Back to Journals » Cancer Management and Research » Volume 12
Indocyanine Green Clearance Test for the Preoperative Assessment of Chemotherapy-Related Hepatic Injury in Patients with Colorectal Liver Metastasis
Authors Wang LJ , Yan XL, Li J, Wang K, Xing BC
Received 6 March 2020
Accepted for publication 19 April 2020
Published 8 May 2020 Volume 2020:12 Pages 3237—3245
DOI https://doi.org/10.2147/CMAR.S252693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Li-Jun Wang, Xiao-Luan Yan, Juan Li, Kun Wang, Bao-Cai Xing
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Hepatopancreatobiliary Surgery Unit I, Peking University Cancer Hospital & Institute, Beijing 100142, People’s Republic of China
Correspondence: Bao-Cai Xing
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Hepatopancreatobiliary Surgery Unit I, Peking University Cancer Hospital & Institute, 52 Fucheng Road, Haidian, Beijing 100142, People’s Republic of China
Email [email protected]
Purpose: The aim of the present study was to determine the value of the indocyanine green (ICG) clearance test in the preoperative assessment of chemotherapy-related hepatic injury in patients with liver metastasis from colorectal cancer.
Methods: A total of 218 patients who underwent hepatic dissection due to colorectal cancer liver metastasis at the Peking University Cancer Hospital between January 2016 and December 2017 were retrospectively evaluated; 179 patients who received chemotherapy prior to hepatic resection were further examined. Patient- and chemotherapy-related factors were analyzed in both groups with normal and abnormal ICG retention rate at 15 min (ICG-R15), and their intraoperative and postoperative outcomes were compared.
Results: The chemotherapy group had a higher mean ICG-R15 value and a higher number of patients who exhibited abnormal ICG-R15, compared with the no-chemotherapy group. Under the guidance of ICG test, no patients experienced severe complications in the abnormal ICG-R15 group compared with the normal ICG-R15 group, and the overall morbidity was also not significantly different between the two groups. However, the incidence of severe complications was higher in patients who underwent major resection with a normal ICG-R15 value compared with patients with an abnormal ICG-R15 value who underwent minor resection (P< 0.05). Multivariable logistic regression analyses revealed that body mass index (BMI) ≥ 28 and oxaliplatin use were independent predictors of abnormal ICG-R15. The ICG-R15 value was significantly higher in the two-risk factors group compared with the no-risk factor group (P=0.012), but not statistically different compared with the one risk factor group.
Conclusion: Screening of patients with chemotherapy-associated liver injury using the IGC test may help in performing safe hepatectomy by avoiding major resection. BMI ≥ 28 and oxaliplatin use were independent preoperative predictors of abnormal ICG-R15.
Keywords: indocyanine green, resection, liver metastasis, colorectal cancer, morbidity
Introduction
In total, ~50% of patients with colorectal cancer present with liver metastases during diagnosis or after treatment for the locoregional disease.1,2 Surgical resection remains the best choice of colorectal liver metastases (CRLM) treatment, and the reported 5-year survival rate is 30–50%.3,4 Nowadays, patients who receive chemotherapy before hepatectomy are common in the aim of conversion therapy or neoadjuvant chemotherapy to reduce possible recurrence.2,5 Therefore, making accurate preoperative evaluations of the impact of chemotherapy on liver reserve function and perioperative results are of great importance.
The indocyanine green retention rate at 15 min (ICG-R15) has been one of the most widely used quantitative liver function tests since the 2000s.6 After bolus ICG injection, the dye connects with plasma proteins, which is quickly taken by hepatocytes, and is ultimately excreted into bile. The abnormal structure or function of hepatic lobules may lead to a decrease in ICG clearance rate, which represents impaired liver reserve function.
The Makuuchi decision tree for hepatectomy based on ICG-R15 value contributes to improved surgical mortality in hepatocellular carcinoma patients.7 Liver cirrhosis is a permanent hepatic lobule change, and the ICG-R15 value may accurately predict the dysfunction in liver reserve.8 However, chemotherapy-related liver injury is a dynamic process, and whether ICG-R15 can predict the dysfunction of liver reserve in these patients remains controversial.9 The ICG test has been found to be helpful in identifying patients with damaged hepatic function following chemotherapy and may assist in the estimation of the postoperative risk of mortality.10 Another study showed that the increase in ICG-R15 is a predictor of complications after hepatectomy.11 However, no positive correlation between morbidity and the higher ICG-R15 values was identified in another study.12 So, the association between abnormal ICG-R15 findings and morbidity remains unclear.
Therefore, the present study aimed to compare the ICG-R15 values between patients with or without preoperative chemotherapy and investigate the perioperative outcomes of patients who had abnormal ICG-R15 values. The current study then aimed to identify factors related to abnormal ICG-R15 values in patients who were receiving preoperative chemotherapy, thereby evaluating the value of the ICG test in the assessment of the chemotherapy-related liver injury.
Materials and Methods
Subjects
The data of a consecutive series of 218 cases (155 males, 63 females) with a median age of 58.5 years (range 26–78) who received liver resection in Peking University Cancer Hospital between January 2016 and December 2017 due to CRLM were retrospectively analyzed. The inclusion criteria were: i) resection of liver metastases was performed with curative intent; and ii) the patients completed the treatment. Cases who had a history of hepatic resection or underlying chronic liver disease, including hepatitis B or C virus infection or alcohol abuse, were excluded to minimize confounding factors. The requirement for ethics approval for this study was waived by the ethics committee of Beijing Cancer Hospital due to the retrospective nature of the study and no patients’ privacy disclosure involved. This study was performed in accordance with the Declaration of Helsinki, and informed consent was signed by all patients for their data to be used for research purposes.
Preoperative Chemotherapy
Preoperative chemotherapy referred to any chemotherapy within 90 days prior to hepatic resection. Accordingly, 179 (82.1%) patients who received preoperative chemotherapy were included for further analysis. Systemic chemotherapy regimens were oxaliplatin- or irinotecan-based regimens either in combination with 5-fluorouracil and leucovorin or capecitabine. The chemotherapeutic dosage was decided in compliance with colorectal cancer guideline.13 Combined molecular-targeted agents (cetuximab or bevacizumab) may be used for patients with initially unresectable tumors or those with resectable tumors with a high recurrence risk. The chemotherapy regimen, cycle, lines and use of combination molecular-targeted agents were recorded.
Operative Technique
Hepatic resection was usually performed 3–4 weeks after chemotherapy cessation. According to the tumor size, number and sites, a hepatic resection to different extents, for example, wedge resection, segmentectomy, sectoriectomy and hemihepatectomy, was performed. A non-anatomic resection strategy was preferred which was defined as the removal of the tumor with a margin of microscopically normal liver tissue without regard to hepatic anatomy. Major resection referred to the removal of three or more hepatic segments. Surgical complications were evaluated by the Clavien-Dindo classification.14 Severe complications were defined as those of Clavien-Dindo grade 3 or above. Combined radiofrequency ablation (RFA) may be applied in deep tumors so as to avoid excessive removal of the normal parenchymal tissues. The ablated tumors were ≤2 cm in diameter.
Measuring the ICG-R15 Levels
The ICG test was usually performed within 5 days before hepatic resection and ≥2 weeks from the time of the last chemotherapy. ICG-R15 was determined preoperatively in all patients with total bilirubin (TBil) levels <50 μmol/l using a noninvasive densitometer (DDG-3300; Nihon Kohden Co.). An intravenous bolus of 0.5 mg/kg of ICG (Dianogreen; Yichuang Medicine Co. Ltd.) was injected into the antecubital vein within 30 sec. Blood ICG concentrations were monitored non-invasively via an optical probe attached to the patient’s nose. The ICG-R15 was automatically calculated by drawing the decay curve of the ICG density.
Statistical Analysis
Continuous variables were reported as the mean ± standard deviation. Student’s t-test, Mann–Whitney U-test, Fisher’s exact test or a χ2 test was used for intergroup comparisons when appropriate. Multivariate analysis was performed for significant variables. P<0.05 was considered to indicate a statistically significant difference. All statistical calculations were performed with SPSS 25.0 (SPSS, Chicago, IL).
Results
Comparison of All Patients with or Without Preoperative Chemotherapy
A total of 218 patients who fulfilled the inclusion criteria were included in the study, of whom 179 received chemotherapy before hepatic resection and 39 did not receive chemotherapy. The patients’ clinic characteristics are presented in Table 1. There were no significant differences between the two groups revealed by prothrombin time (PT), prothrombin time activity (PTA), albumin (Alb) and TBil in the blood test. A significantly higher number of patients in the chemotherapy group had abnormal ICG-R15 compared with the no-chemotherapy group. The mean ICG-R15 value was also significantly higher in the chemotherapy group compared with the no-chemotherapy group.
 |
Table 1 Basic Characteristics of All Patients Receiving Preoperative Chemotherapy or No Chemotherapy (n=218) |
Comparison Between Patients Receiving Chemotherapy with Normal and Abnormal ICG-R15 Values
A total of 179 patients who received preoperative chemotherapy were classified into the normal ICG-R15 (<10%) and abnormal ICG-R15 (>10%) groups. Patient- and chemotherapy-related factors were compared between the two groups (Table 2). No significant differences in factors such as age, sex ratio, PT, PTA and Alb were found between the two groups. However, the proportion of obese patients [body mass index (BMI) ≥28] was higher in the abnormal ICG-R15 group compared with the normal ICG-R15 group. The mean TBil in the abnormal ICG-R15 group was higher compared with the normal ICG-R15 group. In chemotherapy-related factors, more patients in the abnormal ICG-R15 group had more than three cycles of chemotherapy (77.4%) compared with the normal ICG-R15 group (56.1%). The proportion of oxaliplatin used was significantly higher in the abnormal ICG-R15 group compared with the normal ICG-R15 group (P<0.05). However, the chemotherapy lines and the use of molecular-targeted agents were not significantly different between the two groups. In the subgroup of patients who had received oxaliplatin, the proportion of bevacizumab used was not higher in the normal ICG-R15 group compared to the abnormal ICG-R15 group.
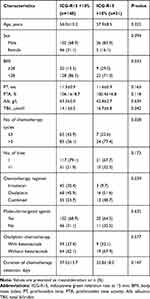 |
Table 2 Analysis of Clinical Factors in Groups with Normal and Abnormal ICG-R15 for Patients Who Received Preoperative Chemotherapy (n=179) |
Intraoperative Outcomes and Postoperative Morbidity
The intraoperative outcomes and postoperative morbidity are presented in Table 3. In the abnormal ICG-R15 group, more patients had undergone minor hepatectomy compared with the normal ICG-R15 group (P=0.034). The proportions of minor liver resections including wedge resection, segmentectomy (or plus wedge resection), and sectoriectomy (or plus wedge resection) in the normal ICG-R15 group were 64.2% (65/107), 30.8% (33/107), and 8.4% (9/107), respectively, while those in the abnormal ICG-R15 group were 57.1% (16/28), 32.1% (9/28), and 10.7% (3/28), respectively (P >0.05). In patients with multiple tumors in whom combination RFA was used in deeply situated tumors, there was no significant difference in the ratio between the couple groups. Moreover, there was no significant difference in mean blood loss between the couple groups.
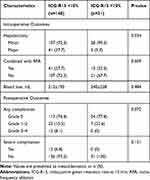 |
Table 3 Comparisons of Intraoperative and Postoperative Characteristics in Patients with Abdominal and Normal ICG-R15 Value After Chemotherapy (n=179) |
The mortality rate was zero in both groups. Complications after hepatic resection were recorded according to the Clavien-Dindo classification (Table 3). The overall incidence of complications was 23.6% and 22.6% in the normal and abnormal ICG-R15 groups, respectively, which was not significantly different. In total, 12 patients in the normal ICG-R15 group experienced severe complications (grade 3/4), including reoperation (n=1), transitory liver failure (n=5), ICU readmissions (n=2), intra-abdominal collection (n=2) and intrathoracic collection (n=2). No patients in the ICG-R15-abnormal group experienced severe complications, which was not significantly different compared with the opposite group. The patient- and treatment-related factors were compared in terms of overall complications and severe complications (Supplementary Table 1).
To minimize the influence of the extent of hepatectomy on the results of the morbidity, patients with normal ICG-R15 value were next divided into a minor resection group and a major resection group. The incidence of overall complication and severe complication in the two groups was compared to patients with abnormal ICG-R15 value who underwent minor resection, respectively (Figure 1). Only three patients with abnormal ICG-R15 value underwent major resection, so the result in this group was not compared due to the limited number. The incidence of overall complication and severe complication was not significantly different in patients with abnormal ICG-R15 value and normal ICG-R15 value who underwent minor resection (P>0.05). While in patients who underwent major resection with normal ICG-R15 value, the incidence of severe complication was 17.1%, which was significantly higher than patients with abnormal ICG-R15 value who underwent minor resection (P<0.05).
Factors Predicting Abnormal ICG-R15
Four variables, including BMI, chemotherapy cycles, oxaliplatin use and TBil value, which were significantly different between the two groups were included in multivariate logistic regression analyses, which revealed that BMI ≥28 and oxaliplatin use were independent preoperative factors predicting abnormal ICG-R15 (Table 4).
 |
Table 4 Multivariate Analysis of Clinical Factors Related to Abnormal ICG-R15 Value in Patients Receiving Preoperative Chemotherapy (n=179) |
Patients were classified into three groups according to the number of risk factors combined. The value was significantly higher in the two-risk factors group compared with the no-risk factor group (P = 0.012), but not significantly different compared with the one risk factor group (Figure 2).
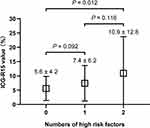 |
Figure 2 Comparison of the mean ICG-R15 value between patients with zero, one and two risk factors after chemotherapy. Abbreviation: IGC-R15, indocyanine green retention rate at 15 min. |
Discussion
The influence of chemotherapy-related liver injury needs to be evaluated accurately for safe hepatectomy. The traditional Child-Pugh score,15 which is a scoring system to measure liver function using five variables, including total bilirubin, serum albumin, international normalized ratio, ascites and hepatic encephalopathy, is not powerful enough in screening patients with chemotherapy-related liver injury, as most patients exhibit Child-Pugh grade A. The ICG-R15 is one of the most widely used hepatic function tests, which compensates for the shortcoming of general scores.6 Diagnosing chemotherapy-related liver injury requires liver biopsy or the so-called blue liver or yellow liver during surgery exploration; however, these are invasive methods and lag behind surgery planning. Hence, a noninvasive method that evaluates the hepatic functional reserve before hepatic resection in patients who received chemotherapy is of great importance.
The present study found that the ICG-R15 value was higher in patients who received chemotherapy compared with those who had not, which indicates that the ICG-R15 value may reflect chemotherapy-related hepatic injury. The pathological diagnosis of chemotherapy-related hepatic injury is based on pathological score, which only reflects structural changes, including sinusoidal pathological score and non-alcoholic fatty liver disease activity score.16 However, due to the diversity of the surgery timing after chemotherapy cessation and the difference in sampling parts of the normal liver tissue, the pathology score does not fully reflect the existence and degree of chemotherapy-related hepatic injury. For a normal liver, the ICG value is usually <10%;17,18 thus, it is highly suggestive of the existence of chemotherapy-induced structural or functional abnormalities in the liver for ICG-R15 >10%. So, the present study simply used the abnormal ICG-R15 value to represent the pathological chemotherapy-related hepatic injury.
The change of ICG-R15 values during chemotherapy cessation is much greater within 2-week after chemotherapy.19 Thus, the present study regularly performed the ICG test after 3–4 weeks of chemotherapy cessation, leaving sufficient time for hepatic function recovery. Therefore, if the ICG value remains abnormal after that period, the chemotherapy-induced liver injury exists and the risk of surgery may be increased. Thus, the maximum extent of hepatic parenchymal resection is lower for patients with damaged liver function than that of patients with normal hepatic reserve function. For safe hepatic resection, a relatively cautious attitude toward patients with abnormal ICG-R15 was taken, and only three patients underwent major hepatic resections, such as a right hepatectomy, with ICG-R15 values not much higher than 30%. The postoperative outcomes revealed that these patients’ morbidity was not higher compared with that of the normal ICG-R15 group. Since the extent of hepatectomy and hepatic reserve function are the most important determinants of postoperative complication besides advances in hepatic surgical technique and perioperative care. We designed to divide the patients into four groups based on minor/major resection combined with normal or abnormal ICG-R15 value. However, no patients were categorized into major resection with abnormal ICG-R15 value group due to the limited number. The subgroup analysis shows that the incidence of severe complication in patients who underwent major resection with normal ICG-R15 value was increasing, which was significantly higher than patients with abnormal ICG-R15 value but underwent minor resection (P<0.05). This indicated that the extent of hepatectomy has a greater impact on the severe postoperative complications compared with the liver reserve function. However, if patients with ICG abnormalities also underwent major hepatectomy, the results may be more serious, although it cannot be analyzed due to the limited number. Previous studies have shown that patients with abnormal ICG value who underwent major hepatic resection may experience postoperative liver failure or even a risk of mortality.20,21 No patients in the abnormal ICG-R15 group experienced severe complications, which may be due to the low proportion of major hepatectomy caused by the “whistling” effect of the ICG test in this group. Besides, a total of five patients with abnormal ICG-R15 value did not undergo hepatectomy (performed RFA or palliative chemotherapy instead) which was not included in the study during the same period, and three of them needed to perform major hepatectomy, which probably reduced the potential probabilities of serious complications in this group. Hence, a surgical plan based on the ICG index may avoid potential serious risks due to extensive hepatic resection.
Meanwhile, according to the Makuuchi criteria, only limited resection or enucleation of no more than one Couinaud’s segmentectomy was deemed tolerable if the ICG-R15 is >30%; however, three patients in the present study underwent safe hepatectomy with more than one Couinaud segment, but no more than three segments. As perioperative management improved and surgical technology matured, resection slightly beyond the Makuuchi standard became relatively safe in patients with chemotherapy-related injury. However, notably, whether patients can safely undergo a major resection with an ICG-R15 >30% still needs further exploration with great caution. Recently, RFA has become a less invasive choice, which may be used to raise resection and survival rates.22,23 In our center, RFA was typically applied in deep tumors in order to avoid excessive removal of the normal parenchymal tissues irrespective of the ICG-R15 value. Since the extent of hepatic resection has a greater impact on complications, RFA may play an important role in “parenchymal-sparing hepatectomy”, especially in patients with abnormal liver function. Hence, if a patient with ICG-R15 abnormality requires major resection, a minor resection combined with RFA could be considered.
As mentioned above, screening of patients with chemotherapy-related liver injury to avoid major resection may be helpful for performing a safe hepatectomy. The present study showed that a BMI ≥28 and oxaliplatin use were independent preoperative factors related to abnormal ICG-R15 in patients who received preoperative chemotherapy. Diabetes and obesity are known factors related to the fatty liver with metabolism reduction.24,25 It is therefore not surprising that BMI was an independent predictive factor for abnormal ICG-R15 in the present study. It has been reported that irinotecan is related to steatohepatitis and hepatic steatosis.9 Using irinotecan in the current study rarely led to ICG abnormality, and most patients with severely impaired ICG had associated oxaliplatin use, either alone or in combination with irinotecan. Oxaliplatin that leads to ICG-R15 abnormality has been reported in several studies,26,27 and the mechanism may be associated with hepatic lobule changes. Although irinotecan mostly resulted in hepatocellular changes, the hepatic reserve function was sufficient to compensate for the injured cell after a period of waiting; hence, the proportion of patients receiving irinotecan presenting with ICG abnormality was lower. In the present study, the subgroup of patients who had received oxaliplatin was further examined; however, bevacizumab use was not different between the two groups. The superiority of bevacizumab in protecting against chemotherapy-related liver injury was not observed in this group. A meta-analysis28 demonstrated that bevacizumab could reduce SOS and fibrosis induced by oxaliplatin; however, the mechanism still needs to be further investigated. How multiple cycles of chemotherapy lead to liver injury is easy to understand, and a previous study reported a significant increase in morbidity, which was likely associated with chemotherapy-related liver injury accumulation.29 However, in the present study, more than three cycles of chemotherapy did not reach a statistically significant difference in the multivariate analyses. The reason may be related to the number of cases included or because the chemotherapy cycle did not have as much effect as the other factors after sufficient time for liver injury recovery. In addition, the results showed that the mean TBil in the ICG-R15 abnormal group was higher than that in the ICG-R15 normal group, the reasons cannot be distinguished between the TBil interference with ICG test or the poor liver reserve function itself. However, under the condition that TBil ≤50 μmol/l, the multi-factor analysis showed that the increase of TBil was not an independent risk factor of abnormal ICG-R15 value, suggesting that the increase of TBil may only be a result of chemotherapy-related liver injury. Above all, the present study suggests that in clinical practice, special attention should be paid to the possible risk of liver injury when selecting chemotherapy regimens before surgery, particularly when using oxaliplatin in obese patients.
The present study also had several limitations due to the retrospective nature. A small number of patients with abnormal ICG-R15 who did not undergo hepatectomy because of tumor progression or were concerned about morbidity were not enrolled in the final analysis, which may have influenced the final results. In addition, the present study is a retrospective study; therefore, the SOS and NAFLD scores were not collected prospectively, which may limit the effectiveness of the results. Despite its limitations, this study provided more data in the field of preoperative evaluation of chemotherapy-related hepatic injury using the ICG test10–12 and was helpful in chemotherapy regimen selection.
Conclusion
Screening of patients with chemotherapy-associated liver injury using the IGC test may help in performing safe hepatectomy by avoiding major resection. And BMI ≥28 and oxaliplatin use were independent preoperative predictors of abnormal ICG-R15.
Acknowledgments
This study was supported by grants from the Beijing Natural Science Foundation of China (no. 7192035), Beijing Municipal Administration of Hospitals Incubating Program (no. PX2016002), and Chinese Society of Clinical Oncology (CSCO)-MERCK SERONO Oncology Research Fund (no. Y-MX2015-34).
Disclosure
The authors declare that they have no competing interests.
References
1. Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15(3):938–946. doi:10.1200/JCO.1997.15.3.938
2. Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. doi:10.1016/j.ejca.2006.04.012
3. Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi:10.2147/CLEP.S34285
4. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, Phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi:10.1016/S1470-2045(13)70447-9
5. Muratore A, Zorzi D, Bouzari H, et al. Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14(2):766–770. doi:10.1245/s10434-006-9146-1
6. Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39(2):107–116. doi:10.1111/j.1872-034X.2008.00441.x
7. Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169(6):589–594. doi:10.1016/S0002-9610(99)80227-X
8. Ohwada S, Kawate S, Hamada K, et al. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg. 2006;93(3):339–346. doi:10.1002/bjs.5258
9. Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-related hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94(3):274–286. doi:10.1002/bjs.5719
10. Krieger PM, Tamandl D, Herberger B, et al. Evaluation of chemotherapy-related liver injury in patients with colorectal cancer liver metastases using indocyanine green clearance testing. Ann Surg Oncol. 2011;18(6):1644–1650. doi:10.1245/s10434-010-1494-1
11. Hiwatashi K, Ueno S, Sakoda M, et al. The evaluation of liver function and surgical Influence by ICGR15 after chemotherapy for colorectal liver metastases. J Cancer. 2016;7(5):595–599. doi:10.7150/jca.13759
12. Wakiya T, Kudo D, Toyoki Y, et al. Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-related liver injury in patients with colorectal cancer liver metastasis. Ann Surg Oncol. 2014;21(1):167–172. doi:10.1245/s10434-013-3203-3
13. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi:10.1093/annonc/mdw235
14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
15. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
16. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi:10.1002/hep.20701
17. De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8(7):355–367. doi:10.4254/wjh.v8.i7.355
18. Okochi O, Kaneko T, Sugimoto H, Inoue S, Takeda S, Nakao A. ICG pulse spectrophotometry for perioperative liver function in hepatectomy. J Surg Res. 2002;103(1):109–113. doi:10.1006/jsre.2001.6328
19. Takamoto T, Hashimoto T, Sano K, et al. Recovery of liver function after the cessation of preoperative chemotherapy for colorectal liver metastasis. Ann Surg Oncol. 2010;17(10):2747–2755. doi:10.1245/s10434-010-1074-4
20. Nonami T, Harada A, Kurokawa T, Nakao A, Takagi H. Hepatic resection for hepatocellular carcinoma. Am J Surg. 1997;173(4):288–291. doi:10.1016/S0002-9610(96)00399-6
21. Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84(9):1255–1259. doi:10.1002/bjs.1800840917
22. Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. doi:10.1148/radiol.2211001624
23. van Amerongen MJ, van der Stok EP, Futterer JJ, et al. Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Euro J Surg Oncol. 2016;42(4):523–530. doi:10.1016/j.ejso.2016.01.013
24. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi:10.1056/NEJMra011775
25. Brouquet A, Benoist S, Julie C, et al. Risk factors for chemotherapy-related liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery. 2009;145(4):362–371. doi:10.1016/j.surg.2008.12.002
26. Komori H, Beppu T, Baba Y, et al. Histological liver injury and surgical outcome after FOLFOX followed by a hepatectomy for colorectal liver metastases in Japanese patients. Int J Clin Oncol. 2010;15(3):263–270. doi:10.1007/s10147-010-0046-9
27. Narita M, Oussoultzoglou E, Chenard MP, et al. Liver injury due to chemotherapy-induced sinusoidal obstruction syndrome is associated with sinusoidal capillarization. Ann Surg Oncol. 2012;19(7):2230–2237. doi:10.1245/s10434-011-2112-6
28. Volk AM, Fritzmann J, Reissfelder C, Weber GF, Weitz J, Rahbari NN. Impact of Bevacizumab on parenchymal damage and functional recovery of the liver in patients with colorectal liver metastases. BMC Cancer. 2016;16:84. doi:10.1186/s12885-016-2095-6
29. Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247(1):118–124. doi:10.1097/SLA.0b013e31815774de
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

