Back to Journals » OncoTargets and Therapy » Volume 9
Induction of apoptosis by Armillaria mellea constituent armillarikin in human hepatocellular carcinoma
Authors Chen Y , Chen C, Huang H
Received 9 January 2016
Accepted for publication 20 May 2016
Published 1 August 2016 Volume 2016:9 Pages 4773—4783
DOI https://doi.org/10.2147/OTT.S103940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Faris Farassati
Yu-Jen Chen,1–4 Chien-Chih Chen,5 Huey-Lan Huang6
1Department of Medical Research, 2Department of Radiation Oncology, Mackay Memorial Hospital, 3Institute of Traditional Medicine, School of Medicine, National Yang-Ming University, 4Institute of Pharmacology, Taipei Medical University, Taipei, 5Department of Biotechnology, HungKuang University, Taichung, 6Department of Bioscience Technology, College of Health Science, Chang Jung Christian University, Tainan, Taiwan
Abstract: Armillaria mellea is a honey mushroom often used in the traditional Chinese medicine “Tianma”. Currently, this medicinal mushroom is also used as a dietary supplement in numerous Western and Eastern countries. Armillarikin was isolated from A. mellea, and we previously discovered that it induced cytotoxicity in human leukemia cells. In this study, we further investigated the cytotoxicity of armillarikin against liver and intrahepatic bile duct cancer cells. Armillarikin was cytotoxic against human hepatocellular carcinoma Huh7, HA22T, and HepG2 cells based on the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium and alamarBlue® assays. Armillarikin treatment also induced the collapse of the mitochondrial transmembrane potential of these cells. Furthermore, armillarikin-induced apoptotic cell death was demonstrated by sub-G1 chromosomal DNA formation by using flow cytometry. In addition, the apoptosis was inhibited by the pan-caspase inhibitor, Z-VAD-fmk. Immunoblotting also revealed the armillarikin-induced activation of procaspase-3, -8, and -9 and upregulation of the apoptosis- and cell cycle arrest-related phospho-histones 2 and 3, respectively. Moreover, reactive oxygen species scavengers also inhibited the armillarikin-induced apoptosis in human hepatocellular carcinoma, suggesting that reactive oxygen species formation played an important role in the armillarikin-induced apoptosis of human hepatocellular carcinoma. In conclusion, our study indicates the potential of armillarikin as an effective agent for hepatoma or leukemia therapies.
Keywords: armillarikin, Armillaria mellea (A. mellea), hepatocellular carcinoma (HCC), apoptosis, reactive oxygen species (ROS)
Introduction
Armillaria mellea is a golden colored honey mushroom that belongs to the Tricholomataceae family of fungi. It is grown in numerous areas of the world, including the US. It is an edible mushroom that is used for its medicinal and health-promoting properties worldwide. For example, the fermented mycelia extracts are used as dietary supplement products. In Chinese herbal medicine, A. mellea is an important component of “Tianma” (“Tian-ma” or “Tien-ma”, literally translated as “heavenly hemp”)1–3 because it grows symbiotically with Gastrodia elata blume. The orchid G. elata relies on A. mellea to provide its nutrition and both have become common components of Tianma. In Chinese herbal medicine, A. mellea is indicated for the treatments of palsy, dizziness, hypertension, headache, insomnia, vertigo, and neurasthenia.
Over the past few decades, hepatocellular carcinoma (HCC, also called malignant hepatoma) has been one of the five most deadly cancers worldwide4 and is especially prevalent in less developed countries. For example, liver cancer was the second most deadly cancer in males in 2012,4 and the first or second most deadly cancer in a decade in Taiwan. HCC is more prevalent in Asian countries, but its incidence has also increased in Western countries due to chronic hepatitis or cirrhosis associated with hepatitis B virus, hepatitis C virus, alcoholic fatty liver disease, and other risk factors, such as aflatoxin, obesity, type II diabetes, and smoking.4,5 Current aggressive therapies include surgical treatment, chemotherapy, transhepatic arterial chemoembolization, radiation therapy, radiofrequency ablation, and targeted therapy with multiple drugs. However, effective cures for HCC are urgently needed because of the associated poor prognosis with a relatively low 1-year survival rate and less than 10% 5-year survival rate.6–8
Several compounds found in A. mellea have potential antitumor or cytotoxicity effects, including armillarikin,2,9,10 armillaridin,3,11–13 other melleolides,9,12,13 and arnamial.14 Other biological activities of A. mellea extracts or components include induction of maturation in human dendritic cells,15 upregulation of concanavalin A- or lipopolysaccharide-stimulated lymphocyte proliferation,16 antifungal activity, and antioxidant properties.17
Programmed cell death, such as apoptosis, autophagy, and necroptosis, is a common form of anticancer drug-induced cytotoxicity. In our previous study, we demonstrated reactive oxygen species (ROS)-mediated apoptosis of human leukemia cells induced by armillarikin.2 Therefore, we speculated whether armillarikin would also be useful for hepatoma treatment and, here, demonstrated its cytotoxicity and apoptotic effects against HCC cells.
Materials and methods
Cell culture and treatment
The human HCC cell lines Huh7, HepG2, and HA22T were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), nonessential amino acids (Gibco), and penicillin/streptomycin (Gibco) at 37°C in an atmosphere of 5% CO2. The armillarikin was purified as previously described.2,9,18 In brief, the ethanol (EtOH) extract of A. mellea was separated by using silica gel column chromatography, which was eluted with n-hexane:ethyl acetate (EtOAc, 1:1). The final extract obtained was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich Co., St Louis, MO, USA) as a 1,000-fold stock solution. The armillarikin powder was stable at 4°C for at least 2 years as shown by the similar growth inhibition we observed after this period in the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) viability assays of the same cell line. For drug treatment, the HCC cell lines were treated with the vehicle DMSO or various concentrations of armillarikin for 1–3 days as indicated. For some experiments, the armillarikin-treated cells were also incubated in the presence or absence of 20 μM of the broad-spectrum caspase inhibitor, Z-VAD-fmk (R&D Systems, Inc., Minneapolis, MN, USA) or the antioxidant reagent, N-acetyl cysteine (NAC, 2.5 mM, Sigma-Aldrich Co., St Louis, MO, USA). The Z-VAD-fmk and NAC were dissolved in DMSO or water as 1,000- and 20-fold stock solutions, respectively.
Cell viability assay
The HCC cells were treated with 6.25–50 μM armillarikin for 24–72 hours and then the viability was measured by using the MTS assay (Promega Corporation, Fitchburg, WI, USA) following the manufacturer’s instructions. The absorbance optical density values of the DMSO vehicle-treated control cells were considered as 100% activity.
Cell morphology and micronuclei observation
After incubation with armillarikin, the morphology of the HCC cells was photographed directly or after fixation with methanol and staining with Giemsa (Gibco). For the micronuclei observation, the cells were fixed with methanol and stained with Hoechst 33342 2.5 μg/mL (Thermo Fisher Scientific) as described in the manual of the fluorescence microscopy software (AxioVision, Zeiss, Oberkochen, Germany).
Flow cytometry
The HCC cells were treated with DMSO or armillarikin, collected, and then resuspended in hypotonic buffer (0.1% each of sodium citrate and Triton X-100) containing 5 μg/mL propidium iodide (Sigma) as described by Nicoletti et al19 to detect the apoptotic cells with hypodiploid chromosomal DNA (sub-G1 or DNA laddering) by using flow cytometry (fluorescence-activated cell sorting, FACSCalibur).20 The percentage of the sub-G1 cells was calculated. To detect the collapsed mitochondrial transmembrane potential of the cells by using flow cytometry, they were mixed with 0.02 μM 3,3′-dihexyloxacarbocyanine iodide (Molecular Probes, Eugene, OR, USA) and analyzed according to the manufacturer’s instructions. All the flow cytometric data were analyzed by using the FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Immunoblotting
After treatment with armillarikin, the HCC cells were collected and dissolved in lysis buffer (Sigma-Aldrich) containing protease inhibitors (Roche Biochemicals, Indianapolis, IN, USA). After the debris had been removed and quantified, 50 μg of the cell lysates was separated by using 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, the separated proteins were immunoblotted with antibodies against caspase-8 (BD Biosciences, San Jose, CA, USA), caspase-3 (Active Motif), cleaved nuclear poly adenosine diphosphate-ribose polymerase (Cell Signaling, Beverly, MA, USA), caspase-9, apoptosis-related phospho-histone H2A.X (phospho-histone 2, Upstate), and phospho-histone 3 (Santa Cruz Biotechnology Inc., Dallas, TX, USA) while the loading control was actin (Sigma-Aldrich).
Results and discussion
Growth inhibition of HCC cells by armillarikin
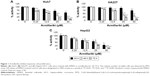
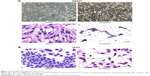
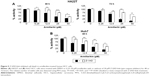
We demonstrated the armillarikin-induced cytotoxicity and apoptosis in human leukemia cells in our previous study. To evaluate the cytotoxicity of armillarikin against HCC cells, we treated the human HCC cell lines, Huh7, HepG2, and HA22T cells with DMSO (vehicle control) or 6.25–50 μM armillarikin for 1–3 days. Ethical approval was not required by the IRB of Chang Jung Christian University due to observations of cytotoxic activity of armillarikin in HCC cell lines in our study. The 0.1% DMSO showed no obvious effect on cell viability and morphology compared to the untreated control (data not shown). The cell viability was measured by using the MTS (Figure 1) and alamarBlue® (Invitrogen, Carlsbad, CA, USA) (Figure S1) assays. Armillarikin inhibited the cell growth of the three HCC cell lines in a time- and dose-dependent manner. The highest antiproliferative interval was 48–72 hours for most of the three HCC cells. To determine whether the decreased cell viability was induced by the cell death, we performed the following experiments. We observed the morphology of the HA22T and Huh7 HCC cells treated with armillarikin to confirm its cytotoxicity in these cells (Figure 2). In addition, armillarikin induced the collapse of the mitochondrial transmembrane potential in armillarikin-treated HepG2 and HA22T HCC cells (Figure 3), indicating induction of HCC cell death. These data suggest that armillarikin was cytotoxic against the HCC cells.
Induction of apoptosis by armillarikin in human hepatoma cells
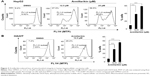
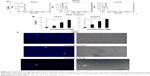
Armillarikin caused HCC cell death and, therefore, we subsequently determined whether it also induced apoptosis. As shown in Figure 4, armillarikin induced a high percentage of hypoploid (or sub-G1) cells in the Huh7 and HepG2 HCC cell lines according to cell cycle analysis using flow cytometry, indicating that armillarikin was apoptotic against the majority of the cells. To further confirm this result, we examined the micronuclei morphology. As shown in Figure 4C, fragmented nuclei were observed in the immunofluorescence nuclei staining images after armillarikin treatment of Huh7 cells.
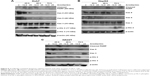
Caspases play key roles in the apoptosis process and, therefore, the armillarikin-induced apoptosis was further investigated using the broad-spectrum caspase inhibitor, Z-VAD-fmk. As shown in Figure 5, Z-VAD-fmk attenuated the growth inhibition induced by armillarikin in both HA22T and Huh7 HCC cells, indicating that caspase-associated death pathways mediated the armillarikin-induced apoptosis. To further confirm this result, we detected the cleavage of caspases and apoptosis-related protein expression by using Western blotting. As shown in Figure 6, the full-length procaspase-9, -3, and -8 expression levels were decreased while the cleaved form of poly adenosine diphosphate-ribose polymerase (a caspase-3 substrate) and the cell cycle arrest-related phospho-histone 3 and DNA fragmentation-related phospho-H2A.X were increased following armillarikin treatment of human HCC cells.21 Furthermore, the vehicle control at 24, 48, and 72 hours confirmed there were no obvious differences in the proteins we detected (data not shown). These data confirmed the armillarikin-induced apoptosis of the HCC cells.
ROS-mediated apoptosis by armillarikin in human HCC cells
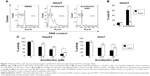
To further investigate the other signaling molecules likely involved in the armillarikin-induced apoptosis, we used the ROS scavenger, NAC. Cotreatment with NAC attenuated both armillarikin-induced apoptosis and growth inhibition in HA22T cells (Figure 7A–C). These data suggest that ROS production occurs upstream of armillarikin-induced apoptosis in human HCC cells.
Armillarikin is known to have cytotoxic effects against HCC, leukemia, lung, and colon cancers,2,9,10 but our current findings clearly elucidated that the mechanism underlying the ROS-mediated apoptosis induced by armillarikin in HCC is similar to that operating in human leukemia cells.2 Furthermore, this study demonstrates an example of the regulation of ROS-mediated apoptosis induced by DNA-damaging reagents,22,23 similar to that shown by natural products, such as marine algae and Baizhu extracts, and caffeic acid phenethyl ester from propolis.24–27 Similar to armillarikin, these compounds act as pro-oxidant agents for ROS production, which correlated with apoptosis induction. The detailed mechanism of the potential pro-oxidant effects of armillarikin will be elucidated in future studies. Because the production of ROS is deleterious to healthy cells, the induction of oxidative stress that leads to apoptosis is favorable in cancer tumor treatment.28 Therefore, antioxidant/pro-oxidant compounds might have contradictory effects on normal and tumor cells.
Recently, we also published a report that armillaridin is an A. mellea constituent with both antitumor effects on human esophageal carcinoma in vivo3 and inhibitory effects on the differentiation of human activated macrophages.11 Therefore, the effects of both armillarikin and armillaridin support the potential usefulness of A. mellea as an antitumor medicinal mushroom worth future development.
Conclusion
In this study, we discovered that armillarikin inhibited the growth of human HCC Huh7, HepG2, and HA22T cells. This phenomenon was accompanied by cell death and the collapse of the mitochondria due to loss of mitochondrial transmembrane potential after armillarikin treatment. In addition, we found a high percentage of hypodiploid cells (with sub-G1 DNA), detected by the DNA content determination. We further demonstrated the induction of apoptosis by examining the micronuclei, and this was further confirmed by the interference in the apoptotic effect induced by the broad caspase inhibitor, Z-VAD-fmk. Moreover, armillarikin-induced apoptosis was reconfirmed by the immunoblotting of apoptosis-related proteins; cleavage of procaspase-9, -3, and -8; increased cleavage of the caspases 3 substrate, poly adenosine diphosphate-ribose polymerase; and an increase in the cell cycle arrest-related phospho-histone 3 and phosphorylation of the apoptotic marker, H2A.X phosphorylation.21 Finally, apoptosis induction by armillarikin was mediated by ROS, as the ROS scavenger, NAC, hindered both the high percentage of hypodiploid cells and growth inhibitory effect of armillarikin.
In conclusion, the armillarikin-induced apoptosis was mediated by ROS and accompanied by mitochondrial collapse and activation of caspase-8 and -3 in HCC cells. Further experiments need to be conducted to determine the potential antitumor effects of armillarikin. Our study demonstrated that armillarikin may be considered as a potential candidate for further development as a therapy or adjuvant treatment for HCC.
Acknowledgments
We would like to thank Dr King-Song Jeng for providing reagents and the use of the relevant equipment. This work was supported by grants from the Mackay Memorial Hospital (MMH-E-103-13), National Science Council of Taiwan (NSC 100-2314-B-309-004- and NSC 100-2314-B-195-007-MY3), and ROC Ministry of Science and Technology (MOST 103-2313-B-309-001 and MOST 104-2320-B-309-001).
Disclosure
The authors report no conflicts of interest in this work.
References
Heo JC, Woo SU, Son M, et al. Anti-tumor activity of Gastrodia elata blume is closely associated with a GTP-Ras-dependent pathway. Oncol Rep. 2007;18(4):849–853. | ||
Chen Y-J, Wu S-Y, Chen C-C, et al. Armillaria mellea component armillarikin induces apoptosis in human leukemia cells. J Funct Foods. 2014;6(0):196–204. | ||
Chi CW, Chen CC, Chen YJ. Therapeutic and radiosensitizing effects of armillaridin on human esophageal cancer cells. Evid Based Complement Alternat Med. 2013;2013:459271. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Herath NI, Leggett BA, MacDonald GA. Review of genetic and epigenetic alterations in hepatocarcinogenesis. J Gastroenterol Hepatol. 2006;21(1 Pt 1):15–21. | ||
Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28(17):2889–2895. | ||
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. | ||
Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48(4):1312–1327. | ||
Chen CC, Kuo YH, Cheng JJ, et al. Three new sesquiterpene aryl esters from the mycelium of Armillaria mellea. Molecules. 2015;20(6):9994–10003. | ||
Yin X, Feng T, Liu JK. Structures and cytotoxicities of three new sesquiterpenes from cultures of Armillaria sp. Nat Prod Bioprospect. 2012;6:245–248. | ||
Liu TP, Chen CC, Shiao PY, Shieh HR, Chen YY, Chen YJ. Armillaridin, a honey medicinal mushroom, Armillaria mellea (higher Basidiomycetes) component, inhibits differentiation and activation of human macrophages. Int J Med Mushrooms. 2015;17(2):161–168. | ||
Bohnert M, Nutzmann HW, Schroeckh V, et al. Cytotoxic and antifungal activities of melleolide antibiotics follow dissimilar structure-activity relationships. Phytochemistry. 2014;105:101–108. | ||
Bohnert M, Miethbauer S, Dahse HM, Ziemen J, Nett M, Hoffmeister D. In vitro cytotoxicity of melleolide antibiotics: structural and mechanistic aspects. Bioorg Med Chem Lett. 2011;21(7):2003–2006. | ||
Misiek M, Williams J, Schmich K, et al. Structure and cytotoxicity of arnamial and related fungal sesquiterpene aryl esters. J Nat Prod. 2009;72(10):1888–1891. | ||
Kim SK, Im J, Yun CH, et al. Armillariella mellea induces maturation of human dendritic cells without induction of cytokine expression. J Ethnopharmacol. 2008;119(1):153–159. | ||
Sun Y, Liang H, Zhang X, Tong H, Liu J. Structural elucidation and immunological activity of a polysaccharide from the fruiting body of Armillaria mellea. Bioresour Technol. 2009;100(5):1860–1863. | ||
Lung MY, Chang YC. Antioxidant properties of the edible Basidiomycete Armillaria mellea in submerged cultures. Int J Mol Sci. 2011;12(10):6367–6384. | ||
Yang JS, Su YL, Wang YL, et al. Isolation and structures of two new sesquiterpenoid aromatic esters: armillarigin and armillarikin1. Planta Med. 1989;55(5):479–481. | ||
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–279. | ||
Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Diffe. 2009;16(1):3–11. | ||
Talasz H, Helliger W, Sarg B, Debbage PL, Puschendorf B, Lindner H. Hyperphosphorylation of histone H2A.X and dephosphorylation of histone H1 subtypes in the course of apoptosis. Cell Death Differ. 2002;9(1):27–39. | ||
Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ. DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene. 2003;22(50):8168–8177. | ||
Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273(41):26900–26907. | ||
Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49(11):5615–5619. | ||
Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12(2):143–149. | ||
Huang HL, Chen CC, Yeh CY, Huang RL. Reactive oxygen species mediation of baizhu-induced apoptosis in human leukemia cells. J Ethnopharmacol. 2005;97(1):21–29. | ||
Huang HL, Wu SL, Liao HF, et al. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. J Agric Food Chem. 2005;53(5):1776–1781. | ||
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. |
Supplementary material
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.