Informed, written consent was obtained from the participants before enrolment.
Statistical Analysis
The patients were grouped according to sex and age at inclusion (< 40 years, 40 −54, 55–69 and ≥ 70 years). For each sex, the median for continuous variables was compared between age groups with the Kruskal–Wallis test at inclusion, 1 and 8 years. At the same time points, categorical variables were compared according to the chi-square of independence. To facilitate comparisons with other published studies, the mean values are also presented. For continuous variables, 95% confidence interval of the median was calculated using bootstrap samples (2000 samples generated with replacement). For proportions, the standard error of proportion was used. To counteract inflation of type I error due to multiple testing, a Bonferroni-Holm20 correction was used for each time point individually. The p-values are ranked from smallest to highest and each p-value is compared against a significance level that is adjusted for the number of multiple tests as well as the rank of the p-value: α(rank)=α/(n-rank+1), where α is set to 0.05 and n is the number of multiple tests. The analyses included all patients, regardless of whether they had been assessed at all time-points or not. The term significant is used for differences that are statistically significant at a 95% confidence level.
All statistical analyses were conducted with MATLAB R2015a (MATLAB and Statistics Toolbox Release 2015a, The MathWorks, Inc., Natick, Massachusetts, United States) and SPSS Statistics Version 25 (IBM Corporation).
Results
General Characteristics of the Study Population at Onset of Disease
Figure 1 gives an overview of the number of participants with available DAS28 data at each follow-up time together with causes of exclusion between the visits. Of the participants present at inclusion, 22% were not available for the 8-year examination. Among the 624 patients who were not assessed at 8 years, the main causes for non-participation were death (70%), unwillingness to participate (9%), development of other disease (6%), relocation (6%), and unknown causes (9%). The non-participating patients were on average 11 years older at inclusion (mean age 66 years, p<0.001), more likely to be men (27% of the men vs 20% of the women were missing, p<0.001). An additional 265 patients had missing DAS28 data at this time point even though they were still included in the study (no information available regarding the cause of missing data).
The patients in this new onset RA cohort had a median age of 60 years (range 15–93 years) at inclusion, 68% were women and 67% were seropositive (Table 1). In total, 33% were current smokers. The median DAS28 score of participants at inclusion was 5.3, containing a median presentation of 7 tender joints and 10 swollen joints. The most represented age group among both women and men was the 55–69 years-old, with 634 (33%) women and 355 (39%) men. The least represented group were younger men, n=78 (9% of the male participants). The ratio of women to men decreased with age, being 4:1 in the youngest age group falling to less than 2:1 among those older than 70 years (Tables 2 and 3). Sex differences are summarized in Table 4.
 |
Table 1 Demographic, Clinical and Radiographic Characteristics of All Patients, and Divided into Women and Men at Inclusion |
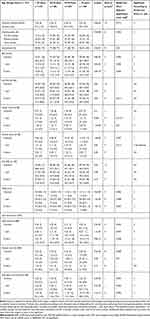 |
Table 2 Clinical Measures for Women at Inclusion, 1 Year and 8 Years |
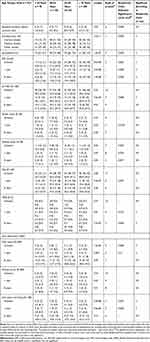 |
Table 3 Clinical Measures for Men at Inclusion, 1 Year and 8 Years |
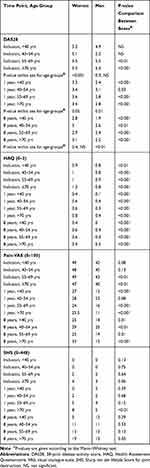 |
Table 4 Clinical Outcomes, Given as Median Values, Compared Across Age Groups and Between Sexes |
Autoantibodies, Symptom Duration Before Diagnosis and Smoking Habits
The results are shown separately in women (Table 2) and men (Table 3). The majority of the patients studied were seropositive, with the lowest proportions in the age group ≥ 70 years (women 56%: men 51%) and the highest in the age group 40–54 years, (women 73%: men 78%). The median time between self-reported onset of symptoms and inclusion was shortest for women and men ≥ 70 years, and longest for women < 40 years. Smoking habits at inclusion differed significantly between age groups for both women and men: for both sexes, the proportion of “never smokers” was largest in the age group < 40 years (57%). Except for those < 40 years, more men than women were smokers. The proportion of “current smokers” within age groups differed between the sexes, with most women smokers in the age group 55–69 years (32%) and most men smokers in the ≥ 70 age group (50%).
Disease Activity Measured as DAS28 and Its Components
Figure 2 shows the median DAS28 for women (A) and men (B) over 8 years from diagnosis. The number of participants on which the DAS28 statistics were calculated are presented in Supplementary Table 1, by sex and age group. In all age groups and for both sexes DAS28, as well as number of tender and swollen joints, decreased most during the first year after inclusion in the study. At inclusion, DAS28 differed significantly between age groups for women ((p<0.001, Figure 2A and Table 4) and women aged older than 55 years had the highest DAS28 (median 5.5, Table 4). For men, DAS28 at inclusion did not vary significantly across age groups, but men <40 years had a significantly lower disease activity over time compared with men from other age groups (Figure 2B), due to lower ESR and GH-VAS (Tables 2 and 3). The DAS28 components are shown in Tables 2 and 3; ESR increased significantly with age at inclusion and after 1 and 8 years, for both sexes. Significant differences in GH-VAS between the age groups were detected only in men at 1 year. The number of swollen joints increased significantly with age at inclusion and after 1 year in women, but not in men. There was no significant difference in tender joints at inclusion, either in men or women. The number of tender joints increased very slightly, but significantly, with age in women at 1 and 8 years.
Pain and Disability
In both sexes and in all age groups, pain-VAS and HAQ decreased during the first year. After 1 year, pain-VAS was relatively constant in both women and men within the age groups (Table 2 and Table 3). A significant difference between age groups was only seen in men at 1 year; men < 40 years and men ≥ 70 years had the lowest scores and men 40–54 years had the highest. Men < 40 years had significantly lower HAQ after one year compared with all other groups. HAQ was higher among women compared with men for all age groups and at all time points (p<0.001, Table 4). For both sexes, the most favorable HAQ was seen for those < 40 years (p<0.001).
Joint Destruction
At one year after inclusion, 2613 (92%) patients were assessed and of those 71% had radiographic assessment of hands and feet. At the 8-year follow-up, 2213 patients (78%) were assessed and 73% of these had radiographic assessments.
The SHS differed significantly between age groups for women at all time points (Table 2) and at inclusion and 1 year for men (Table 3). Joint destruction increased steadily over time, and women ≥ 70 years consistently had the highest SHS at all time points compared with all other groups. Men < 40 years had less joint destruction at inclusion and at 1 year compared with all other groups (Table 3). There were no differences in SHS between men and women under the age of 70 years, whereas older women had marginally higher SHS than older men (Table 4).
Medical Treatment
Figure 3 shows the percentage of patients receiving methotrexate only, sulfasalazine only or bDMARD alone or in combination (Figure 3 A-F) and/or GC (Figure 3 G–H). Methotrexate was the most common csDMARD, and bDMARDs were the least common treatment at inclusion. The usage of bDMARDs increased over time and younger patients were more likely than older patients to receive bDMARDs. Treatment with GC, given alone or together with a DMARD, was similar across all age groups and between sexes, except that they were less often prescribed for men < 40 years. Less than 20% of the patients received GC in monotherapy, however those patients were preferentially women and men ≥ 70 years (Supplementary Figure 1). At 8 years, men ≥ 70 years had, compared with all other groups, the highest proportion of no treatment, ie neither DMARD nor GC.
Discussion
This study investigated the influence of sex and age of onset on the disease course and treatment of more than 2000 early RA patients during 8 years. The striking result that emerges is the great consistency of disease courses measured (Figure 2) in view of the variety of management strategies covered (Figure 3, Supplementary Figure 1). All patients showed significant improvement in most clinical measures immediately on commencement of treatment and throughout the first year, after which disease activity (DAS28) remained fairly constant. Nonetheless, we find that our choice of 4 age bands, formulated specifically to match the maturation of hormone profiles in patients of both sexes, not only provides a useful breakdown of the stages of disease ensuing for patients of different age at onset, but also reveals sex differences that may otherwise be masked.
The notable example of such a sex difference is in the group of men <40 years old at onset: from 6 months and onwards, they showed more improvement in DAS28 than all other groups, both male and female, despite there being no significant difference at inclusion. Nevertheless, at 8 years, there was no difference in joint destruction compared to older men. However, this is the age group that was least represented in our study (n=78, representing 9% of the male participants and only 3% of the entire BARFOT cohort) and these results should be interpreted with care, as these findings might be confounded by the low sample size. At inclusion, women older than 55 years had a significantly elevated median DAS28 compared with age-matched men. During the eight years of follow-up, there was almost no difference in DAS28 between the age groups in women, whereas men younger than 40 years consistently had a lower DAS28 compared with the other age-groups. Tender and swollen joint count decreased strongly and consistently throughout the follow-up and were between 0 and 1 for all groups at 8 years. As previously reported,4 the remaining components of DAS28, ESR and GH-VAS, had a greater impact on DAS28 and might not reflect just joint inflammation. It is however noteworthy that ESR in both men and women falls dramatically on commencement of treatment, in all age groups. Moreover, our results are in line with the knowledge that ESR increases with age, particularly in women,21 which could mean that the higher DAS28 in older women was probably attributed to the measures of disease activity rather than to the disease activity per se. Thus, differences in age and sex should be taken into account when interpreting DAS28.
Women generally had higher HAQ compared with men as has been previously found.22,23 Camacho et al found that a sex-independent higher rate of increased disability (HAQ) associated with increased age of symptom onset. The same study showed that after 5 years the HAQ scores were higher than at baseline for patients 55 years and older.22 Arnold et al13 found that older-onset patients (≥64 years) start and end the first year after diagnosis worse in terms of DAS28 and HAQ compared with younger patients. In the current study, irrespective of age, HAQ decreased during the first year and was thereafter relatively stable Women ≥ 70 years showed a slight increase after 1 year but the HAQ scores at 8 years were still below baseline level at inclusion.
In contrast to all other measures, radiographically assessed joint destruction increased steadily over time. In women, the SHS increased significantly with age group at all time points, mainly due to joint space narrowing. However, joint space narrowing is not specific for RA. Coexisting osteoarthritis has been shown to contribute to a higher joint space narrowing score at inclusion in patients over 55 years of age relative to their younger counterparts.24 In men, the increase with age was significant at inclusion and after one year but this difference was no longer evident at the 8 years timepoint. Previously, Ahlmén et al25 found no significant differences in SHS between women and men, whereas other studies found men to have a greater radiological destruction at 24 months compared with women.12 In the present study, we found no significant differences between men and women, except for the ≥ 70 years women who had higher SHS than men at all time points. Further, this group had consistently the highest SHS among women. There was no difference in the erosion score for men and only a marginal increase for women older than 55 years at one-year follow-up. This indicates that the SHS changes seen in women ≥ 70 years were mainly due to increased joint space narrowing. Hence, by introducing age as a factor, the different trajectories of changes in joint architecture experienced by men and women are disclosed.
For the individual patient, treatment is chosen after careful considerations that include disease activity, age, estimated prognosis, desire to have children for those of reproductive age, and comorbidities. As expected, methotrexate was by far the most commonly used DMARD in the BARFOT cohort. We found that the proportion of younger patients, in particular women less than 40 years, who were treated with methotrexate was lower compared with all other age groups. This could be due to the fact that Methotrexate has teratogenic side effects and is not prescribed before conception or during pregnancy and lactation.26 bDMARDs were introduced during the collection of the BARFOT cohort, which means that only the patients included during 1999–2006 (about half of the patients) had access to treatment with bDMARDs. As seen in other studies,11–14 the prescription of bDMARDs was highest for younger patients and decreased with age at onset. GC in monotherapy was used in < 20% of the patients, however, the patients who were most likely to receive this treatment were women and men ≥ 70 years. Patients younger than 40 years were the least likely to receive GC as the only treatment. Otherwise, no differences for GC as monotherapy or in combination with a DMARD were found. Our findings are in coherence with an epidemiological study on DMARD prescription, covering 87% of the German population, which found that treatment during the first year after onset of RA differed with respect to both sex and age;14 women were less likely to receive cDMARD, bDMARD and GC during the first year compared with men. The same study found that patients ≥ 65 years were less likely to receive DMARDs and more likely to receive only GC compared with patients < 35 years even though these patients had the highest DAS28 levels.4 These findings were contradicted in a large multinational cohort of patients with manifest RA, where no differences in treatment strategies between the sexes were found even though women had a worse disease outcome.3 One possible explanation for GC as monotherapy is the occurrence of comorbidities, since RA patients with late onset have a heavier burden of comorbidities compared with younger ones.11 Notably, GC treatment has been reported to be more common in patients with late onset.11,12
Access to bDMARDs and targeted synthetic DMARDs have improved the disease outcome immensely and today the treatment aim is clinical remission, or at least low disease activity.7 The perfect treatment is an act of balance between too little and too much immunosuppression, and particularly among older RA patients there is often a tension between the likely benefit of treating the active disease and the risk of adverse events due to frailty or comorbidities.27,28
Separating out those participants who were >70 years old at diagnosis confirmed previous reports that the frequency of RF was lower in the elderly, who more frequently had large joint involvement and disease distribution was more equal between the sexes.1,29 Thus, our study reinforces the question posed by other previous studies whether RA in elderly is the same disease as RA in younger persons.12,13
Our study has some limitations. The patients were collected to the BARFOT cohort 1992–2006 and treatment strategies as well as classification criteria have changed. Long-term follow-up studies of RA outcomes are necessary and these limitations are unavoidable. As shown earlier, the patients included in the 2000s (half of the patients) were more often treated with MTX and biologics than those included in 1990s.30 However, despite greater reduction in disease activity for the more actively treated patients, the gender difference persisted during 8 years, with men achieving lower DAS28 than women. Therefore we suggest the present findings also to be valid for more actively treated patients and more recent cohorts.
Comorbidities such as hypertension and diabetes may have influenced at least partially the responsiveness to glucocorticoids. All patients, irrespective of comorbidity, were included in the study. However, the information regarding comorbidities was not consistently recorded, which makes it impossible to estimate their impact on our results.
Of the participants present at inclusion, 22% were not available for the 8-year examination. The non-participating patients were on average 11 years older at inclusion and more likely to be men. They did not differ from the participants in regard to any of the disease activity indicators, with the exception of JSN at inclusion (median 2 vs 0 in the participants, p<0.001) which is non-specific for RA and may indicate comorbidity for osteoarthritis and associate with higher age. Taken together, the loss of non-participants might have rendered our findings regarding age differences at 8-years as slightly conservative, potentially more so for men. The BARFOT cohort encompasses more than 2800 patients. However, the division of the patients into eight subgroups resulted in small numbers of participants in some of the subgroups, especially for the younger men which is an important group for the purpose of this study. This shortcoming limited both the number of statistical analyses we were able to perform and the power of those we performed, and the results regarding younger men should be interpreted with caution. Nonetheless, we consider this approach to have provided us with useful insights.
The aim of this study was to investigate the influence of sex and age on disease development and treatment choices, but our understanding of the influence of age alone on disease development is blurred by the variety of treatment choices across age groups in the cohort.
Conclusions
This large cohort study of patients with early RA over the 8 years following their diagnosis reveals that the disease course for all ages and both sexes was similar, regardless of the details of treatments given, but useful insight was provided by differential analysis using specific function-related age bands and sex as factors.
Men < 40 years of age had the most favorable functional outcome over the study period. The significantly lower DAS28 over time was manifest despite no obvious difference at diagnosis. This group (together with women < 40 years) was also more likely to be treated with bDMARDs. Results for this group should be cautiously interpreted due to the low sample size.
Women ≥70 years experienced the smallest improvement with respect to disability and joint destruction over the study period, and were, together with men in the same age group, more likely to receive treatment with only GC.
Whether the differences in outcome are due to the phenotype of the disease or its treatment is not clear. However, late age onset might be a risk factor for a worse disease outcome and appropriate treatment is important irrespective of age. Our results indicate that there is a need for specific treatment guidelines for patients older than 70 years.
Acknowledgment
Kristina Albertsson, for assessment of radiographs, and the BARFOT Study Group.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of rheumatoid arthritis in Sweden: a nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken). 2013;65(6):870–878. doi:10.1002/acr.21900
2. Tengstrand B, Ahlmen M, Hafstrom I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J Rheumatol. 2004;31(2):214–222.
3. Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11(1):R7. doi:10.1186/ar2591
4. van Onna M, Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord. 2016;17(1):184. doi:10.1186/s12891-016-1038-3
5. Eriksson JK, Johansson K, Askling J, Neovius M. Costs for hospital care, drugs and lost work days in incident and prevalent rheumatoid arthritis: how large, and how are they distributed? Ann Rheum Dis. 2015;74(4):648–654. doi:10.1136/annrheumdis-2013-204080
6. Singh JA, Saag KG, Bridges SL
7. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
8. van Hulst LT, Kievit W, van Bommel R, van Riel PL, Fraenkel L. Rheumatoid arthritis patients and rheumatologists approach the decision to escalate care differently: results of a maximum difference scaling experiment. Arthritis Care Res (Hoboken). 2011;63(10):1407–1414. doi:10.1002/acr.20551
9. Ogasawara M, Tamura N, Onuma S, et al. Observational cross-sectional study revealing less aggressive treatment in Japanese elderly than nonelderly patients with rheumatoid arthritis. J Clin Rheumatol. 2010;16(8):370–374. doi:10.1097/RHU.0b013e3181fe8b37
10. Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65(9):1226–1229. doi:10.1136/ard.2005.051144
11. Oishi S, Wendling D, Sibilia J, et al. Treatment of active rheumatoid arthritis: comparison of patients younger vs older than 75 years (CORPUS cohort). Hum Vaccin Immunother. 2018;14(11):2612–2617. doi:10.1080/21645515.2018.1522470
12. Innala L, Berglin E, Moller B, et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2014;16(2):R94. doi:10.1186/ar4540
13. Arnold MB, Bykerk VP, Boire G, et al. Are there differences between young- and older-onset early inflammatory arthritis and do these impact outcomes? An analysis from the CATCH cohort. Rheumatology (Oxford). 2014;53(6):1075–1086. doi:10.1093/rheumatology/ket449
14. Steffen A, Holstiege J, Klimke K, Akmatov MK, Batzing J. Patterns of the initiation of disease-modifying antirheumatic drugs in incident rheumatoid arthritis: a German perspective based on nationwide ambulatory drug prescription data. Rheumatol Int. 2018;38(11):2111–2120. doi:10.1007/s00296-018-4161-7
15. Hafstrom I, Ajeganova S, Andersson ML, et al. A Swedish register-based, long-term inception cohort study of patients with rheumatoid arthritis - results of clinical relevance. Open Access Rheumatol. 2019;11:207–217. doi:10.2147/OARRR.S218448
16. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
17. Prevoo ML, van ‘T Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi:10.1002/art.1780380107
18. Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the stanford health assessment questionnaire. Scand J Rheumatol. 1988;17(4):263–271. doi:10.3109/03009748809098795
19. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–263.
20. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–508. doi:10.1111/opo.12131
21. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J (Clin Res Ed). 1983;286(6361):266. doi:10.1136/bmj.286.6361.266
22. Camacho EM, Verstappen SM, Lunt M, Bunn DK, Symmons DP. Influence of age and sex on functional outcome over time in a cohort of patients with recent-onset inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Care Res (Hoboken). 2011;63(12):1745–1752. doi:10.1002/acr.20609
23. Andersson ML, Forslind K, Hafstrom I. Comparing five year out-come in two cohorts of patients with early rheumatoid arthritis - A BARFOT Study. Open Rheumatol J. 2015;9(1):8–15. doi:10.2174/1874312901409010008
24. Khanna D, Ranganath VK, Fitzgerald J, et al. Increased radiographic damage scores at the onset of seropositive rheumatoid arthritis in older patients are associated with osteoarthritis of the hands, but not with more rapid progression of damage. Arthritis Rheum. 2005;52(8):2284–2292. doi:10.1002/art.21221
25. Ahlmen M, Svensson B, Albertsson K, Forslind K, Hafstrom I. Influence of gender on assessments of disease activity and function in early rheumatoid arthritis in relation to radiographic joint damage. Ann Rheum Dis. 2010;69(1):230–233. doi:10.1136/ard.2008.102244
26. Favalli EG, Biggioggero M, Crotti C, Becciolini A, Raimondo MG, Meroni PL. Sex and management of rheumatoid arthritis. Clin Rev Allergy Immunol. 2019;56(3):333–345. doi:10.1007/s12016-018-8672-5
27. Serhal L, Lwin MN, Holroyd C, Edwards CJ. Rheumatoid arthritis in the elderly: characteristics and treatment considerations. Autoimmun Rev. 2020;19(6):102528. doi:10.1016/j.autrev.2020.102528
28. Frisell T, Baecklund E, Bengtsson K, et al. Patient characteristics influence the choice of biological drug in RA, and will make non-TNFi biologics appear more harmful than TNFi biologics. Ann Rheum Dis. 2018;77(5):650–657. doi:10.1136/annrheumdis-2017-212395
29. Kobak S, Bes C. An autumn tale: geriatric rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10(1):3–11. doi:10.1177/1759720X17740075
30. Andersson MLE, Forslind K, Hafstrom I. Patients with early rheumatoid arthritis in the 2000s have equal disability and pain despite less disease activity compared with the 1990s: data from the BARFOT Study over 8 years. J Rheumatol. 2017;44(6):723–731. doi:10.3899/jrheum.161235
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



