Back to Journals » International Journal of General Medicine » Volume 15
Independent Association of 25[OH]D Level on Reduced Glutathione and TNF-α in Patients with Diabetes and/or Hypertension
Authors AlRadini FA , El-Sheikh AAK, Al Shahrani AS , Alzamil NM , Fayed AA, Alsayed E , Alharbi SS, Altulihee MA, Andijani SA, AlShaiddi WK, Alamri FA
Received 18 May 2022
Accepted for publication 19 August 2022
Published 5 September 2022 Volume 2022:15 Pages 7065—7075
DOI https://doi.org/10.2147/IJGM.S375282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Faten A AlRadini,1 Azza AK El-Sheikh,2,3 Abeer S Al Shahrani,1 Norah M Alzamil,1 Amel A Fayed,1 Eman Alsayed,4 Shatha S Alharbi,5 Msaad A Altulihee,5 Shaimaa A Andijani,5 Wafa K AlShaiddi,6 Fahad A Alamri7
1Department of Clinical Sciences, College of Medicine, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia; 2Department of Basic Sciences, College of Medicine, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia; 3Department of Pharmacology, Faculty of Medicine, Minia University, El-Minia, Egypt; 4Department of Clinical Pathology, Faculty of Medicine, Minia University, El-Minia, Egypt; 5Department of Family and Community Health, King Abdullah bin Abdulaziz University Hospital, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia; 6Department of Pathology and Laboratory Medicine, King Abdullah bin Abdulaziz University Hospital, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia; 7Global Center of Mass Gathering Medicine, Ministry of Health, Family Medicine, Primary Health Center, Riyadh, Saudi Arabia
Correspondence: Abeer S Al Shahrani, Department of Clinical Sciences, College of Medicine, Princess Nourah bint Abdulrahman University, Riyadh, 11671, Saudi Arabia, Tel +966118239031, Email [email protected]; [email protected]
Purpose: Oxidative and inflammatory pathways play a significant role in the pathophysiology of a wide variety of non-communicable diseases such as type 2 diabetes mellitus (T2DM) and hypertension. However, the effect of serum 25-hydroxyvitamin D (25[OH]D) on these pathways is still controversial. To evaluate the association of 25[OH]D on antioxidant and pro-inflammatory biomarkers, reduced glutathione (GSH) and tumor necrosis factor (TNF)-α, in T2DM and hypertensive patients.
Patients and Methods: This is a cross-sectional study of a consecutive sample of patients attending the the Family Medicine clinic at King Abdullah bin Abdulaziz University Hospital (KAAUH). Participants were screened for eligibility according to the following criteria: aged above 18 years and diagnosed with T2DM and/or hypertension for at least one year. Patients receiving any kind of vitamin D or calcium supplements within the last three months were excluded, as were those with a history of renal failure, cancer, liver, thyroid, or any other chronic inflammatory diseases.
Results: In total 424 T2DM and/or hypertensive patients (mean age 55± 12 years) were recruited. In addition to routine physical and laboratory examinations, levels of serum 25[OH]D, GSH and TNF-α were measured. The prevalence of 25[OH]D deficiency (< 50 nmol/L) was 35.1%, which was independent from GSH and TNF-α levels. In T2DM, hypertensive and patients having both diseases, GSH levels were 349.3± 19, 355.4± 19 and 428.8± 20 μmol/L, respectively. Uncontrolled T2DM and hypertension patients showed significantly higher GSH compared with the controlled group. Males showed slightly higher level of TNF-α compared with females and uncontrolled hypertensive patients had relatively higher TNF-α level when evaluated against controlled hypertensive patients.
Conclusion: 25[OH]D level is independent of oxidative stress and inflammation, assessed by levels of GSH and TNF-α, respectively, in T2DM and hypertensive Saudi patients.
Keywords: vitamin D, diabetes, hypertension, reduced glutathione, TNF-α
Introduction
Type 2 diabetes mellitus (T2DM) is considered as one of the most widespread non-communicable diseases worldwide, particularly in Saudi Arabia where its prevalence has drastically increased from 8.5% in 1992 to 39.5% in 2022 among Saudi population.1 Hypertension is also considered another global major non-communicable disease, with an alarming overall estimated prevalence of nearly a quarter of the adult population in Saudi Arabia.2 Several studies implicated that oxidative stress and inflammatory pathways are incriminated in the initiation, progress and/or complications of both diseases.3,4 Deficiency in reduced glutathione (GSH), one of the strongest antioxidants that largely contributes as a pivotal player in body antioxidant defense systems, has an undeniable role in the pathogenesis of T2DM as well as hypertension.5,6 Oxidative stress caused by GSH deficiency may trigger the induction of inflammatory markers such as tumor necrosis factor (TNF)-α that may further induce other inflammatory mediators contributing to the progress of T2DM and hypertension.7,8 Still, it is possible that inflammatory process precedes oxidative stress, as the initiative/cause crosstalk between these pathways in T2DM and hypertension is a relationship that is too complex to define.9
Deficiency in vitamin D, or its hydroxylated active form 25-hydroxyvitamin D (25[OH]D), is another global health issue affecting more than 1 billion people worldwide and is prevalent in nearly 60% of Saudi population.10 An association has been found between 25[OH]D deficiency and several chronic diseases, including autoimmune disorders, inflammatory bowel diseases, hepatic inflammation, bronchial asthma, neuro-cognitive dysfunctions and malignancies.11 Still, a link between vitamin D status with T2DM and/or hypertension is not fully established. Several studies indicated the presence of a correlation between the initiation, progression, or complications of these diseases with 25[OH]D deficiency,12–15 while other studies showed that there was no correlation.16,17 Consequently, the relationship between 25[OH]D deficiency and the oxidant/inflammatory status in T2DM and/or hypertension also remains controversial. Thus, the objective of the current study was to explore the prevalence of 25[OH]D deficiency among T2DM and hypertensive Saudi patients, and to evaluate the impact of such deficiency on the patients’ oxidative and inflammatory status, represented by serum levels of GSH and TNF-α, respectively.
Materials and Methods
Study Design, Participants, and Setting
This is a cross-sectional study of a consecutive purposive sample of patients attending Family Medicine Clinic at King Abdullah bin Abdulaziz University Hospital (KAAUH) from January 2020 to December 2021. Participants were screened for eligibility according to the following criteria: aged above 18 years and diagnosed with T2DM and/or hypertension for at least one year. Patients receiving any kind of vitamin D or calcium supplements within the last three months were excluded. In addition, exclusion criteria included patients with a history of renal failure, cancer, liver, thyroid, or any other chronic inflammatory diseases.
The current study was approved by the institutional review board (IRB) from Princess Nourah bint Abdulrahman University (PNU), with number of approval 18–0136 on 17/04/2018. An approval was also taken from KAAUH (R0-2019-K-002). In addition, a written consent and study information were disseminated to each patient and filled by the recruiting physician.
Data Collection and Study Variables
Eligible patients were booked for clinic visits with assigned family physicians, in which consent, patient’s data, and baseline physical examinations were recorded. Additionally, an initial set of clinical laboratory investigations were ordered during that visit. Each patient who agreed to participate and completed baseline data and physical examination was requested to go to the outpatient phlebotomy area located in the same hospital for blood sampling. Moreover, to assure confidentiality, easy identification, and processing of blood samples, each patient was given a code number to hand over to the assigned phlebotomist at the time of drawing of his/her blood sample. Study data were collected and managed using the Research Electronic Data Capture (REDCap),18 which is a secure electronic data capture tool hosted at PNU. The tool was designed based on type of collected data and was filled by assigned family physicians. It consists of three parts: sociodemographic data, clinical findings, and laboratory results. The sociodemographic data involved: age, gender, and smoking history. In addition, patients were inquired about other co-morbidities. Blood pressure was also recorded, where patients with systolic blood pressure (SBP) more than 140 mmHg or diastolic blood pressure (DBP) more than 90 mmHg were classified as uncontrolled hypertension.19,20 Patients whose Hemoglobin A1c (HbA1c) exceeded 7.5% were considered as uncontrolled T2DM. Furthermore, patients’ weights and heights were taken to calculate their body mass index (BMI) according to the following equation: BMI = Weight in kg/square of the height in meters.
Clinical Laboratory Investigations
Vitamin D has two biologically relevant forms; namely D3 and D2, that are converted by hydroxylation into 25[OH]D. The level of the latter is measured in serum to determine vitamin D status of all the patients after their outpatient-clinic visit. Measurement is done using commercial vitamin D kit (Abbott Diagnostics) compatible with Abbott Architect Analyzer i2000 SR (Abbott Laboratories, IL, USA). The principle of this technique is quantitative delayed one-step competitive chemiluminescent microparticle immunoassay (CMIA). Vitamin D status was defined according to IOM (US) reference range21 and was considered normal (sufficient) when 25[OH]D levels were > 50 nmol/L. Other routine laboratory investigations were also done for each patient including complete blood count (CBC) using ADVIA 2120i Hematology System (Siemens Healthcare Diagnostics Inc., NY, USA), as well as fasting blood glucose (FBG), C-reactive protein (CRP) and calcium (Ca) according to the commercial kit manufacturer’s instructions. Level of glycosylated hemoglobin (HbA1c) was measured using Beckman Coulter Unicel DxC Synchron 800 (Beckman Coulter, CA 92821, USA), where patients with HbA1c more than 7.5% were considered as uncontrolled diabetics.22 Using the latter equipment, fasting lipid profile was also measured, including total cholesterol, triglycerides, low-density lipoprotein (LDL) and high-density lipoprotein (HDL).
Assessment of GSH and TNF-α Using ELISA technique
The leftovers of serum samples that were collected from patients during routine laboratory investigations were stored at −80 °C until used. Measurements of GSH and TNF-α were performed using Evolis Fully Automated ELISA Processor (Bio-Rad Laboratories, CA, USA). For GSH assessment, colorimetric GSH assay kit was used (ab239727; BioVision/Abcam), whose action is based on an enzymatic cycling method in the presence of GSH and a chromophore. The reduction of the latter produces a stable compound that can be detected kinetically at 450 nm. For determination of TNF-α in serum, human TNF-α SimpleStep ELISA Kit (ab181421; BioVision/Abcam) was used, which is a sandwich ELISA technique that quantitatively measures TNF-α by capturing the antibodies conjugated to an affinity tag recognized by the monoclonal antibody coating the ELISA plate, forming antibody-analyte sandwich complex that can be detected kinetically at 450 nm.
Statistical Analysis
Shapiro–Wilks Normality Test was used to assess the statistical normality assumption of the continuous variables. As variables were not following the normal distribution, mean ± standard error along with median and 95% confidence intervals were used to express continuous variable. Frequency and percentage were used for categorically measured variables. Spearman’s correlations test was used to assess the correlations between metric variables. The Mann–Whitney U test and Kruskal–Wallis H-test were used to compare continuous variables among groups. The SPSS IBM statistical analysis program (Version#21, Armonk, NY: IBM Corp) was used for the statistical data analysis. The statistical significance level (P-value) was considered at 0.05 if achieved.
Results
Sociodemographic, Physical and Clinical Investigation Findings
Among the total sample recruited in this study, 120 (28.3%) had T2DM, 155 (36.6%) had hypertension and 149 (35.1%) suffered from both T2DM and hypertension. The average age of the total cohort was 54.9±0.6 years and patients with hypertension were significantly older than the other two groups (60.8±0.97 versus 51.9±0.96 and 51.7±0.80). The three groups had comparable gender distribution, smoking habits as well as BMI. As expected, patients with T2DM only showed significantly lower averages in systolic and diastolic blood pressure readings, while they had significantly higher glycemic parameters (FBG and HbA1c). Co-morbidities in terms of cerebrovascular accident/transient ischemic attack (CVA/TIA), cardiovascular disease (CVD), dyslipidemia, and osteoarthritis were significantly more frequently reported among patients with both T2DM and hypertension (Table 1).
 |
Table 1 Sociodemographic, Physical, and Clinical Characteristics of the Studied Population |
Most patients had normal 25[OH]D level (275, 64.9%) while 44 (10.4%) had deficiency as shown in Figure 1. For this reason, we have used 25[OH]D level of 50 nmol/l as a cut-off level between sufficiency and insufficiency.
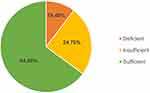 |
Figure 1 Vitamin D status (25[OH]D) among study population. |
Analysis of CBC did not differ significantly among the groups with exception of white blood cells (WBC) that was at its highest level in patients with both T2DM and hypertension and the lipid profile parameters of this group, except for triglycerides, seemed significantly at a lower level when compared with patients with either T2DM or hypertension. The average level of 25[OH]D concentration in the total cohort was 63.6±1.4 nmol/L, with higher level recorded among patients with both T2DM and hypertension (70.1±2.5 nmol/L). Additionally, GSH average was 380.3±11 μmol/L and was substantially higher in the same group (428.8±20 μmol/L) when compared with the patients with only T2DM or hypertension. TNF-α average was 153.2±5 pg/mL and was similar in all three groups.
Association of Vitamin D Status on GSH and TNF-α in T2DM and/or Hypertensive patients
Table 2 displays the independent association of vitamin D status (deficiency versus normal levels of serum 25[OH]D) on GSH and TNF-α among various groups in the studied population. Younger patients (below 60 years) and females had lower levels of GSH whether they were suffering from 25[OH]D deficiency or not. Among patients with normal 25[OH]D levels, patients with uncontrolled T2DM showed a significantly higher level of GSH (420.7 μmol/L (95% CI: 355.3–486.0)) compared with controlled group (367.9 μmol/L (95% CI: 337.9–397.8)) and similarly in patients with 25[OH]D deficiency (uncontrolled T2DM 432.8 μmol/L (95% CI: 324.6–523.1)) versus controlled T2DM (351.7 μmol/L (95% CI: 310.3–393.2), respectively). In the meantime, the GSH level was higher among patients with uncontrolled hypertension (400.1 μmol/L (95% CI: 340.7–459.4)) compared with those with controlled hypertension (332.9 μmol/L (95% CI: 283.9–381.9)), but this difference did not reach the statistically significant level (P>0.05). The GSH showed significantly higher level among patients with both T2DM and hypertension when compared with those suffering from only one of the conditions (P=0.04). Moreover, older patients, males, and those with normal 25[OH]D levels had relatively higher GSH level across all categories of co-morbidities. Patients with uncontrolled T2DM had higher levels of GSH whether they were suffering from 25[OH]D deficiency or not, while the GSH level did not differ between patients with either controlled or uncontrolled hypertension even if they have vitamin D deficiency. Likewise, the independent association of vitamin D status on TNF-α among various groups was evaluated (Table 2) showing no specific defined pattern when comparing patients with 25[OH]D deficiency and those with normal level across all subgroups (age, gender, controlled vs uncontrolled T2DM/hypertension or even co-morbidities). However, males showed slightly higher level of TNF-α compared with females and uncontrolled hypertensive patients had relatively higher TNF-α levels when evaluated against controlled hypertensive patients.
 |
Table 2 Reduced Glutathione (GSH) and Tumor Necrosis Factor (TNF)-α Levels Among different Categories According to Vitamin D Status |
Correlation Between 25[OH]D, GSH and TNF-α Levels with All Tested Parameters
Pairwise correlations were investigated between all laboratory workup and 25[OH]D concentration, GSH and TNF-α. The 25[OH]D concentration was positively correlated with MCV (r = 0.128, P<0.01). The level of GSH was also positively correlated with HbA1c (r = 0.146, P<0.01) and WBC (r = 0.143, P<0.01), while negatively correlated with HDL (r = −0.124, P<0.01). TNF-α did not show any significant correlations in the pairwise comparisons with other parameters (Table 3).
 |
Table 3 Correlation Between Reduced Glutathione (GSH) and Tumor Necrosis Factor (TNF)-α with Other Laboratory and Clinical Findings |
Discussion
The prevalence of 25[OH]D deficiency is one of the alarming health concerns worldwide due to its association with several chronic non-communicable, and even communicable diseases.23 Such deficiency is prevalent in different societies to various extents depending on several factors, including duration of sunlight exposure, skin pigmentation, 25[OH]D precursor availability from dietary nutritional sources, genetic polymorphisms, physical activity, and vitamin D pharmaceutical supplementation.24–26 The association between the prevalence of 25[OH]D with diabetes and/or hypertension also seemed to have ethnic bases.27 In Saudi Arabia, 25[OH]D deficiency was a major concern, as some studies reported nearly 70% prevalence among T2DM patients.13 In the current study, the prevalence of 25[OH]D deficiency was 35.1% in T2DM and/or hypertensive patients. It is noteworthy that patients suffering from hypertension only had lower levels of 25[OH]D. The prevalence of 25[OH]D deficiency reported in the current study is slightly lower than the previously reported levels, which is in line with the documented constant decrease in 25[OH]D deficiency prevalence in this region of Saudi Arabia over the recent years.28,29 This might be attributed to the constant efforts to improve the awareness, knowledge, attitude, and practices towards maintaining normal levels of 25[OH]D among Saudi population.30,31 Moreover, different populations, assays and definitions of deficiency could influence these results.
To date, it was still debatable whether there was an effect of 25[OH] deficiency on the antioxidant and/or inflammatory status of T2DM and/or hypertensive patients. On one hand, some studies showed a relationship between 25[OH]D and the alteration of GSH levels in T2DM.32,33 While one meta-analysis showed that 25[OH]D supplementation had a beneficial effect improving most of the oxidative stress parameters among diabetic patients.34 Furthermore, a study reported that severe 25[OH]D deficiency was accompanied by an increase in TNF-α in diabetic patients suffering from painful peripheral neuropathy.35 For hypertension, 25[OH]D/calcium supplementation was reported to cause an increase in GSH and a decrease in blood pressure in women at risk of hypertension with pregnancy.36 To the contrary, some studies showed that 25[OH]D had no effect. For example, one study reported that 25[OH]D deficiency was not accompanied by an elevation in systemic inflammation in T2DM patients.37 As for hypertension, an animal study performed on spontaneously hypertensive rats showed that 25[OH]D supplementation did not alter the level of GSH.38 Similarly, another study showed that 25[OH]D supplementation failed to improve hypertension clinically in patients by decreasing their blood pressure39 or by decreasing their systemic inflammatory markers.40 In line with the results of the latter studies, in the present study, 25[OH] deficiency was independent from oxidative stress represented by GSH level or inflammatory pathway represented by TNF-α levels in both T2DM and hypertensive Saudi patients. Nevertheless, the alarming prevalence of more than one third of a tested population suffering from 25[OH]D deficiency still should not be taken lightly, as it is a serious co-morbidity when added to the hazards faced by T2DM and hypertensive patients.
In the current study, the absolute level of GSH was around 380 μmol/L on average among all tested groups, which is considered within the normal range reported for GSH levels in humans (290–490 μmol/L).41 In previous studies, the level of GSH was reported to be lower in T2DM42 and hypertensive patients43 compared with normal controls, owing to the oxidative stress induced during the pathogenic course of these diseases. Nevertheless, some studies reported that there was no significant difference between GSH levels in T2DM or hypertensive patients compared with their respective controls.44,45 It is noteworthy that, in the current study, we did not compare T2DM/hypertensives with controls, but rather we investigated a correlation between the level of GSH among diabetics and/or hypertensive patients, correlating it with vitamin D status. Indeed, some studies indicated a crosstalk between 25[OH]D and GSH, where GSH was reported to positively up-regulate the bioavailability of 25[OH)D,46 and, on the other hand, 25[OH)D was shown to up-regulate GSH production in vitro in U937 monocytes exposed to high glucose.47 Still, it seems that such in vitro and animal preliminary studies do not reflect significance in clinical situations, as it did not attain significant medically relevant correlation in the present study. Independent from 25[OH]D levels, the current study showed that the level of GSH had a trend of being relatively higher in patients with uncontrolled T2DM or hypertension compared with controlled patients. In addition, GSH was higher in patients suffering concomitantly from both T2DM and hypertension compared with those having one of the diseases alone. It is possible that such an increase in GSH is a part of a feedback protective mechanism of the reactive oxygen species scavenging system. This feedback effect was accentuated by the fact that GSH level in the current study also increased in correlation with age and with the increase in WBC, indicative of infection, while decreased in correlation with HDL, which is considered the “good” cholesterol.
The pro-inflammatory cytokine TNF-α was considered one of the biomarkers indicative of the severity of T2DM, as higher levels of circulating TNF-α receptors were correlated with higher levels of mortality in these patients.48 In hypertensive patients, TNF-α was also shown to have higher levels compared with controls.49 Supplementation of T2DM patients with 25[OH]D was shown to cause an improvement of their inflammatory status.50 Similarly, in hypertensive patients, a correlation was established between 25[OH]D insufficiency and SBP in hypertensive patients,51 which was contrary to the results of the current study where there was no correlation of the level of 25[OH]D with TNF-α in either T2DM or hypertensive patients. An explanation for such discrepancy may be due to the increment of GSH seen in the present study that might blunt the oxidative stress, which might be a main determinant factor of induction of inflammatory pathway. Nevertheless, in the current study slightly higher levels of the pro-inflammatory cytokine TNF-α were seen in uncontrolled hypertensive patients compared with patients with controlled blood pressure.
Conclusions
The prevalence of 25[OH]D deficiency in T2DM and hypertensive Saudi patients was seen in about one third of the patients only, which is considered an improvement compared with previously reported studies. This was independent of the levels of GSH or TNF-α in both disease groups. GSH seemed to play a critical role as a feedback protective biomarker in uncontrolled patients of both diseases, ameliorating oxidative stress and limiting the induction of TNF-α.
Abbreviations
25[OH]D, 25-hydroxyvitamin D; BMI, body mass index; Ca, calcium; CVA/TIA, cerebrovascular accident/ transient ischemic attack; CVD, cardiovascular diseases; CRP, C-reactive protein; DBP, diastolic blood pressure; FBG, fasting blood glucose; GSH, reduced glutathione; Hb, hemoglobin; HCT, hematocrit; HDL, high density lipoprotein; KAAUH, King Abdullah bin Abdulaziz University Hospital; LDL, low density lipoprotein; MCV, mean corpuscular volume; RBC, red blood cells; RDW, red cell distribution width; SBP, systolic blood pressure; T2DM, type II diabetes mellitus; TG, triglycerides; TNF-α, tumor necrosis factor-α; WBC, white blood cells.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Princess Nourah bint Abdulrahman University, number 18-0136 on 17/04/2018, as well as King Abdullah bin Abdulaziz University Hospital (R0-2019-K-002). Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research project was funded by Health Sciences Research Center, King Abdullah bin Abdulaziz University Hospital, Princess Nourah bint Abdulrahman University, through the Research Funding Program, grant number (G18-00016).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aljulifi MZ. Prevalence and reasons of increased type 2 diabetes in Gulf Cooperation Council Countries. Saudi Med J. 2021;42(5):481–490. doi:10.15537/smj.2021.42.5.20200676
2. Saeed AA, Al-Hamdan NA, Bahnassy AA, Abdalla AM, Abbas MAF, Abuzaid LZ. Prevalence, awareness, treatment, and control of hypertension among Saudi adult population: a national survey. Int J Hypertens. 2011;2011:174135. doi:10.4061/2011/174135
3. Ndisang JF, Vannacci A, Rastogi S. Oxidative stress and inflammation in obesity, diabetes, hypertension, and related cardiometabolic complications. Oxidative Med Cell Longev. 2014;2014:506948. doi:10.1155/2014/506948
4. Pouvreau C, Dayre A, Butkowski EG, de Jong B, Jelinek HF. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res. 2018;11:61–68. doi:10.2147/JIR.S148911
5. Teskey G, Abrahem R, Cao R, et al. Glutathione as a Marker for human disease. Adv Clin Chem. 2018;87:141–159.
6. Matuz-Mares D, Riveros-Rosas H, Vilchis-Landeros MM, Vázquez-Meza H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants. 2021;10(8):1220. doi:10.3390/antiox10081220
7. Xue T, Zhang X, Xing Y, et al. Advances about immunoinflammatory pathogenesis and treatment in diabetic peripheral neuropathy. Front Pharmacol. 2021;12:748193. doi:10.3389/fphar.2021.748193
8. Murray EC, Nosalski R, MacRitchie N, et al. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res. 2021;117(13):2589–2609. doi:10.1093/cvr/cvab330
9. Taye A, El-Sheikh AAK. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur J Clin Investig. 2013;43(7):740–745. doi:10.1111/eci.12092
10. Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M. Vitamin D deficiency in Saudi Arabians: a reality or simply hype: a meta-analysis (2008–2015). J Family Community Med. 2016;25:1–4.
11. Marino R, Misra M. Extra-skeletal effects of vitamin D. Nutrients. 2019;11(7):1460. doi:10.3390/nu11071460
12. Nasr MH, Hassan BAR, Othman N, et al. Prevalence of vitamin D deficiency between type 2 diabetes mellitus patients and non-diabetics in the Arab Gulf. Diabetes Metab Syndr Obes. 2022;15:647–657. doi:10.2147/DMSO.S350626
13. Al Ghadeer HA, AlRamadan MS, Al Amer MM, et al. Vitamin D serum levels in type 2 diabetic patients: a cross-sectional study. Cureus. 2022;14(2):e22558. doi:10.7759/cureus.22558
14. Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–645. doi:10.1097/HJH.0b013e32834320f9
15. Alagacone S, Verga E, Verdolini R, Saifullah SM. The association between vitamin D deficiency and the risk of resistant hypertension. Clin Exp Hypertens. 2020;42(2):177–180. doi:10.1080/10641963.2019.1601204
16. Al-Sumaih I, Johnston B, Donnelly M, O’Neill C. The relationship between obesity, diabetes, hypertension and vitamin D deficiency among Saudi Arabians aged 15 and over: results from the Saudi health interview survey. BMC Endocr Disord. 2020;20(1):81. doi:10.1186/s12902-020-00562-z
17. Legarth C, Grimm D, Wehland M, Bauer J, Krüger M. The impact of vitamin D in the treatment of essential hypertension. Int J Mol Sci. 2018;19(2):455. doi:10.3390/ijms19020455
18. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
19. Carey RM, Calhoun DA, Bakris GL, et al.; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53–e90. doi:10.1161/HYP.0000000000000084
20. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi:10.1097/HJH.0000000000002453
21. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): National Academies Press (US); 2011. PMID: 21796828
22. Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American college of physicians. Ann Intern Med. 2018;168(8):569–576. doi:10.7326/M17-0939
23. Karampela Ι, Vallianou N, Magkos F, Apovian CM, Dalamaga M. Obesity, hypovitaminosis D, and COVID-19: the Bermuda triangle in public health. Curr Obes Rep. 2022;1–10. doi:10.1007/s13679-022-00471-3
24. Kulkarni B, Augustine L, Nair K. Sun exposure as a strategy for acquiring vitamin D in developing countries of tropical region: challenges & way forward. Indian J Med Res. 2022;154:423–432. doi:10.4103/ijmr.IJMR_1244_18
25. Das S, Hasan MM, Mohsin M, et al. Sunlight, dietary habits, genetic polymorphisms and vitamin D deficiency in urban and rural infants of Bangladesh. Sci Rep. 2022;12(1):3623. doi:10.1038/s41598-022-07661-y
26. Anouti FA, Ahmed LA, Riaz A, et al. Vitamin D deficiency and its associated factors among female migrants in the United Arab Emirates. Nutrients. 2022;14(5):1074. doi:10.3390/nu14051074
27. Xia J, Tu W, Manson JE, et al. Combined associations of 25-hydroxivitamin D and parathyroid hormone with diabetes risk and associated comorbidities among U.S. white and black women. Nutr Diabetes. 2021;11(1):29. doi:10.1038/s41387-021-00171-2
28. Al-Daghri NM, Hussain SD, Ansari MGA, et al. Decreasing prevalence of vitamin D deficiency in the central region of Saudi Arabia (2008–2017). J Steroid Biochem Mol Biol. 2021;212:105920. doi:10.1016/j.jsbmb.2021.105920
29. Kaddam IM, Al-Shaikh AM, Abaalkhail BA, et al. Prevalence of vitamin D deficiency and its associated factors in three regions of Saudi Arabia. Saudi Med J. 2017;38(4):381–390. doi:10.15537/smj.2017.4.18753
30. Habib SS, Alhalabi HB, Alharbi KS, et al. Knowledge attitude and practices of university students to vitamin D and vitamin D supplements during times of low sun exposure and post lockdown. Eur Rev Med Pharmacol Sci. 2021;25(23):7297–7305. doi:10.26355/eurrev_202112_27423
31. Zareef TA, Jackson RT. Knowledge and attitudes about vitamin D and sunlight exposure in premenopausal women living in Jeddah, and their relationship with serum vitamin D levels. J Health Popul Nutr. 2021;40(1):38. doi:10.1186/s41043-021-00263-w
32. Jain SK, Kahlon G, Bass P, Levine SN, Warden C. Can L-cysteine and vitamin D rescue vitamin D and vitamin D binding protein levels in blood plasma of African American type 2 diabetic patients? Antioxid Redox Signal. 2015;23(8):688–693. doi:10.1089/ars.2015.6320
33. Gu JC, Wu YG, Huang WG, et al. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J Clin Lab Anal. 2022;36(5):e24430. doi:10.1002/jcla.24430
34. Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, et al. The effect of vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. 2019;139:141–152. doi:10.1016/j.phrs.2018.11.011
35. Xiaohua G, Dongdong L, Xiaoting N, et al. Severe vitamin D deficiency is associated with increased expression of inflammatory cytokines in painful diabetic peripheral neuropathy. Front Nutr. 2021;8:612068. doi:10.3389/fnut.2021.612068
36. Samimi M, Kashi M, Foroozanfard F, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J Hum Nutr Diet. 2016;29(4):505–515. doi:10.1111/jhn.12339
37. Cătoi AF, Iancu M, Pârvu AE, et al. Relationship between 25 hydroxyvitamin D, overweight/obesity status, pro-inflammatory and oxidative stress markers in patients with type 2 diabetes: a simplified empirical path model. Nutrients. 2021;13(8):2889. doi:10.3390/nu13082889
38. Machado CDS, Ferro Aissa A, Ribeiro DL, Antunes LMG. Vitamin D supplementation alters the expression of genes associated with hypertension and did not induce DNA damage in rats. J Toxicol Environ Health A. 2019;82(4):299–313. doi:10.1080/15287394.2019.1592044
39. Theiler-Schwetz V, Trummer C, Grübler MR, et al. Effects of vitamin D supplementation on 24-hour blood pressure in patients with low 25-hydroxyvitamin D levels: a randomized controlled trial. Nutrients. 2022;14(7):1360. doi:10.3390/nu14071360
40. Grübler MR, Zittermann A, Verheyen ND, et al. Randomized trial of vitamin D versus placebo supplementation on markers of systemic inflammation in hypertensive patients. Nutr Metab Cardiovasc Dis. 2021;31(11):3202–3209. doi:10.1016/j.numecd.2021.07.028
41. Tekin S, Seven E. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye. 2021. doi:10.1038/s41433-021-01753-1
42. Picu A, Petcu L, Ştefan S, et al. Markers of oxidative stress and antioxidant defense in Romanian patients with type 2 diabetes mellitus and obesity. Molecules. 2017;22(5):714. doi:10.3390/molecules22050714
43. Pinzón-Díaz CE, Calderón-Salinas JV, Rosas-Flores MM, Hernández G, López-Betancourt A, Quintanar-Escorza MA. Eryptosis and oxidative damage in hypertensive and dyslipidemic patients. Mol Cell Biochem. 2017;440(1–2):105–113. doi:10.1007/s11010-017-3159-x
44. Gawlik K, Naskalski JW, Fedak D, et al. Markers of antioxidant defense in patients with type 2 diabetes. Oxidative Med Cell Longev. 2016;2016:2352361. doi:10.1155/2016/2352361
45. Liu Q, Liu Y, Shi J, et al. Entire peroxidation reaction system of myeloperoxidase correlates with progressive low-density lipoprotein modifications via reactive aldehydes in atherosclerotic patients with hypertension. Cell Physiol Biochem. 2018;50(4):1245–1254. doi:10.1159/000494579
46. Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA
47. Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437(1):7–11. doi:10.1016/j.bbrc.2013.06.004
48. Cheng D, Fei Y, Saulnier P-J, Wang N. Circulating TNF receptors and risk of renal disease progression, cardiovascular disease events and mortality in patients with diabetes: a systematic review and meta-analysis. Endocrine. 2020;68(1):32–43. doi:10.1007/s12020-019-02153-y
49. Verma MK, Jaiswal A, Sharma P, Kumar P, Singh AN. Oxidative stress and biomarker of TNF-alpha, MDA and FRAP in hypertension. J Med Life. 2019;12(3):253–259. doi:10.25122/jml-2019-0031
50. El Hajj C, Walrand S, Helou M, Yammine K. Effect of vitamin D supplementation on inflammatory markers in non-obese Lebanese patients with type 2 diabetes: a randomized controlled trial. Nutrients. 2020;12(7):2033. doi:10.3390/nu12072033
51. Zuk AM, Liberda EN, Tsuji LJS. Examining chronic inflammatory markers on blood pressure measures in the presence of vitamin D insufficiency among indigenous cree adults: results from the cross-sectional multi-community environment-and-health study in Eeyou Istchee, Quebec, Canada. BMJ Open. 2021;11(1):e043166. doi:10.1136/bmjopen-2020-043166
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
