Back to Journals » Infection and Drug Resistance » Volume 13
Indicators of Critical Illness and Predictors of Mortality in COVID-19 Patients
Authors Aly MH , Rahman SS, Ahmed WA , Alghamedi MH, Al Shehri AA, Alkalkami AM , Hassan MH
Received 10 May 2020
Accepted for publication 11 June 2020
Published 26 June 2020 Volume 2020:13 Pages 1995—2000
DOI https://doi.org/10.2147/IDR.S261159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mohamed H Aly,1 Sayed S Rahman,2 Waleed A Ahmed,3 Mansour H Alghamedi,4 Abudlrahman A Al Shehri,5 Amna M Alkalkami,2 Mohammed H Hassan6
1Internal Medicine Department, Security Forces Hospital Makkah (SFHM), Makkah al-Mukarrammah, Saudi Arabia; 2Nephrology Department, Internal Medicine Department, Security Forces Hospital Makkah (SFHM), Makkah al-Mukarrammah, Saudi Arabia; 3Infectious Disease Unit, Internal Medicine Department, Security Forces Hospital Makkah (SFHM), Makkah al-Mukarrammah, Saudi Arabia; 4Gastroenterology and Hepatology Department, Internal Medicine Department, Security Forces Hospital Makkah (SFHM), Makkah al-Mukarrammah, Saudi Arabia; 5Rheumatology Department, Internal Medicine Department, Security Forces Hospital Makkah (SFHM), Makkah al-Mukarrammah, Saudi Arabia; 6Medical Biochemistry Department, Faculty of Medicine, South Valley University, Qena, Egypt
Correspondence: Mohammed H Hassan
Medical Biochemistry Department, Faculty of Medicine, South Valley University, Qena, Egypt
Tel +201098473605
Email [email protected]
Abstract: COVID-19 is an emerging disease all over the world and spreading at an unpredicted rate, resulting in significant influences on global economies and public health. Clinical, laboratory, and imaging characteristics have been partially described in some observational studies. Not enough systematic reviews on predictors of critical illness and mortality in COVID 19 have been published to date. In this review, we had illustrated the prognostic predictors of COVID-19 by gathering published information on the risk factors related to the outcomes of SARS-CoV-2 infections.
Keywords: COVID 2019, SARS-CoV-2, predictors, critical illness, mortality
Introduction
In Wuhan city, the capital of Hubei in December 2019 in China, an outbreak representing in pneumonia of unidentified origin had occurred. In 7 January 2020, scientists of china could isolate a new strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), from the affected patients,1 that causes the disease; coronavirus disease 2019 (COVID-19) as stated by WHO in February 2020.2
Although the outbreak mostly have started from a zoonotic transmission in a large seafood market that also traded in live wild animals, it soon became obvious that efficient person-to-person transmission was also possible.3 The clinical spectrum of COVID-19 disease appears to be wide, ranging from asymptomatic infection, mild upper respiratory tract illness, severe viral pneumonia with respiratory failure or death. The evaluation of risk factors for the severity of the disease and possibility of death is a very important issue for prediction of the possible outcome.
Age and Gender as Predictors for Critical Illness in COVID-19 Infection
A study conducted by Zhou et al5 confirmed that increased age of patients with COVID-19 was associated with death. The decrease in T-cell and B-cell functions depending on age and the additional production of type 2 cytokines, could lead to a lack in control of viral replication and load and more exacerbation in proinflammatory responses that may lead to increase the severity of the disease and poor outcome.6 Petrilli et al7 described characteristics of 4103 patients in New York City with laboratory-confirmed Covid-19 disease, 1999 of them needed hospital admission while 650 of them needed ICU, respiratory support, were discharged to hospital and/or died. They found that older age was one of the most important predictors of hospitalization and a vital predictor of severe outcomes.
Nasiri et al8 showed that sex affected the mortality rate, where the mortality of females with COVID-19 was significantly lower than males [Odds Ratio (3.4); 95% CI 1.2–9.1, P value = (0.01)], on the other hand, there was not any significant difference between male and female regarding ICU admissions. Earlier coronavirus epidemics of SARS and MERS resulted in a similar form of higher mortality in males than females. Karlberg et al9 also showed that the difference in mortality rates according to sex distribution of the affected cases was higher in younger males (0–44 years old) (RR=2), compared to those of age group (45–74years old) (RR-1.45). Also, another study conducted by Alghamdi et al10 showed that regarding MERS, the number of diseases in males was double that of females (52% vs 23%).
Associated Co-Morbidities as Important Risk Factors in COVID-19 Infection
Patients with coronary heart disease have also been found to have severe acute cardiac events and poor outcomes in respiratory viral infections including influenza.11 A study conducted by Zhou et al showed that increasing level of high-sensitivity cardiac troponin I(cTnI) during hospitalization was presented in more than half of cases who died with COVID-19.5
Li et al12 reported the clinical characteristics of 25 cases died with COVID-19. The clinical profile of these patients showed that the most important risk factors for death in these patients represented in, age and underlying diseases. Regarding the underlying diseases associated with death, the most recorded one was chronic hypertension then diabetes mellitus, chronic cardiac diseases, cerebral infarction, kidney disease, chronic obstructive pulmonary disease, malignant tumors and acute pancreatitis. Li et al12 demonstrated that respiratory failure was the leading cause of death in all the patients, which confirmed that lungs of the patients are the most important target organ of SARS-CoV-2. Multiple organ failure could be observed in those patients, the heart came after the lung as the most common damaged organ then the kidney and the liver. The results of that study verified that the death of the patients with COVID-19 may be to a large extent related to impaired cardiopulmonary function. All the patients’ albumin levels, 80% and 68% of patients’ RBC and Hb levels were decreased, which indicates that malnutrition is one of the causes of severe COVID-19.
Measures of inflammation process were found to be much more important than co-morbidities and demographic characteristics in the hospitalized patients with COVID-19.7 Exaggerated inflammatory response were well defined as severe sepsis.13 Even though the extent of inflammation caused by COVID-19 is similar to or different than that caused by sepsis is unknown. Some case reports recommend that patients with critical COVID-19 disease are showing complications from hypercoagulability,5 as pulmonary emboli,14 and microscopic thrombi.15 So, it is notable that obesity was the chronic condition that had the strongest association with critical illness, with odds ratio higher than any other cardiovascular or pulmonary disease because obesity is recognized to be a pro-inflammatory condition.16,17
Guan et al18 observed that among patients with COVID-19, the circulatory and endocrine co-morbidities were common. Patients with one or more co-morbidities were associated with worse clinical outcomes. Of the 1590 cases, 399 (25.1%) had at least one co-morbidity. The prevalence of specific co-morbidities was; hypertension (269; 16.9%), other cardiovascular diseases (53.7%), diabetes (130; 8.2%), cerebrovascular diseases (30; 1.9%), hepatitis B infections (28; 1.8%), chronic obstructive pulmonary disease (24; 1.5%), chronic kidney diseases (21; 1.3%), malignancy (18; 1.1%) and immunodeficiency (3; 0.2%). At least, single co-morbidity was commonly seen in severe cases than in non-severe cases (32.8% vs 10.3%).
In hospitalized COVID-19 patients, the prevalence of kidney impairment (abnormal urine analysis and kidney dysfunction) was high. After adjustment for confounders, kidney impairment was related to higher risk of in-hospital mortality so; Clinicians should increase their attention to kidney impairment in hospitalized COVID-19 patients. Early discovery and effective intervention of kidney impairment can help to reduce mortality of COVID-19 patients in clinical practice.19 Petrilliet al.7 reported particularly strong association of chronic kidney disease with the risk of hospitalization. Also they declared the importance of hypoxia even with supplemental oxygen in distinguishing among patients who will develop critical illness and those who will not.
Abnormal Hematological and Biochemical Markers in COVID-19 Infection
Blood picture of patients with COVID-19 characterized by normal or low count of WBCs and decreased level of lymphocytes.20 Increased levels of WBCs and neutrophils were found in 68% and 72% of patients. Similarly, elevation of procalcitonin (PCT) levels were observed in 90.5% of patients.12
D-dimers are fragments produced when plasmin cleaves fibrin to break down clots. They are routinely used to exclude the diagnosis of thrombosis. However, plasma d-dimer increases by any pathologic or non-pathologic process that increases fibrin production or breakdown.21 A study conducted by Zhou et al5 found d-dimer more than 1 μg/mL is associated with mortality of COVID-19. High levels of d-dimer have been associated with 28-day mortality in patients with sepsis or infection identified in the emergency department.22 Petrilli et al.7 reported striking findings regarding the predictive value of inflammatory markers to distinguish future critical from non-critical illness. Early elevation in d-dimer level had the strongest association with prolonged hospitalization and the need for mechanical ventilation or death.7 Many studies have reported that d-dimer levels are associated with severity and clinical outcome of community-acquired pneumonia.23 The other mechanisms include the pro-inflammatory cytokine responses that considered one of the mediators of atherosclerosis disease that contribute to plaque rupture by the local inflammation, induce procoagulant factors, and haemodynamic changes, which trigger to thrombosis formation and ischemic diseases.24
Also, angiotensin converting enzyme 2 (ACE 2), that considered the receptor for SARS-CoV-2, is expressed on vascular endothelial cells and myocytes.25 Therefore, there is at least theoretical possibility of direct affection of the heart by the virus.
C-reactive protein is a useful marker and gauge of inflammation; it plays an important role in host defense against invading pathogens as well as in inflammation.26 Early rise in the C-reactive protein also had the strongest association with mechanical ventilation or mortality.7
Serum amyloid A (SAA) is a plasma protein that transports lipids during inflammation.27 Li et al12 showed that the increased levels of SAA and CRP before death in 85% and 100% of patients, suggestive of that there is a serious inflammatory cascade in patients having COVID-19. In intend to monitor biochemical indicators which are important in monitoring the severity of the disease and its progress, Li et al12 consulted the results of laboratory test for all the dead patients, among which 16patients had repeated measurements. The SAA maintained a high level in all the patients, this result indicated that raised SAA levels are closely related to the poor prognosis of patients. The first test level of neutrophils was (93.8%), CRP (84.6%), PCT (100%), cTnI (88.9%), d-dimer (91.6%) and lactate dehydrogenase (LDH) (100%) were increased than the last test, but the levels of lymphocytes were decreased (87.5%), suggesting that the increasing of neutrophils, CRP, d-dimer, PCT, cTnI, and LDH levels can be used as markers of disease severity and progress, similarly the decline of lymphocytes counts.
Nasiri et al8 identified common laboratory findings associated with a poor outcome including lymphopenia (50.1%), raised C-Reactive Protein (72%), and increased levels of LDH (41%). Also, thrombocytopenia was found in 11.1% of the cases. The Studies performed by Huang et al,4 and Lippi et al28 have suggested that thrombocytopenia and lymphopenia in patients with COVID-19 are associated with prolonged hospitalization and worse outcomes. Patients with SARS were indicated to have a higher percentage of lymphopenia (68–90%) and thrombocytopenia (20–45%) than those of COVID-19.29 Thrombocytopenia and lymphopenia have been previously associated with a greater risk of mortality in SARS and influenza.30,31
Liver changes were one of the most common finding in the COVID-19 infected subjects (13.3%). However, a significant AST rising was found in number of subjects (19.7%) also level of ALT (14.6%). In some previous viral infections including SARS, changes and impaired liver function have been noticed as collateral damage, perhaps caused by direct damaging of the hepatic tissue by the pathogen.32 Although this could be the same situation with COVID19, an iatrogenic trauma because of medications as the used lopinavir in COVID-19 therapy can play a role.
The method and level of the virus replication and its duration of life are important factors in evaluating the root of transmission and guiding protocols for patients’ protection and isolation. As coronavirus RNA detection is more sensitive than the isolation of the virus itself, most studies gave care to use qualitative or quantitative viral RNA tests as a possible marker for infectious coronavirus. About SARS-CoV, the viral RNA was obtained from the respiratory specimens from about third of patients affected by the virus for at least 4 weeks from the onset of the disease.33 The same regarding MERS-CoV RNA, where the duration of viral detection in the lower respiratory specimens continued as long as 3 weeks.34
A study conducted by Zhou et al5 found that duration of SARS-CoV-2 RNA in survivors could reach for a median of 20 days even though it could still till death in non-survivors. This result has an important role in the decision of patient’s isolation and guidelines regarding the length of treatment protocols. Usually in serious influenza virus infection, continued viral replication was associated with worse outcome that may be fatal, also the delay in starting with suitable antiviral treatment had an important effect on viral replication and detection.35
In patients with COVID-19, the serum level of ferritin was significantly elevated in non-survivors compared with survivors during the clinical stages of the disease, and raised with case deterioration.5 Shoenfeldreported that the H-chain of the ferritin had a role in activating macrophages to raise the secretion of inflammatory cytokines. So, understanding of the pathogenesis of the hyperferritinemic syndrome including the infection with COVID-19 became obvious.36
Chest Imaging Findings as Risk of Critical COVID-19 Infection
Many studies noted chest X-ray and CT abnormalities in their study populations including unilateral pneumonia,38 bilateral pneumonia,4,5,37–39 bilateral infiltrates,40 and ground glass opacities.5,37,39,41,42 Cao et al found a mortality rate of 17/102 patients. They found that ground-glass opacity was associated with mortality, present in 41.2% of cases who died versus 12.9% of those who lived.43 Summary of the risk factors of critical illness and mortality predictors in COVID-19 were presented in (Figure 1).
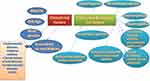 |
Figure 1 Risk factors of critical illness and mortality predictors in COVID-19. |
Conclusions
COVID-19 is an emerging disease all over the world and spreading at an unpredicted rate, resulting in significant influences on global economies and public health. The present review assessed the risk factors related to the outcomes of SARS-CoV-2 infections which were included old age, male gender, and obesity, associated co-morbidities in the form of cardiovascular diseases, diabetes mellitus, chronic kidney disease, liver disease, cerebrovascular diseases and COPD. Laboratory parameters associated with critical COVID-19 disease were lymphopenia, thrombocytopenia, leucocytosis, increased neutrophils, increased C reactive protein and ferritin, increased D dimer, raised ALT and/or AST, decreased albumin, increased cardiac troponin and elevated LDH. Finally, ground-glass opacities when present in the CT chest were considered as the most important imaging indicator for COVID-19 severity.
Funding
N/A.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbio. 2020;l 5:536–544.
2. Chan JWM, Ng CK, Chan YH, et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax. 2003;58:686–689. doi:10.1136/thorax.58.8.686
3. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi:10.1056/NEJMoa2001316
4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1038. doi:10.1016/S0140-6736(20)30566-3
6. Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Supplement 7):504–512. doi:10.1086/432007
7. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4103 patients with COVID-19 disease in New York City. medRxiv. 2020. doi:10.1101/2020.04.08.20057794
8. Nasiri MJ, Haddadi S, Tahvildari A, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic Review and Meta-analysis. medRxiv. 2020. doi:10.1101/2020.03.24.20042903
9. Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–231. doi:10.1093/aje/kwh056
10. Alghamdi IG, Hussain II, Almalki SS, et al. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi:10.2147/IJGM.S67061
11. Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–1720. doi:10.1001/jama.2013.279206
12. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:
13. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. NEngl J Med. 2003;348(2):138–150. doi:10.1056/NEJMra021333
14. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;ehaa254. doi:10.1093/eurheartj/ehaa254
15. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):
16. Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10(3):e0121971. doi:10.1371/journal.pone.0121971
17. Caër C, Rouault C, Le Roy T, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep. 2017;7(1):3000. doi:10.1038/s41598-017-02660-w
18. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a Nationwide Analysis [published online ahead of print, 2020 Mar 26]. Eur Respir J. 2020;55(5):2000547. doi:10.1183/13993003.00547-2020
19. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):
20. National Health Commission of People’s Republic of China. Diag-nosis and treatment of pneumonia caused by novel coronavirus (trial version 4). Available from: https://www.nhc.gov.cn/xcs/zhengcwj/202001/4294563ed35b43209b31739bd0785e67/files/7a9309111267475a99d4306962c8bf78.pdf. Accessed 28 Jan 2020
21. Linkins LA, TakachLapner S. Review of D-dimer testing: good, Bad, and Ugly. Int J Lab Hematol. 2017;39:
22. Rodelo JR, De la Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30(9):1991–1999. doi:10.1016/j.ajem.2012.04.033
23. Dai RX, Kong QH, Mao B, et al. The mortality risk factor of community acquired pneumonia patients with chronic obstructive pulmonary disease: a retrospective cohort study. BMC Pulm Med. 2018;18(1):12. doi:10.1186/s12890-018-0587-7
24. Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi:10.1056/NEJMoa041747
25. Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 incardiacmyocytes and fibroblasts. Am J Physiol Heart Circ Physiol2008. 2008;295(6):2373–2379. doi:10.1152/ajpheart.00426.2008
26. Wu Y, Potempa LA, El KD, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015;396(11):1181–1197. doi:10.1515/hsz-2015-0149
27. Frame NM, Kumanan M, Wales TE, et al. Structural basis for lipid binding and function by an evolutionarily conserved protein, serum amyloid A. J Mol Biol. 2020;S0022-2836(20):30093–30100. doi:10.1016/j.jmb.2020.01.029
28. Lippi G, Plebani M, Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148.
29. Yang M, Hon KL, Li K, Fok TF, Li CK. The effect of SARS coronavirus on blood system: its clinical findings and the pathophysiologic hypothesis. Zhongguo Shi Yan Xue Ye XueZaZhi. 2003;11:217–221.
30. Lopez-Delgado JC, Rovira A, Esteve F, et al. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly. 2013;143:w13788. doi:10.4414/smw.2013.13788
31. Bellelli V, d’Ettorre G, Celani L, Borrazzo C, Ceccarelli G, Venditti M. Clinical significance of lymphocytopenia in patients hospitalized with pneumonia caused by influenza virus. Crit Care. 2019;23(1):330. doi:10.1186/s13054-019-2608-1
32. Peiris JSM, Lai ST, Poon LLM, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet. 2003;361(9366):1319–1325. doi:10.1016/S0140-6736(03)13077-2
33. Xu D, Zhang Z, Jin L, et al. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur J Clin Microbiol Infect Dis. 2005;24(3):165–171. doi:10.1007/s10096-005-1299-5
34. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East Respiratory Syndrome coronavirus infection. Clin Infect Dis. 2016;62(4):477–483. doi:10.1093/cid/civ951
35. Wang Y, Guo Q, Yan Z, et al. Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis. 2018;217(11):1708–1717. doi:10.1093/infdis/jiy115
36. Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. doi:10.1016/j.autrev.2020.102538
37. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
38. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill patients in the seattle region - case series. N Engl J Med. 2020:NEJMoa2004500. doi:10.1056/NEJMoa2004500.
39. Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15(3):e0230548. doi:10.1371/journal.pone.0230548
40. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(4):e26. doi:10.1016/S2213-2600(20)30079-5
41. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:e200950. doi:10.1001/jamacardio.2020.0950.
42. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;ciaa272. doi:10.1093/cid/ciaa272
43. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020:ciaa243. doi:10.1093/cid/ciaa243.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
