Back to Journals » OncoTargets and Therapy » Volume 12
Individualized treatment for a case of recurrent ovarian ependymoma
Received 15 July 2018
Accepted for publication 29 November 2018
Published 20 December 2018 Volume 2019:12 Pages 113—117
DOI https://doi.org/10.2147/OTT.S180309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Zhen Yuan,1 Mei Yu,1 Yu Chen2
1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China; 2Department of Obstetrics and Gynecology, LuHe hospital, Capital Medical University, Beijing, China
Abstract: Ovarian anaplastic ependymoma is a rare gynecologic malignancy. Owing to its rarity, treatment is challenging. Unilateral ovarian ependymoma was found during laparoscopy in a 19-year-old woman. After cytoreductive surgery, three cycles of bleomycin, etoposide, and cisplatin were administered. Four years after chemotherapy, the patient experienced relapse. After the secondary cytoreductive surgery, no further treatment was performed. She is in good condition, ~19 months after secondary cytoreductive surgery using hormone-replacement therapy without chemotherapy. Some cases of ovarian ependymoma can be treated with surgery alone without chemotherapy and the outcome has been satisfying. Choosing treatment based on malignant ovarian germ cell tumors may be not optimal. Therefore, we suggest individualized treatment for ovarian ependymoma.
Keywords: ovarian ependymoma, contralateral ovary, chemotherapy, hormone-based treatment
Introduction
Ovarian ependymoma is a rare malignancy. According to the classification of tumors of female reproductive organs, ovarian ependymoma falls under the neuroectodermal tumors category, which includes monodermal teratomas and somatic-type tumors arising from a dermoid cyst.
The treatment of extra-axial ependymomas has largely revolved around treatment modalities designed for malignant ovarian germ cell tumors, including surgical debulking followed by adjuvant chemotherapy. In this report, we describe the case of a 19-year-old girl with recurrent ovarian ependymoma treated with cytoreductive surgery, without chemotherapy.
Case summary
The local Institution Review Board has assessed this manuscript and given Institution Review Board exemption. The patient has given written informed consent for the inclusion of material pertaining to herself, and she acknowledged that she cannot be identified via the article. A 19-year-old girl, with a complaint of acute lower abdominal pain, was admitted to our institution in March 2012. Ultrasound identified pelvic masses, and the possibility of an ovarian mass torsion was not excluded. Laparoscopic surgery led to the finding of a right ovarian cyst (diameter: 10 cm), a purple-black mass (diameter: 10 cm) with a pedicle to the uterus-rectum-fossa, and miliary nodules on the surface of the sigmoid colon. During emergent operation at our hospital, a frozen section could not be performed to determine diagnosis. The right ovarian cyst and the purple-black mass were removed. Postoperative pathologic test results revealed ependymoma (WHO tumor grade II–III, according to WHO Classification of Tumors of the Central Nervous System, revised fourth edition). An immunohistochemical analysis revealed negative cytokeratin 5.2 (CAM 5.2), negative cytokeratin 7 (CK 7), partially positive glial fibrillary acidic protein (GFAP), and partially positive nestin. The serum CA125 level was 12,572.7 U/mL preoperatively; postoperatively, it returned to normal.
After approval was given by the gynecologic tumor board of our hospital, in April 2012, the patient underwent a second laparoscopy. The operative findings included a solid mass on the surface of the right ovary (diameter: 2 cm); multiple miliary nodules (diameter: 0.5–0.8 cm) on the surface of the vesical peritoneal reflection, posterior cul-de-sac, sigmoid colon, and peritoneum; and a smooth left ovary without papilla. A tissue biopsy of the left ovarian tissue was performed, and intraoperative frozen sections revealed ependymoma. However, the possibility of carcinoma was not excluded. Because both ovaries were involved, fertility could not be spared. After converting to an open laparotomy, cytoreductive surgery was performed, including bilateral salpingo-oophorectomy, hysterectomy, omentectomy, appendectomy, lymphadenectomy of the pelvic and para-aortic lymph nodes, and resection of other lesions. No residual disease was noted after debulking. The postoperative pathologic test revealed ependymoma with active growth (WHO tumor grade II–III), involving both ovaries and the serous membrane of the left fallopian tube, bladder, uterus-rectum-fossa, sigmoid colon, appendix, and peritoneum. An immunohistochemical analysis revealed negative CAM 5.2, partially positive GFAP, Ki-67 (20%), positive nestin, and partially positive p53. A further specific immunohistochemical analysis revealed negative periodic acid–Schiff staining and negative reticular fiber staining. The pathologic stage was IIIa. Three cycles of chemotherapy, including bleomycin, etoposide, cisplatin 30 mg/m2, intravenous drip, d1–3+ etoposide 100 mg/m2, intravenous drip, d1–3+ bleomycin 15 mg/m2, intramuscular, d1–2, were administered postoperatively. The latest chemotherapy cycle was administered in June 2012. After complete remission, the patient was regularly followed up as an outpatient. The serum CA125 level was obtained, and ultrasonography of the pelvis and abdomen was performed at each follow-up visit. To relieve peri-menopausal syndrome, tibolone (1.25 mg every day) was administered orally for 3 years until October 2015. For skin rashes related to tibolone, oral administration of estradiol valerate (1 mg once a day) was started.
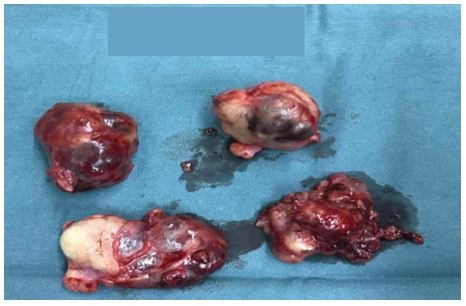
In October 2016, 4 years after chemotherapy, the ultrasound revealed multiple pelvic cystic and solid masses, with a normal serum CA125 level. In November 2016, positron emission tomography-computed tomography scan revealed multiple hypermetabolic cystic and solid masses in front of the rectum and near the left iliac vessel (maximum diameter: 5 cm; Figure 1). In November 2016, secondary cytoreductive surgery was performed. The operative findings included five cystic and solid masses (diameter: 1–5 cm) in the uterus-rectum-fossa (Figures 2 and 3), multiple neoplastic nodules (diameter: 1–3 mm) on the surface of the sigmoid colon, multiple cobblestone-like masses on the serous membrane of the rectum, and three small neoplastic nodules (diameter: 1–3 mm) on the anterior abdominal wall. At completion of this surgery, residual nodules (diameter: 1–3 mm) were observed on the surface of the sigmoid colon and rectum. Immunohistochemical analysis revealed positive GFAP, positive nestin, partially positive S100, positive vimentin, negative CK7, negative etoposide/methotrexate/actinomycin, estrogen (ER) weakly positive at 60%, Ki-67 (index 25%), progesterone (PR) moderately positive at 60%, and locally positive S-100. Another specific immunohistochemical analysis revealed negative periodic acid–Schiff staining and negative reticular fiber staining. The patient refused to receive adjuvant chemotherapy or hormonotherapy. Being counseled on the risk of recurrence, patient chose to be under close follow-up. Serum CA125 level evaluation and ultrasound were performed for monitoring the tumor. To relieve peri-menopausal syndrome, tibolone (2.5 mg every day) was administered orally. The patient was well and without relapse ~19 months after surgery.
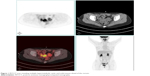  | Figure 1 PET/CT scan revealing multiple hypermetabolic cystic and solid masses ahead of the rectum. |
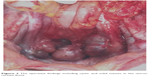  | Figure 2 The operative findings including cystic and solid masses in the uterus-rectum-fossa. |
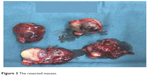  | Figure 3 The resected masses. |
Discussion
Traditionally, ependymomas are thought to originate from the neuroectodermal tissue, most commonly from the spinal tissue or in the cranium. However, in rare circumstances, these tumors can proliferate outside the central nervous system. It has been hypothesized that ependymomas in the pelvis can arise from the pluripotent stem cells of Mullerian origin or from an established ovarian teratoma.1
It has been hypothesized that neuroectodermal tumors of the ovary can arise from the germ cells.2 According to the 2003 WHO histologic classification of malignant ovarian germ cell tumors (MOGCT), the neuroectodermal tumor group belongs to MOGCT, and treatment of ovarian ependymoma is mostly according to that of MOGCT.
The primary treatment strategy for MOGCT is surgery and BEP therapy. With regard to the fertility-sparing surgery for malignant germ cell tumors, biopsies should not be performed if the contralateral ovary appears normal, given that these tumors are usually unilateral.3 Nevertheless, during the initial surgery for the present case, although the left ovary was smooth and without papillary nodules, biopsy results revealed microscopic lesions. Moreover, because of the rarity of ovarian ependyma, the safety of fertility-sparing surgery is unclear. Furthermore, whether a systematic biopsy of the remaining ovary needs to be performed remains to be clarified.
A review of previous reports about treatment is summarized in Table 1. BEP therapy has been used as the standard chemotherapeutic treatment for testicular and ovarian MOGCT and has been reported to significantly improve the outcome. In the present report, our patient was administered three courses of BEP after the first cytoreductive surgery. After undergoing secondary cytoreductive surgery because of relapse, she did not receive any treatment and is doing well without second relapse. This outcome is consistent with that of a previous report, in which the patient did not receive further treatment after operation and was well.4
Some reports have demonstrated that ER and PR are localized in central nervous system tumors. Ependymomas are spinal tumors with the highest incidence of cytosol ER both in male and female patients.5 Some cases of recurrent ependymoma have been shown to respond to tamoxifen.6,7 One case recurred while the patient was receiving ER and PR replacement therapy after oophorectomy, and a cytosolic assay of the recurrent tumor yielded results that were positive for both ER and PR receptors. Consequently, authors suggested that ER replacement therapy after castration stimulates viable malignant ependymal cells to proliferate, and that antiestrogens such as tamoxifen can be used postoperatively.8 Furthermore, some studies have demonstrated antiestrogens’ role in the treatment of ovarian or pelvic ependymoma.4,9 However, the International Menopause Society statement does not mention whether ependymoma is a contradiction for hormone replacement therapy or not.10 Strong positive staining results were observed for ER and PR receptors, in reported cases.4,9 Our patient was administered hormone replacement therapy because of serious peri-menopausal syndrome, and her staining results revealed slightly positive ER and moderately positive PR receptors.
Further studies are required to investigate whether a systematic biopsy of the remaining ovary should be performed for the fertility-sparing surgery, to evaluate the efficacy of BEP therapy for patients with ovarian ependymoma and to assess whether hormone replacement therapy should be administered to patients with weak ER and PR staining results.
Acknowledgment
This study was funded by the National Natural Science Foundation of China (81572576) and the CAMS Initiative for Innovative Medicine (CAMS-2018-12 M-1-002). We thank this patient’s surgeon, Dongyan Cao. Without her help, we could not complete this article.
Disclosure
The authors report no conflicts of interest in this work.
References
Stolnicu S, Furtado A, Sanches A, et al. Ovarian ependymomas of extra-axial type or central immunophenotypes. Hum Pathol. 2011;42(3):403–408. | ||
Aguirre P, Scully RE. Malignant neuroectodermal tumor of the ovary, a distinctive form of monodermal teratoma: report of five cases. Am J Surg Pathol. 1982;6(4):283–292. | ||
Del Carmen MG. Malignant Germ Cell Tumors of the Ovary. In: del Carmen MG, Young RH, Schorge JO, Birrer MJ, editors. Uncommon Gynecologic Cancers. Hoboken, NJ, USA: John Wiley & Sons, Ltd; 2014:120–130. | ||
Zhou F, Song J, Mikolaenko I, Rosenblum M, Shukla PS. Pelvic ependymoma with clinical response to GnRH analog therapy: a case report with an overview of primary extraneural ependymomas. Int J Gynecol Pathol. 2015;34(5):450–458. | ||
Concolino G, Liccardo G, Conti C, Panfili C, Giuffre R. Hormones and tumours in central nervous system (CNS): steroid receptors in primary spinal cord tumours. Neurol Res. 1984;6(3):121–126. | ||
Yoffe R, Khakoo Y, Dunkel IJ, et al. Recurrent ependymoma treated with high-dose tamoxifen in a peripubertal female: impact on tumor and the pituitary–ovarian axis. Pediatr Blood Cancer. 2007;49(5):758–760. | ||
Ben Arush MW, Postovsky S, Goldsher D, El Hasid R, Constantini S. Clinical and radiographic response in three children with recurrent malignant cerebral tumors with high-dose tamoxifen. Pediatr Hematol Oncol. 1999;16(3):245–250. | ||
Auerbach R, Mittal K, Schwartz PE. Estrogen and progestin receptors in an ovarian ependymoma. Obstet Gynecol. 1988;71(6 Pt 2):1043–1045. | ||
Gorski JW, Taylor JS, Zhang J, Liu J, Jazaeri AA. Hormonal based treatment of ovarian anaplastic ependymoma with anastrozole. Gynecol Oncol Rep. 2017;20:93–96. | ||
Sturdee DW, Pines A, International Menopause Society Writing Group, et al. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2011;14(3):302–320. | ||
Deval B, Rousset P, Bigenwald C, Nogales FF, Alexandre J. Treatment of ovarian anaplastic ependymoma by an aromatase inhibitor. Obstet Gynecol. 2014;123(2 Pt 2 Suppl 2):488–491. | ||
Urbano-Ruiz A, Gollop TR, Rossi AGZ. Ovarian ependymoma: a case report. Int J Gynecol Cancer. 2014;24:364. | ||
Zhou F, Song J, Mikolaenko I, Rosenblum M, Shukla PS. Pelvic ependymoma with clinical response to GnRH analog therapy: a case report with an overview of primary extraneural ependymomas. Int J Gynecol Pathol. 2015;34:450–458. | ||
Hino M, Kobayashi Y, Wada M, Hattori Y, Kurahasi T, Nakagawa H. Complete response to paclitaxel, ifosfamide, and cisplatin therapy in a case of ovarian ependymoma. J Obstet Gynaecol Res. 2016;42(11):1613–1617. | ||
Liang L, Olar A, Niu N, et al. Primary glial and neuronal tumors of the ovary or peritoneum: a clinicopathologic study of 11 cases. Am J Surg Pathol. 2016;40(6):847–856. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

