Back to Journals » Cancer Management and Research » Volume 13
Influence Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: Hashimoto’s Thyroiditis Has a Weak Effect on Central or Lateral Lymph Node Metastasis
Authors Zhou L, Chen G, Sheng L , Liu N, Zhang B, Zeng Q, Chen B
Received 12 March 2021
Accepted for publication 24 April 2021
Published 14 May 2021 Volume 2021:13 Pages 3953—3961
DOI https://doi.org/10.2147/CMAR.S310773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Liguang Zhou,1 Gang Chen,2 Lei Sheng,3 Nan Liu,3 Bin Zhang,3 Qingdong Zeng,3 Bo Chen3
1Department of Ultrasound, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China; 2Department of General Surgery, Shandong Guoxin Healthcare Group Xinwen Center Hospital, Taian, People’s Republic of China; 3Department of Thyroid Surgery, General Surgery, Qilu Hospital of Shandong University, Jinan, 250012, People’s Republic of China
Correspondence: Bo Chen
Department of Thyroid Surgery, General Surgery, Qilu Hospital of Shandong University, Wenhuaxi Road 107#, Jinan, 250012, People’s Republic of China
Tel + 86-531-82165383
Email [email protected]
Purpose: To analyze the effect of Hashimoto’s thyroiditis (HT) concurrent with papillary thyroid cancer (PTC) on cervical lymph node metastasis (LNM).
Methods: Two thousand nine hundred twenty-six patients who underwent thyroidectomy and lymph node dissection between January 2015 and December 2018 were enrolled in this study. Patient demographics and clinicopathologic features were analyzed.
Results: Of the total enrolled patients, 598 (20.4%) had concurrent HT. There were 1482 PTC cases with N0, 1033 cases with N1a, and 411 cases with N1b. Patients with HT had lower frequency of extrathyroidal extension (ETE), lymphatic vascular (LV) invasion, high pathological T stage (III+IV) and central LNM rate. Stratifying central LNM by non-ETE or without intrathyroidal spreading, it was further found that central LNM rate in patients with HT was lower than that of patients without HT. However, there was no significant difference in the central LNM rate in patients with PTC stratified by ETE or intrathyroidal spreading. HT with PTC played a weak protective role in N1a, reducing the risk of N1a by 16.4%. Conversely, HT is a risk factor for N1b, increasing the risk by 1.336 times compared to patients without HT. TgAb is an independent risk factor for N1b, which appears related to the promotion of N1b by HT.
Conclusion: In PTC, HT has a protective effect on central LNM and a risk effect on lateral LNM, although the difference was not significant. This weak protective effect on N1a is more obvious in PTC with less aggressive clinicopathologic characteristics. The risk effect of HT on N1b may be associated with TgAb.
Keywords: Hashimoto’s thyroiditis, papillary thyroid cancer, lymph node metastasis, influence factor, extrathyroidal extension, intrathyroidal spreading
Introduction
Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis,1 is the most common inflammatory thyroid disease and the leading cause of hypothyroidism. HT is a problem worldwide, and in developed countries affects 0.1–5% of the population.2–5 HT is characterized by increased TPOAb and/or circulating TgAb, diffuse swelling, uneven echo of parenchymal tissue on ultrasound, follicular cell atrophy, and lymphocytic infiltration pathologically. HT is thought to arise due to a combination of genetic susceptibility and environmental risk factors, which determine the breakdown of immunological tolerance6 and create a favorable setting for malignant transformation.7
Papillary thyroid carcinoma (PTC) is the most common cancer of the endocrine system.1,8,9 In 1955, Dailey et al10 first suggested an association between HT and PTC, linking chronic inflammation to neoplastic changes. Since then, numerous epidemiological studies have confirmed a high level of coexistence between HT and PTC, ranging from 20% to 85%.11–14 In patients with thyroid nodules, the nodules are 2.766 times more likely to be associated with PTC in patients with HT than in those without HT (OR =2.766; 95% CI 1.947–3.929; P < 0.001).15 HT is an endogenous carcinogen for thyroid cancer.1 A recent study with a large sample size confirmed that the rate of incidental PTC in patients with HT undergoing surgical treatment for benign thyroid disease was much higher than that in patients with nodular goiter or with Graves’ disease.16
Lymph node metastasis (LNM) is generally regarded as an indicator of the progression and aggressiveness of malignant tumors. The presence of an autoimmune disease facilitates the occurrence of neoplastic lesions, but it is not entirely clear whether the disease predisposes to cancer development per se or whether it may provide a protective barrier against its spread as LNM or local recurrent disease.17–20 Several studies revealed that patients with PTC and coexisting HT exhibited less aggressive clinicopathologic characteristics, including lower rates of ETE and LNM.14,19–21 However, other reports have shown no relationship between the presence of HT and PTC aggressiveness, including LNM.18,22,23 Even Konturek et al found that patients with PTC and HT had significantly more lymph nodes and metastatic lymph nodes.12
To date, there has been no definite and clear position formulated on predisposition to the development or inhibition of LNM in patients with PTC and HT.
This study was designed to explore the demographic and pathological features of patients presenting with PTC with and without HT, to analyze independent factors for cervical LNM, and to determine the role of PTC concurrent with HT in LNM.
Materials and Methods
Patients
We analyzed the data of consecutive patients with thyroid disease undergoing surgery in our hospital between January 2015 and December 2018. Approval for this study was obtained from the ethics and IRB committee of Qilu Hospital of Shandong University and this study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained retrospectively from patients when they came to the office for follow-up.
Exclusion Criteria
The exclusion criteria were: patients with PTC who did not undergo lymph node dissection (LND); patients who were missing clinicopathological data; patients with recurrent PTC; patients with other benign thyroid diseases and other pathologic types of thyroid cancer; patients positive for thyroid-stimulating hormone receptor antibodies (TRAb); patients with Graves’ disease in their medical history; and patients with an absence of clinical, ultrasound, and morphological signs of HT.
Inclusion Criteria
The inclusion criteria were: patients who underwent thyroidectomy and central lymph node dissection and/or lateral lymph node dissection; patients with PTC confirmed by routine pathology; and patients with data that could be obtained from medical records and analyzed for various clinicopathologic factors, including demographics, tumor features, and postoperative pathological details.
Diagnosis of HT
The final diagnosis of HT was based on the histopathological gold standard of diffuse lymphocytic infiltration with numerous lymphoid follicles and the presence of germinal centers.
The serological or ultrasound evidence had a moderate concordance with the gold standard histological findings.24 If pathological diagnoses were not performed and we had tissue in the tissue bank for that specific patient, we proceeded with a pathological diagnosis when TPOAb was 2.11-fold above the upper reference range value, which is the optimal cutoff, or when ultrasound showed a diffusely enlarged gland with a thickened isthmus and uneven internal echo.
Laboratory Methods
TPOAb and TgAb were measured by chemiluminescent microparticle immunoassay (CMIA, Abbott Laboratories). All thyroid autoantibody measurements were performed following the manufacturer’s recommended procedure. The TgAb measurement range was 1–1000 IU/mL with a normal range of 0–4.11 IU/mL in our institution. The TPOAb measurement range was 1–1000 IU/mL with a normal range of 0–5.61 IU/mL.
Surgery
All patients underwent general anesthesia with intubation and were placed in a supine position with necks hyperextended. The operation was performed as we described previously.25–27 Open surgery was performed in the neck with a transverse arc-shaped incision, and the incision length was determined by the scope of lymph node dissection. Endoscopic thyroid surgery was performed using a modified anterior chest approach or a breast approach. Thyroidectomy was performed according to the meticulous capsular dissection technique. In our center, all patients underwent central lymph node dissection (CLND) regardless of suspected lymph nodes. In other words, we perform prophylactic CLND in patients with clinically node negative (cN0) PTC. CLND was performed cranially to the two superior thyroid arteries and the pyramidal lobe, caudally to the innominate vein, laterally to the carotid sheaths, and dorsally to the prevertebral fascia. Lateral lymph node dissection included at least the II a, III, IV, and V b compartments.
LN Status
Based on the LN status in patients with PTC, patients without positive LNs were classified as group N0, those with only central LNM but no lateral LNM were classified as group N1a, and those with lateral LNM regardless of central LNM were classified as group N1b.
Statistical Analysis
SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL) was used for data analysis and statistical significance was defined as P < 0.05. Continuous variables were first tested for normality. If the data followed a normal distribution, continuous variables were shown as means ± standard deviation (SD), and were analyzed using t-tests. If the data did not follow a normal distribution, median-interquartile ranges (IQR) were used for the Wilcoxon signed-rank test. Categorical variables were compared using the χ2 test or Fisher’s exact test. Logistic regression was used for multivariate analysis. The parallel test of LN status, such as N0, N1a, and N1b, was conducted first. If P > 0.05, the orderly classification logistics regression was adopted and if P < 0.05, the multinomial logistic regression analysis was adopted for multivariate analysis.
Results
From January 2015 to December 2018, a total of 3704 patients underwent surgery for thyroid disease. Among them, 778 patients met the exclusion criteria and were not included in this study. A flow chart of data collection is shown in Figure 1.
 |
Figure 1 Flowchart depicting data collection and division of groups according to lymph node status. |
A total of 2926 patients with PTC were finally enrolled in our study. In this cohort there were 2279 female (77.9%) and 647 male (22.1%) patients. The median age was 45 years. Of the included patients, 598 had concurrent HT and the rate of PTC concurrent with HT was 20.4%. Cervical lymph node status indicated that there were 1482 cases with N0, 1033 cases with N1a, and 411 cases with N1b.
Demographics and Pathological Characteristics of Patients with PTC Stratified by HT
There were 598 PTC patients with concurrent HT and 2328 patients without HT. We compared their demographics and pathological characteristics in Table 1.
 |
Table 1 Demographics and Pathological Characteristics of Patients with PTC Stratified by Hashimoto’s Thyroiditis |
Tumor size, multifocal lesions, unilateral or bilateral lesions, intrathyroidal spreading, lateral LNM, number of positive lateral LNs, and number of total lateral LNs were comparable in the PTC group with HT and the PTC group without HT (all P > 0.05). The group with HT was significantly associated with lower frequency of males (p < 0.001), classical subtype (p = 0.038), ETE (p = 0.006), lymphatic vascular (LV) invasion (p = 0.011), central LNM (p = 0.002), and high pathological T stage (III+IV) (p = 0.006). Compared with the group without HT, the group with HT also had the following characteristics: younger age (p = 0.049); increased TPOAb (p < 0.001); increased TgAb (p < 0.001); increased TSH (p < 0.001); less positive central LNs (p = 0.037) and more total central LNs (p < 0.001).
We further stratified the patients by invasive indicators such as ETE and intrathyroidal spreading and found that the central LNM rate in patients with HT is lower than that in patients without HT only under the condition of a tumor without ETE or intrathyroidal spreading. However, for patients with ETE or intrathyroidal spreading, the central LNM rate had no significant difference regardless of HT (Table 2).
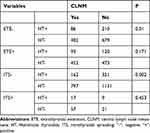 |
Table 2 The Central LNM Rate in Patients with and without HT Stratified by ETE or Intrathyroidal Spreading |
Clinical Pathological Factors for Lymph Node Status in the Cervical Compartment (pN0, pN1a, or pN1b)
Univariate analysis revealed significant differences among N0, N1a, and N1b groups in terms of age (p < 0.001), sex (p < 0.001), TPOAb (p = 0.018), TgAb (p = 0.001), HT (p = 0.008), ETE (p < 0.001), tumor size (p < 0.001), LV invasion (p < 0.001), intrathyroidal spreading (p < 0.001), focality (p < 0.001), unilateral or bilateral lesions (p < 0.001), and PTC pathological subtype (p = 0.033). TSH did not significantly differ between N0, N1a, and N1b groups (p = 0.213) (Table 3).
 |
Table 3 Univariate Analyses of the Clinical Pathological Factors for Lymph Node Status in the Cervical Compartment (pN0, pN1a, and pN1b) |
To exclude the influence of each variable factor, we performed multinomial logistic regression analysis of LNM after parallel line testing of dependent variables. With reference to N0, independent factors for N1a were tumor size (OR, 2.101; 95% CI, 1.794–2.460, P < 0.001), age (OR, 0.972; 95% CI, 0.964–0.979, P < 0.001), female (OR, 0.521; 95% CI, 0.423–0.642, P < 0.001), ETE (OR, 1.446; 95% CI, 1.216–1.719, P < 0.001), and intrathyroidal spreading (OR, 2.480; 95% CI, 1.578–3.896, P < 0.001) (Table 4).
 |
Table 4 Analyses of the Clinical Pathological Factors for PTC Patients with pN1a or pN1b Using Multinomial Logistic Regression |
With reference to N1a, independent factors for N1b were TgAb (OR, 1.001; 95% CI, 1–1.001, P = 0.009), tumor size (OR, 1.832; 95% CI, 1.585–2.117, P < 0.001), female (OR, 0.738; 95% CI, 0.565–0.963, P = 0.025), ETE (OR, 1.415; 95% CI, 1.098–1.825, P = 0.007), and intrathyroidal spreading (OR, 2.295; 95% CI, 1.580–3.332, P < 0.001) (Table 4).
The Effect of PTC Concurrent with HT on LN Status (pN0, pN1a, and pN1b)
Multinomial logistic regression analysis showed that HT was not an independent factor for N1a or N1b. However, HT concurrent with PTC played a weak protective role in N1a (N0 as a reference), reducing the risk of N1a by 16.4% compared with patients with PTC without HT (OR, 0.846; 95% CI, 0.667–1.072; P = 0.167). However, compared with patients with PTC without HT, HT is a risk factor for N1b compared in patients with PTC and HT, and increases the risk 1.336 times (N1a as a reference) (OR, 1.336; 95% CI, 0.952–1.876; P = 0.094) (Table 4).
Discussion
The activated inflammatory response present in HT may result in oncogene mutations that eventually lead to the occurrence of PTC.1,7,8,28 A visible increase in the incidence of PTC and HT co-existence has been noticed over the past 20 years. In this study, the rate of PTC with HT was 20.4%, which is consistent with the 20% to 85% range reported previously.7,8,11,13,29 Patients with PTC and HT have different demographics than do those without HT. In this study, the HT group had a higher frequency of patients that were female, of a younger age, with increased TSH, TgAb, and TPOAb. These results are consistent with other reports.14,15
The association between HT and PTC behavior has been a topic of interest for researchers. Our results show that PTC with HT is less often associated with factors that indicate aggressive tumor behavior such as ETE, LV invasion, TNM stage (III+IV), central LNM rate and the number of positive central LNs, which are consistent with those presented in many other studies.14–16,19–21,25,30 These studies suggest, paradoxically, that HT lymphocytic infiltrate might be an immunological response with a cancer-retarding effect, contributing to a favorable outcome for patients with PTC. However, other studies have shown that HT does not attenuate any aggressive factors such as tumor size, ETE, or multifocal lesions, indicating that there is no relationship between HT and PTC aggressiveness.15,23,31,32
In our study, we routinely performed prophylactic CLND in PTC patients with cN0. Therefore, only 15 patients who did not undergo CLND were excluded from our study. The strategy ruled out the selective bias resulted from CLND of benign lymph node enlargement in HT patients. Patients with PTC were divided into three groups, N0, N1a, and N1b, based on their LN status. We also performed a parallel line test and found that the severity of LNM was not equally spread among the three groups, suggesting that multinomial logistic regression could be used to identify the independent influencing factors for each group. Not surprisingly, the risk factors for N1a and N1b in PTC included larger tumors, being male, ETE, younger age, and intrathyroidal spreading, all of which are recognized LNM risk factors that we validated previously.26
Regarding the effect of HT on LNM, a meta-analysis showed that PTC with HT was related to the absence of LNM, but there is significant statistical heterogeneity among the studies.11 Liang proposed that patients with PTC and HT had early-stage disease and were associated with a low rate of central and lateral LNM (19.6% vs 9.5%) by a chi-square test,14 but they did not explore whether HT was an independent risk factor for LNM using multivariate analysis. Lee found that HT was not an independent predictor of central LNM in multivariate analysis, although central LNM was less frequent in patients with PTC and HT than in patients with PTC without HT in univariate analysis.1 In this study, univariate analysis revealed a significant difference in LNM between the group with HT and that without HT. When age, gender, tumor size, ETE, and intrathyroidal spreading were adjusted, we found HT was not an independent risk factor for LNM. Although the difference was not significant, we found that PTC with HT had a weak inhibitory effect on central LNM, reducing the risk of N1a by 16.4% compared with PTC without HT, and that HT had a weak promoting effect on lateral LNM, increasing the risk of N1b by 1.336 time (N1a as a reference). It was slightly statistically significant in the effect of HT in promoting lateral LNM, compared with the observed inhibitory effect on central LNM, which was trending toward significance (P = 0.094).
Combined with central LNM stratified by ETE and intrathyroidal spreading, we further found that patients with HT had a significantly lower central LNM rate compared with patients without HT only in the absence of aggressive features. There was a comparable central LNM rate in patients with ETE or intrathyroidal spreading regardless of HT. Jara studied central LNM in PTC stratified by demographic and histopathologic characteristics and found that the central LNM rate stratified by female or T1a stage was significantly lower in patients with PTC and HT than that in those with PTC without HT.33 However, there was no observed difference in the central LNM rate in patients with PTC stratified by being male or other T stages that are factors of tumor aggression. Another study consistently reported that the LNM rate of the HT group was significantly lower than that of the non-HT group in terms of PTC with T1, but there was no difference in LNM in T2 and T3 between the two groups.12 These findings suggested that the protective role of HT in central LNM rate was only associated with early stage, or PTC with low aggressiveness, and that this protection may be offset or reversed by factors of tumor progression or high aggressiveness, which is consistent with our results.
Similarly, tumors associated with lateral LNM are more aggressive, so HT did not have a weak protective effect against lateral LNM, which can be shown in central LNM. We speculate that the autoimmune inflammation in HT may be exploited by the metastatic mechanism of PTC with aggressiveness. The immune background of HT involves activation of T-helper and T-cytotoxic lymphocytes and over production of TPOAb and TgAb antibodies. Based on our results, TgAb is an independent risk factor for lateral LNM, as consistent with the other study.34 In this study, the 95% confidence interval of TgAb was relatively narrow, with an OR value of 1.001. This is because clinical data showed a large span of TgAb. Although TgAb was an independent risk factor for N1b, the risk only increased by 1.001 times for every unit increase in the value. Circulating TgAb is associated with HT, and is present in 60–80% of patients with HT. Both retrospective studies and a prospective study suggest that the association of HT with PTC progression is antibody-specific.29,35,36 PTC patients with positive serum TgAb had more aggressive disease and less favorable long-term outcomes than did demographically similar patients without circulating TgAb.37 It is possible that the presence of thyroid autoimmune antibodies makes it easier for differentiated thyroid cancer cells expressing thyroid-specific antigen to locate to the lymph node when they invade the lymphatic vessel.37 Vasileiadis et al found a positive correlation between TgAb and LNM in patients with PTC.38 There was no significant relationship between TSH level and PTC aggressiveness, including LNM.8 This is consistent with our result that TSH was comparable among N0, N1a, and N1b groups and was not an independent factor for LNM.
To the best of our knowledge, this is the first time that the specific role of HT in cervical LNM and its potential mechanism have been described. Additional strengths of this study include the large sample size and the access to pathology reports for all patients. In our study, all cases eventually needed to be confirmed by paraffin section, which improved the diagnostic accuracy of HT. The limitations of this work are that it is a retrospective analysis and that some immunological basic lab studies were not available. We plan to commence a prospective study that includes these data to obtain a better understanding of the potential role of HT on PTC and the immunological connections to central LNM and lateral LNM.
Conclusion
In PTC, HT has a protect effect on central LNM and is a risk factor for lateral LNM, although the differences were not statistically significant. This weak protective effect on N1a is more obvious in PTC with less aggressive clinicopathologic characteristics. The risk effect of HT on N1b may be associated with TgAb. The immune response of HT may have a weak protective effect on initial LNM, but may be exploited by other high-risk factors for LNM during tumor progression. Further studies are needed to obtain a better understanding.
Data Sharing Statement
- The datasets generated and/or analyzed during the current study are not publicly available but may be obtained from the corresponding author upon reasonable request.
Grants and Fellowships
No grants or fellowships supported writing of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee IKH, Soh EY, Lee J. The association between chronic lymphocytic thyroiditis and the progress of papillary thyroid cancer. World J Surg. 2020;44(5):1506–1513. doi:10.1007/s00268-019-05337-9
2. Teng WSZ, Teng X, Guan H, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783–2793. doi:10.1056/NEJMoa054022
3. Rallison ML, Dobyns BM, Meikle AW, Bishop M, Lyon JL, Stevens W. Natural history of thyroid abnormalities: prevalence, incidence, and regression of thyroid diseases in adolescents and young adults. Am J Med. 1991;91(4):363–370. doi:10.1016/0002-9343(91)90153-o
4. Ragusa F, Fallahi P, Elia G, et al. Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101367. doi:10.1016/j.beem.2019.101367
5. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301–316. doi:10.1038/nrendo.2018.18
6. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335(2):99–107. doi:10.1056/NEJM199607113350206
7. Tamimi DM. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002;10(2):141–146. doi:10.1177/106689690201000207
8. Sulaieva O, Chernenko O, Chereshneva Y, Tsomartova D, Larin O. Thyroid stimulating hormone levels and BRAFV600E mutation contribute to pathophysiology of papillary thyroid carcinoma: relation to outcomes? Pathophysiology. 2019;26(2):129–135. doi:10.1016/j.pathophys.2019.05.001
9. Gilmartin ARM. Incidence of thyroid cancer among patients with thyroid nodules. Ir Med J. 2018;111(8):802.
10. Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70(2):291–297. doi:10.1001/archsurg.1955.01270080137023
11. Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto’s thyroiditis and papillary thyroid carcinoma risk. Oncotarget. 2017;8(37):62414–62424. doi:10.18632/oncotarget.18620
12. Konturek A, Barczynski M, Wierzchowski W, Stopa M, Nowak W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2013;398(3):389–394. doi:10.1007/s00423-012-1021-x
13. Konturek A, Barczynski M, Nowak W, Wierzchowski W. Risk of lymph node metastases in multifocal papillary thyroid cancer associated with Hashimoto’s thyroiditis. Langenbecks Arch Surg. 2014;399(2):229–236. doi:10.1007/s00423-013-1158-2
14. Liang J, Zeng W, Fang F, et al. Analisi clinica dell’associazione fra tiroidite di Hashimoto e carcinoma papillare della tiroide in 1392 pazienti. [Clinical analysis of Hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients]. Acta Otorhinolaryngol Ital. 2017;37(5):393–400. doi:10.14639/0392-100X-1709
15. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168(3):343–349. doi:10.1530/EJE-12-0903
16. Paparodis RD, Karvounis E, Bantouna D, et al. Incidentally discovered papillary thyroid microcarcinomas are more frequently found in patients with chronic lymphocytic thyroiditis than with multinodular Goiter or Graves’ disease. Thyroid. 2020;30(4):531–535. doi:10.1089/thy.2019.0347
17. Lun Y, Wu X, Xia Q, et al. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013;148(3):396–402. doi:10.1177/0194599812472426
18. Song E, Jeon MJ, Park S, et al. Influence of coexistent Hashimoto’s thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2018;88(1):123–128. doi:10.1111/cen.13475
19. Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011;22(3):144–149. doi:10.1007/s12022-011-9164-9
20. Kim EY, Kim WG, Kim WB, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009;71(4):581–586. doi:10.1111/j.1365-2265.2009.03537.x
21. Moon S, Chung HS, Yu JM, et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: a meta-analysis of observational Studies. Endocrinol Metab (Seoul). 2018;33(4):473–484. doi:10.3803/EnM.2018.33.4.473
22. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93(3):809–814. doi:10.1210/jc.2007-2215
23. Del Rio P, Cataldo S, Sommaruga L, Concione L, Arcuri MF, Sianesi M. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008;33(1):1–5.
24. Guan H, de Morais NS, Stuart J, et al. Discordance of serological and sonographic markers for Hashimoto’s thyroiditis with gold standard histopathology. Eur J Endocrinol. 2019;181(5):539–544. doi:10.1530/EJE-19-0424
25. Chen B, Wang Y, Xuan S, et al. Endoscopic thyroidectomy: the development in a Chinese center. J Laparoendosc Adv Surg Tech A. 2012;22(1):76–80. doi:10.1089/lap.2011.0336
26. Sheng L, Shi J, Han B, et al. Predicting factors for central or lateral lymph node metastasis in conventional papillary thyroid microcarcinoma. Am J Surg. 2019;220(2):334–340. doi:10.1016/j.amjsurg.2019.11.032
27. Liu N, Chen B, Li L, Zeng Q, Lv B. Subplatysmal or subfascial approach in totally endoscopic thyroidectomy has better postoperative efficacy for voice, sensory, swallowing symptoms and cosmetic result. Cohort study. Int J Surg. 2018;60:22–27. doi:10.1016/j.ijsu.2018.10.034
28. Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98(2):474–482. doi:10.1210/jc.2012-2978
29. Azizi G, Keller JM, Lewis M, et al. Association of Hashimoto’s thyroiditis with thyroid cancer. Endocr Relat Cancer. 2014;21(6):845–852. doi:10.1530/ERC-14-0258
30. Dong S, Xia Q, Wu YJ. High TPOAb levels (>1300 IU/mL) indicate multifocal PTC in Hashimoto’s thyroiditis patients and support total thyroidectomy. Otolaryngol Head Neck Surg. 2015;153(1):20–26. doi:10.1177/0194599815581831
31. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001;25(5):632–637. doi:10.1007/s002680020165
32. Kim KW, Park YJ, Kim EH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto’s thyroiditis. Head Neck. 2011;33(5):691–695. doi:10.1002/hed.21518
33. Jara SM, Carson KA, Pai SI, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery. 2013;154(6):
34. Jo K, Kim MH, Ha J, et al. Prognostic value of preoperative anti-thyroglobulin antibody in differentiated thyroid cancer. Clin Endocrinol (Oxf). 2017;87(3):292–299. doi:10.1111/cen.13367
35. Kim ES, Lim DJ, Baek KH, et al. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid. 2010;20(8):885–891. doi:10.1089/thy.2009.0384
36. Azizi G, Malchoff CD. Autoimmune thyroid disease: a risk factor for thyroid cancer. Endocr Pract. 2011;17(2):201–209. doi:10.4158/EP10123.OR
37. Shen CT, Zhang XY, Qiu ZL, et al. Thyroid autoimmune antibodies in patients with papillary thyroid carcinoma: a double-edged sword? Endocrine. 2017;58(1):176–183. doi:10.1007/s12020-017-1401-7
38. Vasileiadis I, Boutzios G, Charitoudis G, Koukoulioti E, Karatzas T. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann Surg Oncol. 2014;21(8):2725–2732. doi:10.1245/s10434-014-3593-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
