Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 7
Infectious peritonitis profile in peritoneal dialysis at Ibn Sina University Hospital: a 6-year data report
Authors Bekaoui S, Haddiya I , Houthi MS, Berkchi F, Ezaitouni F, Ouzeddoun N, Bayahia R, Benamar L
Received 25 December 2012
Accepted for publication 15 November 2013
Published 2 August 2014 Volume 2014:7 Pages 323—327
DOI https://doi.org/10.2147/IJNRD.S42069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Samira Bekaoui, Intissar Haddiya, Maria Slimani Houti, Fatima Zahra Berkchi, Fatima Ezaitouni, Naima Ouzeddoun, Rabia Bayahia, Loubna Benamar
Department of Nephrology, Dialysis, and Renal Transplantation, Ibn Sina University Hospital, Rabat, Morocco
Background: Infectious peritonitis (IP) is the most common complication in peritoneal dialysis (PD). The purpose of this study is to assess the prevalence of IP and to determine its clinical, biological, and evolutive characteristics.
Patients and methods: We conducted a five year, five months retrospective study from July 2006 to December 2011. All patients on peritoneal dialysis that have been followed on PD for a minimum of 3 months and who presented IP during follow-up were included. Data were analyzed using SPSS 17.0.
Results: The 76 episodes of IP were identified in 36 patients. The peritonitis rate (months × patients/peritonitis), as calculated by the Registre de Dialyse Péritonéale de Langue Française (RDPLF Registry) [French peritoneal dialysis registry] in December 2011, was 18.59. Time to occurrence of peritonitis from the start of peritoneal exchange was 15.44±10 months. The mean age of our patients was 49.1±16.8 years [10–80]: the youngest patient's age was 10, while the oldest was 80 years old (male to female: sex ratio M/F=1,66). Also, 22% of our patients were diabetic. The mean follow-up in PD was 22.6±14 months. Abdominal pain was present in 79% of the cases. Fever and vomiting were noted in 42% and 38% of cases, respectively. The C-reactive protein rate was elevated in 77% of cases, and leukocytosis was found in 27% of cases. Bacteriological proof was present in 73.68% of cases. Gram-positive cocci were involved in 56.6% of microbiologically proven IP cases. Gram-negative bacilli were represented in 37.7%. The outcome was favorable in 89.4%. The PD catheter was removed in 2.63% of the cases. In addition, 7.89% of our patients were transferred to hemodialysis.
Discussion: The rate of IP remains high in our series. More than one-half of the peritonitis cases with positive cultures (56.6%) were caused by Gram-positive cocci. Gram-negative bacilli ranked second (27.7%). These results agree with data in the literature. Moreover, the rate of culture-negative IP in our series is high (26%). Evolution is good in most cases (89%).
Conclusion: Despite the gradual decrease of its rate, peritonitis remains frequent in our center and calls for optimization of means of prevention. The high frequency of negative culture IP in our study urges us toward better collaboration with biologists to target antibiotic therapy and improve IP management.
Keywords: peritoneal dialysis, infectious peritonitis, hemodialysis
Introduction
Peritoneal dialysis (PD) is a renal replacement therapy method that offers several advantages in end-stage renal disease.1 It was first used in Morocco during the 1980s and then rapidly abandoned. However, thanks to a pilot project at Rabat University Hospital, this technique was reintroduced in 2006 in response to medical and social imperatives.2 Since then, we have acquired considerable experience. Currently, PD has an established role in the treatment of end-stage renal disease in Morocco – especially for patients from remote areas where hemodialysis (HD) centers are not available or for those who present with a contraindication to HD such as lack of vascular access and congestive heart failure.
Morocco has a total population of 35,245,000 inhabitants.3 The gross national product and the gross domestic product per capita are estimated at $127 billion US and $5.10 US, respectively.4
In 2007, the Moroccan National Registry of Transplants and Dialysis (MAGREDIAL)5 estimated the incidence of end-stage renal disease to be 100–150 per million inhabitants and the prevalence of HD treatment at 162 per million inhabitants in peripheral regions of the country and 250 patients per million in the center of the country. Regarding renal transplantation, currently, 220 patients have benefited from renal transplantation in Morocco.
Infectious complications, especially infectious peritonitis (IP), are still a matter of concern in our PD practice because of their frequency and the consequences that may ensue. The purpose of this study is to evaluate the prevalence of IP and to determine its clinical, biological, and evolutive characteristics.
Patients and methods
PD technique
Since the opening of the PD unit in July 2006 at the Rabat University Hospital, 61 patients have been placed under continuous ambulatory peritoneal dialysis.
We used the straight double Dacron® cuff Tenckhoff catheter. The catheter was inserted using the median minilaparotomy technique performed by a team member surgeon. Perioperative prophylactic antibiotic therapy (vancomycin 1 g) was administered. Information on the PD technique regarding exchanges, asepsis recommendations, and IP signs is delivered to the patient by our PD team, which includes two nurses and a nephrologist. The training is usually performed in small groups.
The exchanges start three weeks after the initial presentation, except in the case of a dialysis emergency. We usually start our PD program with four daily exchanges, and then we adjust this number, according to the peritoneal equilibration test.
Methods
In January 2012, in the PD unit of the Rabat University Hospital, we conducted a 6-year retrospective study from July 2006 to December 2011. All patients on peritoneal dialysis, who have been followed on PD for a minimum of 3 months and who presented IP during follow-up, were included.
The diagnosis of IP was based on cloudy dialysate containing a white cell level exceeding or equal to 100 elements/mm³.
The IP treatment was based on the protocol of the International Society of Peritoneal Dialysis (ISPD):6 triple probabilistic antibiotherapy, administered via intraperitoneal route and composed of third-generation cephalosporin (ceftazidime 1 g/day) targeting Gram-negative bacilli (GNB), particularly Pseudomonas; an antistaphylococcus of the vancomycin type 1 g/4 days or kefzol 1 g/day; and an antibiotic acting in synergy with these two antibiotics, of the gentamycin type, 160 mg, then 80 mg/48 hours for 6 days (for a total of three doses). This antibiotic therapy was later adjusted, according to the results of microbiological tests. The therapy was continued for 15 days after clearing of the dialysate fluid. Fluconazole was prescribed in some situations to prevent the fungal peritonitis that is secondary to prolonged antibiotic therapy.
We evaluated the IP rate and analyzed its different characteristics:
- Epidemiological characteristics – We collected the following data from the patients’ medical records: age, gender, initial nephropathy, length of follow-up on PD, and the time to the first peritonitis episode from the start of the peritoneal exchanges.
- Clinical characteristics – We searched for the presence of fever, abdominal pain, and vomiting.
- Biological characteristics – We recorded the presence of leukocytosis and elevated C-reactive protein levels.
- Microbiological characteristics – We recorded the results of bacteriological and mycological tests of the dialysate fluid (direct examination and cultures on special environments).
- Evolutive characteristics – Favorable evolution was defined as clearing of the dialysate fluid before the end of the first week of antibiotic. Bad evolution conducted to catheter withdrawal. Moreover, catheter withdrawal is performed systematically in the case of fungal peritonitis.
The statistical analysis was performed using SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Quantitative data was expressed by means ± standard deviation, and qualitative data was expressed by percentages.
Results
During the 68-month study period, we observed 76 episodes of infectious peritonitis in 36 patients. The time to the occurrence of peritonitis from the start of the peritoneal exchange was 15.44±10 months (-1 – 52). The peritonitis rate (months × patients/peritonitis) calculated by the Registre de Dialyse Péritonéale de Langue Française (the French peritoneal dialysis registry) (RDPLF Registry) was 18.59; this rate diminished over the years of the study (Figure 1). The mean patient age was 49.1±16.83 years (17- 84), with a predominance of males (sex ratio:1.66). Also, 22 percent of the patients were diabetic. Mean follow-up time on PD was 22.6±14.1 months (3 -51).
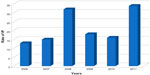  | Figure 1 Rate of IP. |
Clinically, abdominal pain was present in 79% of cases; vomiting and fever were noted in 42% and 38% of cases, respectively.
Biological testing revealed leukocytosis in 27% of cases, with a predominance of polynuclear neutrophils in 98% of cases and lymphocytes in 2% of cases. C-reactive protein levels were elevated in 77.7% of cases.
Microbiological confirmation was obtained in 73.68% of cases (56 cases). Dialysate culture was negative in 26.32% of cases (20 cases). Of the 56 microbiologically documented cases of IP, 94.64% (53 cases) were monomicrobial. In 5.35% of cases (three cases), we found a polymicrobial infection. Of the 53 monomicrobial IP cases, Gram-positive cocci (GPC) were found in 56.6% of cases (30 cases); GNB was found in 37.7% of cases (20 cases). Coagulase-negative staphylococcus (CNS) was responsible for 53.3% of IP cases due to GPC. We found no cases of either methicillin-resistant Staphylococcus or of multidrug-resistant Pseudomonas. Tables 1 and 2 summarize the microbiological profile of the monomicrobial IP cases collected. Three monomicrobial IP were caused by Bacillus, Koch’s Bacillus, and Candida albicans.
  | Table 1 Distribution of GPC |
  | Table 2 Distribution of GNB |
Moreover, three polymicrobial IP-associated CNS and group B streptococcus, CNS and Pseudomonas, and Pseudomonas and group B streptococcus evolution were favorable in 68 cases (89.4%). Eight cases of IP (10.6%) were refractory to antibiotic therapy, requiring a catheter change in two cases (2.63%), and six cases (7.89%) of definitive transfer to HD. There were no cases of septicemia or death following IP.
Discussion
In PD, IP is the most common complication, with its frequency more marked in continuous ambulatory peritoneal dialysis than in automated PD.7 In our series, the mean IP rate is 18, which is close to the ISPD-recommended rate.6 This rate remains high in comparison to the series published in the literature in Arab countries, such as Algeria8 (25.4%) and Tunisia9 (33.4%). However, our IP rate is better than the one reported from Sudan10 (14%). During the 6 years of our study, the peritonitis rate decreased from that found at the opening of the PD unit. It went from one infectious peritonitis episode every 13 patient-months in 2006 to one episode every 34 patient-months by the end of the study.
At the beginning of our PD program in June 2006, high peritonitis rates were mostly due to our lack of experience and knowledge of this technique. Furthermore, the team members involved in PD only included a nephrologist and a nurse. Besides, this technique was performed in an inappropriate, small, narrow place in our dialysis unit.
Being aware of these constraints, in 2008, we started some changes regarding our PD management. We first registered to the RDPLF Registry to share and to analyze our experience. Since 2009, we have been displaying our results in meetings and congresses. At the same time, we got in touch with international PD experts for advice. We also recruited more doctors (nephrologists) and nurses to work exclusively in DP and got a larger space with more equipment, such as lavatories and closets.
In 2010, continuous medical education focused on PD. Finally, a special attention was drawn to the exit site and the catheter placement, and we established protocols regarding catheter management.
More than one-half of the IP cases with positive cultures were caused by GPC. GNB ranked second. These results agree with data in the literature.8,11–13
GPC were mainly represented by CNS. The GNB were represented predominantly by Pseudomonas aeruginosa. This finding is similar to the results reported from Algeria.8 The study of the antibiotic sensitivity of the microorganisms showed that wild strains were involved. Indeed, all the staphylococci were methicillin-sensitive, and we found no cases of multidrug-resistant organisms, even among the GNB (Pseudomonas aeruginosa), which suggests the community origin of the bacterial species isolated. Considering the habitual reservoir of the latter, it may be supposed that contamination occurs essentially via the cutaneous or environmental flora of the patient, rather than from endogenous flora.
Pluribacterial peritonitis is rare: 4.6% in the Algerian series of Ammari et al,11 9.4% in the French series of Laurain et al,12 and 5.35% in our series. Classically, this type of IP is considered to have an unfavorable evolution, but this was not the case for the three IP episodes in our study, which progressed well. However, this observation is conditioned by the fact that in two cases out of the three, there was an association of GPC, which is supposed to have a better prognosis than an association of GNB.14
The rate of negative-culture peritonitis in our series was 26.3%, which remains high when compared to the ISPD6 guidelines that require a rate inferior to 20%. In Algeria,8 this rate is estimated at 14%. In the Sudan,10 it is at 53%. This could be partially explained by the fact that some of our patients, who live in remote areas, may take antibiotics before coming to the hospital to meet the bacteriological tests.
IP is the primary cause of transfer to HD. According to the RDPLF Registry,10 IP accounts for 22.2% of all causes of definitive transfer to HD. In our study, six patients (7.89%) were definitively transferred to HD, although other causes (physical intolerance, associated pathologies, etc) were often involved in the transfer decision.
IP rarely results in death – only 4% in the ISPD.6 Fungal and GNB peritonitis lead to a higher mortality than those due to GPC.14 In our series, no death occurred in the course of IP.
IP is the major concern of PD practitioners because of the complications that it may engender. Therefore, IP prevention is crucial and relies on aseptic insertion of the PD catheter, prevention of exit-site infection, search for and treatment of the chronic nasal carriage of the Staphylococcus aureus, and not only proper patient training on hygiene measures and manipulation errors, but also the periodic retraining of patients and personnel involved in PD care.
Conclusion
Despite the gradual decrease of its rate, peritonitis remains frequent in our center and calls for optimization of means of prevention. The high frequency of negative-culture IP in our study urges us toward better collaboration with biologists to target antibiotic therapy and to improve IP management.
Disclosure
The authors report no conflicts of interest in this work.
References
Ryckelynck JP, Lobbedez T, Ficheux M, et al. [News in peritoneal dialysis]. Presse Med. 2007;36(12 Pt 2):1823–1828. French [with English abstract]. | |
Haddiya I, Skalli Z, Lioussfi Z, et al. [Peritoneal dialysis: a satisfactory experience of a misknown technique in Rabat University Hospital]. Nephrol Ther. 2010;6(7):569–575. French [with English abstract]. | |
Demographics of Morocco available at URL: http://en.wikipedia.org/wiki/Demographics of Morocco. | |
Morocco DGP per capita available at URL: http://www.indexmundi.com/morocco/gdp per capita (ppp).html. | |
Magredial ministère de la santé. Available at: http://www.sante.gov.ma/…/colloque%20magredial/…/3-magre. | |
Li PK, Szeto CC, Piraino B, et al; International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393–423. | |
Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl. 2006;(103):S55–S62. | |
Chelghoum S, Arzour H, Khellaf G, et al. [Peritonitis in peritoneal dialysis: microbiological characteristics and causes]. Nephrol Ther. 2011;7(5):325. French. | |
Mahfoudh H, Jarraya F, Hdiji A, et al. [Peritoneal dialysis in Sfax (Tunisia): An eight-year data report]. Nephrol Ther. 2011;7(5):322. French. | |
Elhassan EA, Kaballo B, Fedail H, et al. Peritoneal dialysis in the Sudan. Perit Dial Int. 2007;27(5):503–510. | |
Ammari H, Ghaffor M. [Bacteriology of peritonitis in continuous ambulatory peritoneal dialysis patients (CAPD)]. Revue française des laboratoires. 2005;369:35–39. French. | |
Laurain C, Durand PY, Albert M, et al. [Infection peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: microbiological review during a four-year period]. Pathol Biol (Paris). 2004;52(10):575–578. French [with English abstract]. | |
Verger O, Ryckelynck JP, Duman M, et al. French peritoneal dialysis registry (RDPLF): outline and main results. Kidney Int Suppl. 2006;(103):S12–S20. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
