Back to Journals » Clinical Interventions in Aging » Volume 14
Individualized Prediction Of Stroke-Associated Pneumonia For Patients With Acute Ischemic Stroke
Authors Huang GQ, Lin YT, Wu YM , Cheng QQ, Cheng HR, Wang Z
Received 28 July 2019
Accepted for publication 15 October 2019
Published 7 November 2019 Volume 2019:14 Pages 1951—1962
DOI https://doi.org/10.2147/CIA.S225039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Gui-Qian Huang,1,* Yu-Ting Lin,2,* Yue-Min Wu,1 Qian-Qian Cheng,3 Hao-Ran Cheng,1 Zhen Wang1
1Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, People’s Republic of China; 2Department of Pulmonary Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, People’s Republic of China; 3School of Mental Health, Wenzhou Medical University, Wenzhou 325000, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen Wang
Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, People’s Republic of China
Tel +86 577-555780166
Fax +86 577-55578033
Email [email protected]
Background: Stroke-associated pneumonia (SAP) is a serious and common complication in stroke patients.
Purpose: We aimed to develop and validate an easy-to-use model for predicting the risk of SAP in acute ischemic stroke (AIS) patients.
Patients and methods: The nomogram was established by univariate and multivariate binary logistic analyses in a training cohort of 643 AIS patients. The prediction performance was determined based on the receiver operating characteristic curve (ROC) and calibration plots in a validation cohort (N=340). Individualized clinical decision-making was conducted by weighing the net benefit in each AIS patient by decision curve analysis (DCA).
Results: Seven predictors, including age, NIHSS score on admission, atrial fibrillation, nasogastric tube intervention, mechanical ventilation, fibrinogen, and leukocyte count were incorporated to construct the nomogram model. The nomogram showed good predictive performance in ROC analysis [AUROC of 0.845 (95% CI: 0.814–0.872) in training cohort, and 0.897 (95% CI: 0.860–0.927) in validation cohort], and was superior to the A2DS2, ISAN, and PANTHERIS scores. Furthermore, the calibration plots showed good agreement between actual and nomogram-predicted SAP probabilities, in both training and validation cohorts. The DCA confirmed that the SAP nomogram was clinically useful.
Conclusion: Our nomogram may provide clinicians with a simple and reliable tool for predicting SAP based on routinely available data. It may also assist clinicians with respect to individualized treatment decision-making for patients differing in risk level.
Keywords: stroke-associated pneumonia, acute ischemic stroke, nomogram, prediction
Introduction
Stroke-associated pneumonia (SAP) is a common poststroke complication with a prevalence rate of 11.3–31.3%.1–4 Despite remarkable advances in the care of acute stroke patients, the outcomes of SAP are still poor, including prolonged hospitalization, high incidence of severe disability, and high in-hospital mortality rate.5,6
The majority of previous studies focused on prophylactic antibiotic treatment in cases of stroke-associated infection, including SAP, but the results indicated failure to reduce the incidence of pneumonia and improve clinical outcomes.7–9 Furthermore, it is difficult to diagnose SAP because of the low sensitivity of X-ray examination and sputum culture.10,11 Therefore, a more objective and easily applicable model for predicting the development of pneumonia in stroke patients is required.
Previous studies indicated that various risk factors, including older age, male gender, dysphagia, decreased monocytic human leukocyte antigen-DR isotype (HLA-DR), stroke-induced immunodepression syndrome, and chronic obstructive pulmonary disease (COPD) were associated with the incidence of SAP.4,6,12,13 In addition, several recent studies developed clinical scores to predict SAP in stroke patients. For example, the 10-point A2DS2 score [age ≥75 years=1, atrial fibrillation=1, dysphagia=2, male sex=1, National Institutes of Health Stroke Scale (NIHSS) score of 0–4=0, score of 5–15=3, score ≥16=5] was derived for the prediction of poststroke pneumonia in a German cohort and showed high sensitivity and specificity;14 subsequently, it was validated in Chinese stroke patients.2 Recently, the ISAN score was developed by Smith et al5 to assess the risk of SAP, based on 23,199 stroke cases in the UK. This score included the parameters of sex, age, pre-stroke independence, and NIHSS score on admission, and exhibited good discrimination in ischemic stroke derivation and validation samples for predicting SAP. For patients with acute middle cerebral artery infarction, the PANTHERIS score is a simple method for predicting SAP based on age, Glasgow Coma Scale score on admission, systolic blood pressure (SBP), and leukocyte count within 24 hrs of admission.1
However, high sensitivity and specificity are not sufficient for clinical prediction models; timely individualized medical decision-making, cost-effectiveness, representativeness, and comprehensiveness of data collection should also be taken into consideration in clinical practice. The purpose of this study was intended to establish and validate a simple, convenient, accurate, and clinically practical model for predicting the risk of SAP in stroke patients, and meanwhile compare its performance with other prediction models.
Methods
Patient Selection
This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and was conducted in accordance with the Declaration of Helsinki. We enrolled consecutive patients who had been admitted to the Department of Neurology, First Affiliated Hospital of Wenzhou Medical University within 24 hrs after onset of ischemic stroke between January 2018 and January 2019. Inclusion criteria in the study were as follows: 1) age ≥18 years; 2) diagnosis of acute ischemic stroke (AIS) confirmed by cranial computed tomography (CT) or magnetic resonance imaging (MRI) within 24 hrs after admission; and 3) written informed consent obtained from the patient or their legal representatives. The exclusion criteria were as follows: 1) diagnosis of transient ischemic attacks (TIAs); 2) preexisting dysphagia; 3) active infection or pyrexia within 2 weeks before admission; 4) a history of hematological diseases, severe hepatic diseases, cancer, or immunosuppressant treatment; and 5) lack of complete medical records.
Of the total of 1344 patients who fulfilled the inclusion criteria, 361 were excluded, such that 983 AIS patients were included in the analysis. Using a computer random number generator, two-thirds of the patients (N = 643) were randomized into the training cohort to construct the predictive nomogram model, and the remaining 340 patients were assigned to the validation cohort to evaluate the performance of the model (Figure 1).
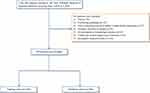 |
Figure 1 Study flow diagram. Abbreviations: AIS, acute ischemic stroke; TIA, transient ischemic attack. |
Data Collection
We collected demographic and clinical data from our electronic medical records system, including age, sex, stroke classification (TOAST criteria), arterial blood pressure on admission, history of stroke, thrombolytic therapy, mechanical ventilation, current smoking status, and current drinking status. Pre-existing comorbidities, including hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, congestive heart failure, and COPD were recorded. In addition, the neurological deficit on admission was measured by well-trained neurologists using the NIHSS score. Based on previously defined cutoff points,14,15 patients were further divided into the following categories according to baseline NIHSS score: 0–4, mild; 5–15, moderate; and ≥16, severe. The baseline laboratory examinations, including fibrinogen, fasting blood glucose, homocysteine (Hcy), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), erythrocytes, leukocytes, platelets, serum creatinine (SCr), the glomerular filtration rate (GFR), total cholesterol, and hemoglobin, were obtained within 24 hrs of admission.
Dysphagia status was determined based on baseline swallow screening. In the stroke center of our institute, all stroke patients received a full clinical swallowing examination by dysphagia-trained nursing staff within 24 hrs after admission. This assessment consisted of a standardized clinical evaluation of consciousness, oromotor function, articulating function, and a water drinking test, to quantify the severity of dysphagia.16 The dysphagic and comatose patients then underwent nasogastric tube interventions to prevent aspiration. Therefore, the presence of a nasogastric tube could reflect both dysphagia status and coma status.
Outcome Measures
In our study, SAP was diagnosed in accordance with the modified Centers for Disease Control and Prevention criteria of hospital-acquired pneumonia,17 based on clinical and laboratory parameters of respiratory tract infection, and was confirmed by both chest X-ray and CT.11 Furthermore, SAP was diagnosed by two treating neurologists who were blinded to other clinical and laboratory findings during the first 7 days of hospitalization after stroke onset. This study only recorded hospital-acquired pneumonia; pneumonia before the stroke was not considered.
Statistical Analyses
Statistical analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA), R for Windows (version 3.4.1; http://www.r-project.org/), and MedCalc software (version 13.0; MedCalc Software, Ostend, Belgium). The differences in continuous variables between SAP and non-SAP patients were assessed with Student’s t-test or the non-parametric Mann–Whitney U-test, and the χ2 or Fisher’s exact test was used to compare categorical variables. Receiver operating characteristic (ROC) curve analysis was used to determine the best cutoff values for continuous variables that were significantly different in the training cohort. In the training cohort, univariate logistic regression analysis was used to screen risk factors related to SAP; variables with a p-value <0.05 were considered as significant factors associated with the occurrence of SAP. And these significant factors thereafter added to multivariate-adjusted binary logistic regression analysis to identify independent clinical predictors of SAP. The SAP nomogram was formulated based on these clinical predictors in multivariate analysis by the package of rms and was validated for discrimination and calibration. Validation of the final nomogram was conducted by a bootstrap method with 1000 resampling. The area under the receiver operating characteristic curve (AUROC) was drawn to evaluate and compare the discrimination ability of the nomogram with that of other prediction models. Calibration curves were obtained by plotting observed probabilities against predicted probabilities, to evaluate the predictive accuracy of the final nomogram. In addition, to determine whether the SAP nomogram could improve the outcomes of patients, decision curve analysis (DCA) was performed using the rmda package. With regard to clinical usefulness, DCA can be used to quantify and compare the potential net benefits of the predictive models,18 thus showing the clinical consequences of a treatment strategy.18–20 All statistical tests were two-sided, and p<0.05 was considered statistically significant.
Results
Clinical Characteristics Of The Study Cohort
A total of 983 patients were enrolled in this study between January 2018 and January 2019. Of these patients, 120 (12.2%) were diagnosed with SAP. The incidence rate of pneumonia after AIS was similar between the two cohorts: 70 (10.9%) of 643 patients in the training cohort, and 50 (14.7%) of 340 patients in the validation cohort. Regarding basic clinical characteristics and laboratory variables, there were no significant differences between the training and validation cohorts (Table 1).
 |
Table 1 Baseline Characteristics Of AIS Patients In Training And Validation Cohort |
Baseline Characteristics Of Patients In The Training Cohort Stratified By SAP
Descriptive analyses (Table 2) showed that patients diagnosed with SAP were older (73.3 vs 65.5 years old) and had higher rates of atrial fibrillation (44.3% vs 9.9%), nasogastric tube intervention (50.0% vs 7.2%) and mechanical ventilation (17.1% vs 4.2%) than their counterparts without SAP. Patients with SAP had a significantly higher NIHSS score on admission (9.0 vs 2.0), and higher fibrinogen (4.4 g/L vs 3.6 g/L), leukocyte counts (9.7×109/L vs 7.3×109/L). We selected 10.0×109/L as the leukocyte cutoff point based on previous studies.21,22 The best cutoff value for fibrinogen in the training cohort, as revealed by ROC analysis, was 3.68 g/L. As a categorical variable, more patients with SAP exhibited a high leukocyte count (55.7% vs 15.7%) and high fibrinogen level (68.6% vs 36.0%) on admission than subjects without SAP (both p<0.001; Table 2).
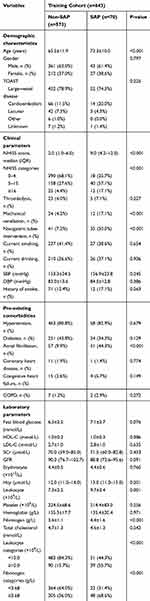 |
Table 2 Baseline Characteristics Of AIS Patients With SAP And Non-SAP In Training Cohort |
Construction Of The Predictive Nomogram For SAP
Multivariable adjusted binary logistic regression analysis demonstrated that age, category of NHISS score on admission, atrial fibrillation, nasogastric tube intervention, mechanical ventilation, leucocyte, and fibrinogen categories were independently associated with SAP (all p<0.05; Table 3). Therefore, the results indicated that these seven variables (age, admission NHISS, atrial fibrillation, nasogastric tube intervention, mechanical ventilation, leucocyte, and fibrinogen) were independent clinical predictors of pneumonia in AIS patients. Then, we constructed a predictive nomogram for SAP using these seven independent predictors listed above, based on R software (Figure 2). Firstly, each predictor was marked on the nomogram and a vertical line to the “Points” axis was drawn to obtain the matching points on the point scale. Then, the corresponding points of each predicator were added up to get the total points. Finally, we located the total points on the “Total Points” axis and drew a vertical line down to the “Probability of stroke-associated pneumonia” axis. The corresponding approximate probability of SAP was obtained. In addition, an example is given in Supplementary Figure 1 to help understand how the model works.
 |
Table 3 Univariate And Multivariate Analysis Of The Associations Between SAP And Baseline Characteristics In Training Cohort |
Validation Of The Predictive Nomogram
ROC analysis indicated that the AUROC of the SAP nomogram in the training and validation cohorts was 0.845 (95% CI 0.814–0.872) (Figure 3A) and 0.897 (95% CI: 0.860–0.927) (Figure 3B), respectively, which suggested good discriminative capacity of this nomogram. In addition, in the training cohort, the calibration plot showed an optimal agreement between the probability of SAP predicted by the nomogram and the actual observations (Figure 4). Regarding the predicted probability versus the actual probability, the mean absolute error in the training cohort was 0.020. Furthermore, in the validation cohort, the calibration plot of observed versus predicted probability of SAP also showed excellent concordance; the mean absolute error was 0.019.
Predictive Efficacy Assessment
To assess the predicted probability of SAP, each patient was also tested for SAP based on the ISAN, A2DS2 and PANTHERIS scores. As shown in Figure 3B, the AUROC value of the nomogram prediction (0.897; 95% CI: 0860–0.927) was greater than those for the A2DS2 (0.791; 95% CI: 0.744–0.833), ISAN (0.779; 95% CI: 0.731–0.822), and PANTHERIS scores (0.785; 95% CI: 0.736–0.829) (all p<0.05).
DCA of the SAP nomogram, and of the ISAN, A2DS2, and PANTHERIS scores, was performed to determine whether these models can improve patient outcomes (Figure 5). The DCA results indicated that the developed nomogram had a marked net benefit for predicting SAP when the threshold probability was >4%. Furthermore, the net benefit was comparable, nomogram always had a greater net benefit than the other models for predicting SAP.
Discussion
In this study, we developed and validated a novel and simple nomogram model for individualized risk management of pneumonia in acute stroke patients. The nomogram incorporated routinely collected data, including age, NIHSS score, nasogastric tube intervention, mechanical ventilation, atrial fibrillation, leukocyte, and fibrinogen, and showed good discrimination and calibration abilities in the training and validation cohorts, respectively. This nomogram would allow the risk probability of poststroke pneumonia to be scored easily in daily clinical practice. In addition, it would be helpful for physician in differentiating risk management of stroke patients by weighing the net benefit of individualized clinical decision-making.
Consistent with previous reports, the present study showed that age, basal NIHSS score, nasogastric tube intervention, mechanical ventilation, and atrial fibrillation contributed to the development of SAP. As shown in previous studies, older age was associated with higher risk of poststroke pneumonia.6,23 This may have been because older people tend to have more comorbidities and impaired swallowing function.24 Furthermore, stroke severity, as measured by the NIHSS, was associated with SAP (OR=1.159). Many studies showed similar results, i.e., that patients with high NIHSS score were more likely to develop pneumonia after stroke,25–27 which were consistent with our findings. Some studies showed that atrial fibrillation was significantly more prevalent in patients who developed pneumonia in the acute stroke stage, with ORs ranging from 1.96 to 3.30.28–30 Accordingly, atrial fibrillation is included as a predictor of poststroke pneumonia in the A2DS2 score.14 Furthermore, mechanical ventilation showed a strong association with the development of pneumonia after stroke. A retrospective study including critically ill ischemic stroke patients requiring invasive mechanical ventilation showed that 40% of the patients developed poststroke pneumonia.31 Another study showed that AIS patients who received mechanical ventilation on admission had an almost fourfold greater likelihood of developing SAP compared to those without mechanical ventilation.32 Gujjar et al explained mechanical ventilation can impair normal mucociliary clearance, allowing bacteria to colonize the airways more easily and thus increase the likelihood of pneumonia.33 Furthermore, patients receiving mechanical ventilation have longer hospital stays, which increases their risk of exposure to pathogens.34
Stroke can cause dysfunction in oropharyngeal and gastric regions, and in the lower esophageal sphincter.35 Therefore, to prevent aspiration in stroke patients with reduced levels of consciousness or severe dysphagia, some studies suggested that nutrition should be provided through a nasogastric tube rather than by oral feeding.35,36 However, although nasogastric tubes can decrease the risk of aspiration during eating, patients fed in this manner remained at a high risk of pneumonia after stroke.37–39 Related studies showed that prolonged nasogastric tube insertion was associated with an increased incidence of pneumonia and worsening of the prognosis in stroke patients.40,41 This may be because the presence of a nasogastric tube can exacerbate lower esophageal sphincter dysfunction, which may lead to reflux of gastric contents.37 In addition, infected reflux may promote microaspiration and colonization by Gram-negative bacteria.42 It has also been suggested that cleaning of the oral cavity by chewing and swallowing can prevent oropharyngeal colonization by pathogenic organisms in the elderly.43 However, in tube-fed patients, the dysfunctional oropharynx loses this protective function, thereby increasing the risk of microaspiration and pneumonia due to higher bacterial load in the saliva.38
Recently, elevated fibrinogen levels were found in patients with stroke and cardiovascular diseases.44,45 Luyendyk et al reported that fibrinogen played an important role in intensive inflammation and chronic low-grade inflammation.46 Therefore, we hypothesized that fibrinogen may reflect inflammatory status in stroke patients. Neutrophils are very important for the immune reaction against bacteria in pneumonia.47 Fibrinogen, as a plasma protein, supports neutrophil activation by interacting with the human leukocyte adhesion glycoprotein αMβ2 integrin.48 A previous animal study showed that fibrinogen could be synthesized and secreted by lung alveolar epithelial cells during inflammatory stimulation.49 Therefore, although leukocyte count is an important risk factor for inflammation, fibrinogen may show better specificity and sensitivity for predicting poststroke pneumonia. Therefore, we included both fibrinogen and leukocyte as predictors to predict SAP more accurately.
Previous studies have introduced various scoring systems to predict poststroke pneumonia. In terms of discrimination, the ISAN, A2DS2, and PANTHERIS scores performed comparably in our validation samples, but all showed poorer discrimination ability compared to the SAP nomogram. The ISAN score is simple, assessing only four clinical factors on presentation to the emergency department. A recent Chinese study50 analyzed data from 19,333 patients in the National Stroke Registry and confirmed that the ISAN score is effective for predicting SAP in patients with ischemic stroke. However, the ISAN score does not include blood biochemical parameters that can reflect the severity of inflammation in stroke patients. Therefore, we included leukocyte count and fibrinogen level on admission in the SAP nomogram, which captured early inflammation levels in the peripheral circulation and lungs of stroke patients. Furthermore, a series of recent prospective studies explored the predictive validity of the A2DS2 scoring system. Nam et al51 reported that a high A2DS2 score is an independent risk factor for SAP. Some Chinese studies confirmed that the A2DS2 score can be used to stratify the risk of occurrence of SAP in acute stroke patients.52,53 However, dysphagia was the main predictor of A2DS2 score, and we concluded that nasogastric tube intervention is a more direct and sensitive indicator than dysphagia, because nasogastric tube intervention can reflect both dysphagia and consciousness disturbance. In addition, although nasogastric tube intervention and mechanical ventilation are well-known risk factors for SAP, the current prediction models do not include these two key variables. Therefore, the advantage of our SAP prediction model is in the incorporation of these two important variables. The PANTHERIS score is a 12-point SAP assessment for patients with acute infarction admitted to the neurology intensive care unit. However, it has some limitations, including lack of NIHSS score and evaluation of swallowing function, which are important risk factors for SAP. Our SAP nomogram addresses this deficiency, and may therefore be more reliable than the PANTHERIS score.
As SAP mostly occurs within the first few days after stroke onset, adoption of timely prophylactic measures is vital once stroke has occurred. To achieve early and accurate stratification of patients at high risk of SAP, predictive models should be simple, reliable, and carefully applied.5 Therefore, seven predictors that can be obtained on the day of admission and comprehensively reflect the patient’s condition were included in our model, making the SAP nomogram both quick and easy to apply.
Among the currently available prediction tools, the nomogram exhibited high accuracy and excellent ability to predict outcomes, and was confirmed as one of the most important decision-making models in modern medical practice.54,55 To our knowledge, this is the first nomogram for prediction of SAP in stroke patients based on routinely collected data on admission. Our findings highlighted the role of nasogastric tube intervention, mechanical ventilation, and fibrinogen level in the pathogenesis of poststroke pneumonia. Our nomogram model showed better discrimination and calibration capabilities for predicting SAP among AIS patients compared to the A2DS2, ISAN, and PANTHERIS scores.
There were some limitations to our study. First, due to our limited data and lack of external validation, there will be some potential bias in our results. Therefore, future multicenter studies are needed to further validate the reliability and generalizability of final nomogram. Second, our study did not systematically document all details of the nasogastric tube interventions, such as the timing of insertion, which may influence the development of respiratory infections in stroke patients. Finally, further prospective studies are needed to validate the reliability and stability of the nomogram.
Conclusion
In conclusion, we have established and validated a reliable nomogram to predict the individualized risk of poststroke pneumonia with good discrimination and accuracy based on routinely collected data. The proposed nomogram might be a simple and useful tool for clinicians in making timely individualized clinical decision according to each patient’s individual risk.
Abbreviations
AIS, acute ischemic stroke; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; Hcy, homocysteine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SAP, stroke-associated pneumonia; SBP, systolic blood pressure; SCr, serum creatinine concentration; TIA, transient ischaemic attacks.
Data Sharing Statement
The data supporting this study are available from the corresponding author for reasonable request.
Acknowledgments
We thank the study participants and the clinical staff at all participating hospitals for their support and contribution to this project. This work was supported by the Projects of Provincial Natural Science Foundation of Zhejiang (no. LY19H090013).
Author Contributions
Gui-Qian Huang and Zhen Wang conceived and designed this project; Yu-Ting Lin, Qian-Qian Cheng, Hao-Ran Cheng collected the data; Gui-Qian Huang, Yu-Ting Lin, and Yue-Min Wu conducted the data analysis; Gui-Qian Huang draft the paper. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Harms H, Grittner U, Droge H, Meisel A. Predicting post-stroke pneumonia: the PANTHERIS score. Acta Neurol Scand. 2013;128(3):178–184. doi:10.1111/ane.2013.128.issue-3
2. Ji R, Shen H, Pan Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44(5):1303–1309. doi:10.1161/STROKEAHA.111.000598
3. Kishore AK, Vail A, Chamorro A, et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46(5):1202–1209. doi:10.1161/STROKEAHA.114.007843
4. Gong S, Zhou Z, Zhou M, et al. Validation of risk scoring models for predicting stroke-associated pneumonia in patients with ischaemic stroke. Stroke Vasc Neurol. 2016;1(3):122–126. doi:10.1136/svn-2016-000025
5. Smith CJ, Bray BD, Hoffman A, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. 2015;4(1):e001307. doi:10.1161/JAHA.114.001307
6. Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77(14):1338–1345. doi:10.1212/WNL.0b013e31823152b1
7. Meisel A, Smith CJ. Prevention of stroke-associated pneumonia: where next? Lancet. 2015;386(10006):1802–1804. doi:10.1016/S0140-6736(15)00127-0
8. Shim R, Wong CH. Ischemia, immunosuppression and infection–tackling the predicaments of post-stroke complications. Int J Mol Sci. 2016;17(1):64. doi:10.3390/ijms17010064
9. Hetze S, Engel O, Romer C, et al. Superiority of preventive antibiotic treatment compared with standard treatment of poststroke pneumonia in experimental stroke: a bed to bench approach. J Cereb Blood Flow Metab. 2013;33(6):846–854. doi:10.1038/jcbfm.2013.6
10. Hassan A, Khealani BA, Shafqat S, et al. Stroke-associated pneumonia: microbiological data and outcome. Singapore Med J. 2006;47(3):204–207.
11. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46(8):2335–2340. doi:10.1161/STROKEAHA.115.009617
12. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–2763. doi:10.1161/01.STR.0000190056.76543.eb
13. Hoffmann S, Harms H, Ulm L, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671–3682. doi:10.1177/0271678X16671964
14. Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43(10):2617–2623. doi:10.1161/STROKEAHA.112.653055
15. Baird AE, Dambrosia J, Janket S, et al. A three-item scale for the early prediction of stroke recovery. Lancet. 2001;357(9274):2095–2099. doi:10.1016/S0140-6736(00)05183-7
16. Ramsey DJ, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34(5):1252–1257. doi:10.1161/01.STR.0000066309.06490.B8
17. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. doi:10.1016/0196-6553(88)90053-3
18. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi:10.1186/1472-6947-8-53
19. Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. 2016;34(21):2534–2540. doi:10.1200/JCO.2015.65.5654
20. Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi:10.1016/j.eururo.2018.08.038
21. Park S, Lee SJ, Kim K, et al. Inclusion of hemoglobin level in prognostic score provides better prognostic stratification in patients with acute promyelocytic leukemia (APL). Int J Hematol. 2013;97(3):388–396. doi:10.1007/s12185-013-1276-1
22. Xiang JX, Hu LS, Liu P, et al. Impact of cigarette smoking on recurrence of hyperlipidemic acute pancreatitis. World J Gastroenterol. 2017;23(47):8387–8394. doi:10.3748/wjg.v23.i47.8387
23. Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke. 2007;38(8):2284–2291. doi:10.1161/STROKEAHA.106.478156
24. Petroianni A, Ceccarelli D, Conti V, Terzano C. Aspiration pneumonia. Pathophysiological aspects, prevention and management. A review. Panminerva Med. 2006;48(4):231–239.
25. Vargas M, Horcajada JP, Obach V, et al. Clinical consequences of infection in patients with acute stroke: is it prime time for further antibiotic trials? Stroke. 2006;37(2):461–465. doi:10.1161/01.STR.0000199138.73365.b3
26. Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol. 2007;254(10):1323–1329. doi:10.1007/s00415-007-0520-0
27. NanZhu Y, Xin L, Xianghua Y, Jun C, Min L. Risk factors analysis of nosocomial pneumonia in elderly patients with acute cerebral infraction. Medicine. 2019;98(13):e15045. doi:10.1097/MD.0000000000015045
28. Yuan MZ, Li F, Tian X, et al. Risk factors for lung infection in stroke patients: a meta-analysis of observational studies. Expert Rev Anti Infect Ther. 2015;13(10):1289–1298. doi:10.1586/14787210.2015.1085302
29. Yu YJ, Weng WC, Su FC, et al. Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci. 2016;33:124–128. doi:10.1016/j.jocn.2016.02.039
30. Nam KW, Kim TJ, Lee JS, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–1892. doi:10.1161/STROKEAHA.118.021228
31. de Montmollin E, Ruckly S, Schwebel C, et al. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: impact on short and long-term outcomes. J Infect. 2019;79:220–227. doi:10.1016/j.jinf.2019.06.012
32. Chapman C, Morgan P, Cadilhac DA, Purvis T, Andrew NE. Risk factors for the development of chest infections in acute stroke: a systematic review. Top Stroke Rehabil. 2018;25(6):445–458. doi:10.1080/10749357.2018.1481567
33. Gujjar AR, Deibert E, Manno EM, Duff S, Diringer MN. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology. 1998;51(2):447–451. doi:10.1212/WNL.51.2.447
34. Odderson IR, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133. doi:10.1016/S0003-9993(95)80121-9
35. Schaller BJ, Graf R, Jacobs AH. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. 2006;101(7):1655–1665. doi:10.1111/j.1572-0241.2006.00540.x
36. Warusevitane A, Karunatilake D, Sim J, Lally F, Roffe C. Safety and effect of metoclopramide to prevent pneumonia in patients with stroke fed via nasogastric tubes trial. Stroke. 2015;46(2):454–460. doi:10.1161/STROKEAHA.114.006639
37. Gomes GF, Pisani JC, Macedo ED, Campos AC. The nasogastric feeding tube as a risk factor for aspiration and aspiration pneumonia. Curr Opin Clin Nutr Metab Care. 2003;6(3):327–333. doi:10.1097/01.mco.0000068970.34812.8b
38. Langdon PC, Lee AH, Binns CW. High incidence of respiratory infections in ‘nil by mouth’ tube-fed acute ischemic stroke patients. Neuroepidemiology. 2009;32(2):107–113. doi:10.1159/000177036
39. Matz K, Seyfang L, Dachenhausen A, Teuschl Y, Tuomilehto J, Brainin M. Post-stroke pneumonia at the stroke unit - a registry based analysis of contributing and protective factors. BMC Neurol. 2016;16:107. doi:10.1186/s12883-016-0627-y
40. Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Dysphagia and factors associated with respiratory infections in the first week post stroke. Neuroepidemiology. 2014;43(2):140–144. doi:10.1159/000366423
41. Ho CH, Lin WC, Hsu YF, Lee IH, Hung YC. One-year risk of pneumonia and mortality in patients with poststroke dysphagia: a nationwide population-based study. Stroke Cerebrovasc Dis. 2018;27(5):1311–1317. doi:10.1016/j.jstrokecerebrovasdis.2017.12.017
42. Spilker CA, Hinthorn DR, Pingleton SK. Intermittent enteral feeding in mechanically ventilated patients. The effect on gastric pH and gastric cultures. Chest. 1996;110(1):243–248. doi:10.1378/chest.110.1.243
43. Leibovitz A, Plotnikov G, Habot B, Rosenberg M, Segal R. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med Sci. 2003;58(1):52–55. doi:10.1093/gerona/58.1.M52
44. Eidelman RS, Hennekens CH. Fibrinogen: a predictor of stroke and marker of atherosclerosis. Eur Heart J. 2003;24(6):499–500. doi:10.1016/S0195-668X(02)00810-2
45. Williams SR, Hsu FC, Keene KL, et al. Shared genetic susceptibility of vascular-related biomarkers with ischemic and recurrent stroke. Neurology. 2016;86(4):351–359.
46. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi:10.1182/blood-2018-07-818211
47. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi:10.1038/nri1785
48. Forsyth CB, Solovjov DA, Ugarova TP, Plow EF. Integrin alpha(M)beta(2)-mediated cell migration to fibrinogen and its recognition peptides. J Exp Med. 2001;193(10):1123–1133. doi:10.1084/jem.193.10.1123
49. Simpson-Haidaris PJ, Courtney MA, Wright TW, Goss R, Harmsen A, Gigliotti F. Induction of fibrinogen expression in the lung epithelium during pneumocystis carinii pneumonia. Infect Immun. 1998;66(9):4431–4439.
50. Zhang R, Ji R, Pan Y, et al. External validation of the prestroke independence, sex, age, national institutes of health stroke scale score for predicting pneumonia after stroke using data from the china national stroke registry. Stroke Cerebrovasc Dis. 2017;26(5):938–943. doi:10.1016/j.jstrokecerebrovasdis.2016.10.043
51. Nam KW, Kwon HM, Lim JS, Lee YS. Leukoaraiosis is associated with pneumonia after acute ischemic stroke. BMC Neurol. 2017;17(1):51. doi:10.1186/s12883-017-0830-5
52. Li L, Zhang LH, Xu WP, Hu JM. Risk assessment of ischemic stroke associated pneumonia. World J Emerg Med. 2014;5(3):209–213. doi:10.5847/wjem.j.issn.1920-8642.2014.03.009
53. Zhang X, Yu S, Wei L, et al. The A2DS2 score as a predictor of pneumonia and in-hospital death after acute ischemic stroke in chinese populations. PLoS One. 2016;11(3):e0150298. doi:10.1371/journal.pone.0150298
54. Battersby NJ, Bouliotis G, Emmertsen KJ, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. 2018;67(4):688–696. doi:10.1136/gutjnl-2016-312695
55. Tan WS, Ahmad A, Feber A, et al. Development and validation of a haematuria cancer risk score to identify patients at risk of harbouring cancer. J Intern Med. 2019;285(4):436–445. doi:10.1111/joim.2019.285.issue-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.