Back to Journals » Journal of Inflammation Research » Volume 15
Independent and Joint Association of Statin Therapy with Adverse Outcomes in Heart Failure Patients with Atrial Fibrillation Treated with Cardiac Resynchronization Therapy
Authors Yu Y, Ding L, Deng Y, Huang H, Cheng S, Cai C, Gu M, Chen X, Ning X, Niu H, Hua W
Received 16 September 2022
Accepted for publication 29 November 2022
Published 9 December 2022 Volume 2022:15 Pages 6645—6656
DOI https://doi.org/10.2147/JIR.S390127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yu Yu,* Ligang Ding,* Yu Deng,* Hao Huang, Sijing Cheng, Chi Cai, Min Gu, Xuhua Chen, Xiaohui Ning, Hongxia Niu, Wei Hua
Cardiac Arrhythmia Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100037, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Hua, Cardiac Arrhythmia Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100037, People’s Republic of China, Email [email protected]
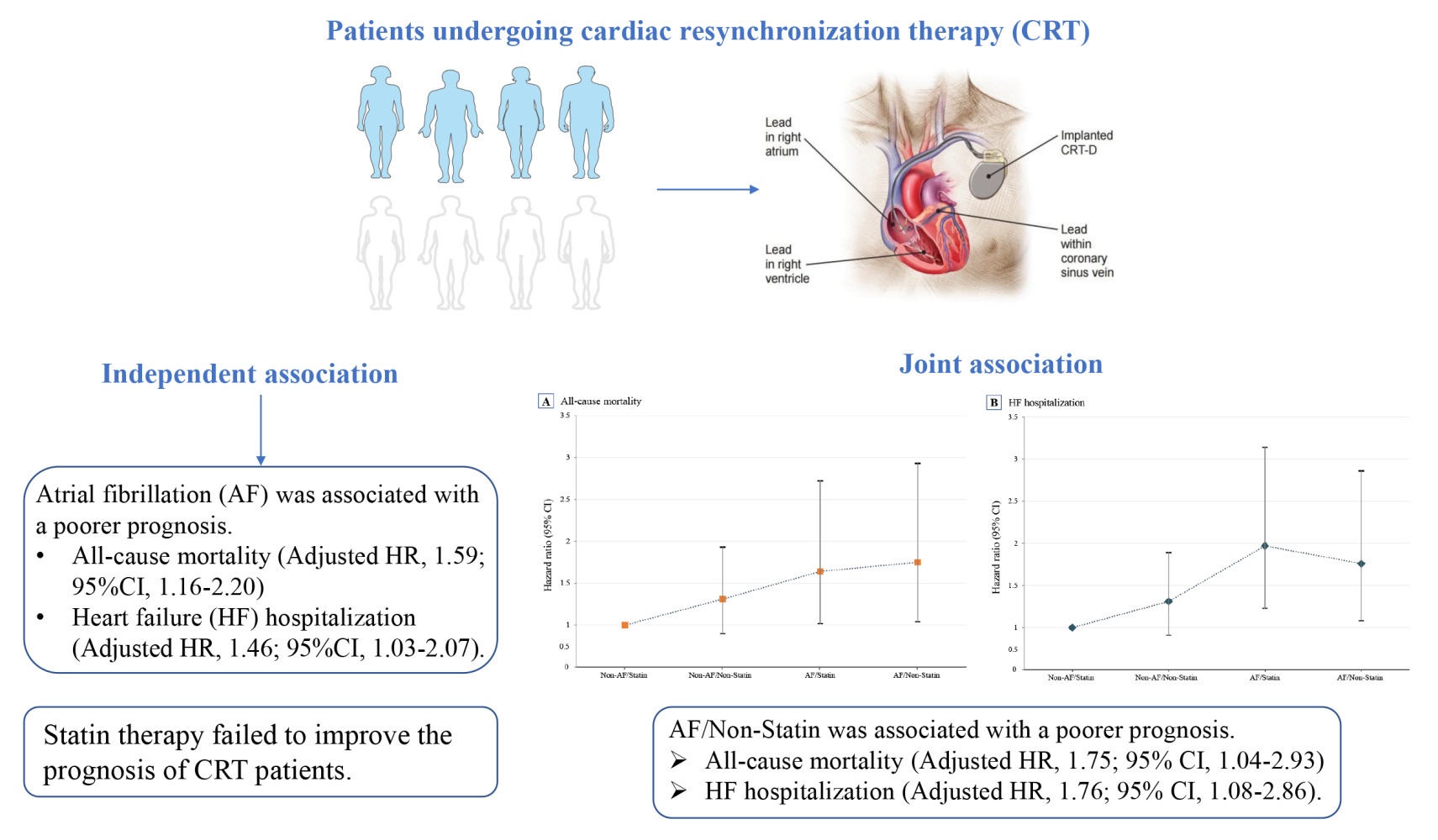
Background: The joint association of atrial fibrillation (AF) and statin therapy with adverse outcomes in heart failure (HF) patients with cardiac resynchronization therapy (CRT) has not been fully investigated so far. The purpose of this study was to explore the independent and joint association of AF and statin therapy with adverse outcomes.
Methods: Study patients were divided into four groups according to AF status and statin use: Non-AF/Statin, Non-AF/Non-Statin, AF/Statin, and AF/Non-Statin. Multivariate Cox proportional hazards regression models were used to evaluate the independent and joint association of AF and statin therapy with poor prognosis.
Results: Among 685 CRT patients, there were 180 deaths (26.5%) and 198 HF hospitalization (29.6%) during the 14 years of follow-up. AF was associated with a 46% increased risk of all-cause mortality (HR, 1.46; 95% CI, 1.03– 2.07) and a 59% increased risk of HF hospitalization (HR, 1.59; 95% CI, 1.16– 2.20) than those without AF. However, statin therapy failed to improve the prognosis. In the joint analysis, compared with the Non-AF/Statin group, the AF/Non-Statin group suffered a higher risk of all-cause mortality (HR, 1.75; 95% CI, 1.04– 2.93) and HF hospitalization (HR, 1.76; 95% CI, 1.08– 2.86). Furthermore, adding AF to the traditional risk factor model significantly improved the predictive value for death (C-statistic from 0.654 to 0.691) and HF (C-statistic from 0.613 to 0.675).
Conclusion: AF was associated with poor prognosis, and statin use failed to improve the prognosis. Further analysis showed that statin therapy is ineffective in improving prognosis and fails to attenuate the adverse effects of AF.
Keywords: atrial fibrillation, cardiac resynchronization therapy, joint association, prognosis, statin therapy
Graphical Abstract:
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with heart failure (HF),1 with 135–400 patients per million people per year worldwide being suitable candidates for CRT.2,3 However, there are significant differences in the prognosis of patients treated with CRT,4 and approximately 30% of patients fail to achieve benefit from CRT, and these patients are referred to as CRT non-responders.5 In particular, atrial fibrillation (AF) is a leading cause of poor prognosis for CRT patients, as episodes of AF result in a significant reduction in the percentage of biventricular pacing (BVP).6
Statins have been shown to have rapid anti-inflammatory and antioxidant effects that have important implications for the prognosis of cardiac patients.7 Established evidence suggests that patients undergoing cardiac surgery benefit from perioperative statin therapy in reducing the risk of all-cause mortality, stroke, and AF.8–10 In addition, several studies have reported that statin use was associated with reduced mortality in patients with implantable cardioverter-defibrillator (ICD) and a reduced risk of fatal ventricular tachycardia in patients with CRT.11,12 However, a recent study found that statin use was associated with poorer survival in patients with CRT.13 Therefore, the evidence on the impact of statin use in CRT patients is limited and inconsistent. Of note, patients with AF may be more likely to use statins than those free of AF due to AF-related comorbidities, including coronary artery disease (CAD), stroke, and hyperlipidemia.14,15 Moreover, statin therapy is associated with a reduced risk of all-cause mortality in patients with AF.16 From this perspective, a critical hypothesis is generated that whether AF-free status combined with statin therapy can further improve the prognosis of CRT patients. However, epidemiological evidence remains scarce on the joint association of AF status and statin therapy with prognosis in patients with CRT.
This study was designed to investigate the independent and joint association of AF status and statin therapy with adverse outcomes in HF patients with CRT. The knowledge generated may contribute to improving the prognosis of HF patients with CRT.
Methods
Study Design and Population
This study is a single-center, retrospective, observational cohort study. A total of 686 patients with HF who underwent CRT implantation at the Arrhythmia Center of Fuwai Hospital between March 2007 and March 2019 were consecutively enrolled in this study. All patients involved in the study signed an informed consent form. The procedure of CRT implantation has been described in detail previously.17 The inclusion and exclusion criteria for the study are shown in detail in Table S1. The Ethics Committee approved this study of Fuwai Hospital, Chinese Academy of Medical Sciences (IRB2012-BG-006). The study was conducted in accordance with the Declaration of Helsinki18 and followed the guidelines for reporting cohort studies of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). After excluding the sample with missing AF data, a total of 685 patients were entered into the final analysis, as illustrated in Figure 1.
 |
Figure 1 Flowchart of the study population. Abbreviations: CRT, cardiac resynchronization therapy; AF, atrial fibrillation. |
Data Collection and Measurements
Baseline characteristics of study patients were extracted through the hospital’s electronic medical system. The covariates in this study included demographic information, disease history, medication history, cardiac function parameters, and laboratory tests and imaging examinations. Disease history included AF, ischemic cardiomyopathy (CMP), left bundle branch block (LBBB), CAD, stroke, hypertension, diabetes, hyperlipidemia, and ventricular tachycardia/ventricular fibrillation (VT/VF). Medication history includes the presence of inpatient medications and post-discharge medications, consisting of statin, β-blocker, diuretics, amiodarone, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), anticoagulants, and antiplatelet. NYHA cardiac function classification is determined by the clinician based on the patient’s symptoms, medical history, and clinical tests and examinations of cardiac structure and function. Left ventricular ejection fraction (LVEF) and left ventricular end-diastolic diameter (LVEDD) were measured by echocardiography. Data entry was performed using a double-blind entry method.
Definition of AF and Statin Therapy
Information on AF and statin therapy was obtained from the electronic case system of Fuwai Hospital. The diagnosis of AF was mainly based on electrocardiogram (ECG) performance. In standard 12-lead ECG or single-lead ECG without discernible repeating sinus P-wave, traced to f-wave and irregular RR interval, the above ECG lasts more than 30s, then the diagnosis of AF was confirmed.19 Pre-existing AF was obtained based on previous hospital records or ECG reports. The clinician derived the diagnosis of new-onset AF based on the interpretation of the ECG report during the hospitalization.
The data on statin therapy was derived from electronic medical records during hospitalization. Statins are mainly used for the treatment of CAD, stroke, and hyperlipidemia, as recommended by clinical guidelines.20 The statins in the study included atorvastatin, rosuvastatin, simvastatin, pravastatin, and pitavastatin.
Study Outcomes and Follow-Up
The study outcomes were all-cause death and HF hospitalization. The follow-up deadline was January 2021. Follow-up for study outcomes was from CRT implantation until the date of first HF rehospitalization or death. Information on study outcomes was collected through hospital outpatient records and medical telephone calls.
Statistical Analysis
Baseline characteristics were divided into four groups according to AF status and statin use, with continuous variables presented as mean ± standard deviation (SD) and categorical variables presented as percentages. For continuous variables, p-values for group comparisons were derived from the analysis of variance (ANOVA), and the chi-square test was used to compare the categorical variables. Hazard ratios (HR) and 95% confidence intervals (CI) for the association of AF status and statin use with all-cause mortality and HF hospitalization were estimated using multivariate Cox proportional hazards regression models, respectively. Adjusted variables in multivariate Cox proportional hazards regression models were based on clinical relevance or univariate association with outcomes, and further screening was performed to determine the final regression model based on the number of available events.21 In the joint analysis, participants were classified into four groups based on AF status and statin use, and multivariate Cox proportional hazards regression models were used to assess the association of the combined variables with all-cause mortality and HF hospitalization. The C-statistics, integrated discrimination improvement (IDI), and net reclassification improvement (NRI) were used to reveal the incremental predictive value of AF and statin therapy for study outcomes.22 All analyses were performed with R statistical software version 4.0.2 and a two-sided P<0.05 was statistically significant.
Results
Baseline Characteristics
A total of 685 patients with CRT implantation were included in the study. In Table 1, Baseline characteristics were divided into four groups according to AF status and statin use: Non-AF/Statin, Non-AF/Non-Statin, AF/Statin, and AF/Non-Statin groups. Patients in the AF/Non-Statin group tended to be younger, have lower rates of smoking, ischemic CMP, LBBB, CAD, hypertension, diabetes, hyperlipidemia, BVP percentage, LVEF ≤35%, and antiplatelet drug use; and higher rates of VT/VF, amiodarone and anticoagulant use, as well as higher values of NT-proBNP than other groups. In this CRT cohort, patients without AF shared a greater proportion (nearly 80%). The proportion of patients in the Non-AF/Statin group was 34.6%, and 44.5% in the Non-AF/Non-Statin group. In contrast, the proportion of patients in the AF/Statin group was only 9.9%, and the proportion in AF/Non-Statin was 10.9% (Figure 2).
 |
Table 1 Baseline Characteristics of the Total Population and Stratified by AF Status and Statin Use |
 |
Figure 2 Joint prevalence of atrial fibrillation and statin use among patients with cardiac resynchronization therapy, 2007 to 2019. |
Independent Association of AF Status, Statin Use and Prognosis
During the 14-year follow-up, 180 (26.5%) CRT patients experienced death, and 198 (29.6%) CRT patients experienced HF hospitalization. CRT patients with a history of AF had a higher incidence of all-cause mortality (25.2% vs 31.7%) and HF hospitalization (27.3% vs 38.3%). In Table 2, after adjusting for potential confounders, CRT patients with a history of AF were associated with a 46% increased risk of all-cause mortality (HR, 1.46; 95% CI, 1.03–2.07) and a 59% increased risk of HF hospitalization (HR, 1.59; 95% CI, 1.16–2.20). Meanwhile, CRT patients in the Statin group had a lower incidence of all-cause mortality (28.5% vs 24.0%) and HF hospitalization (31.6% vs 27.2%). Although CRT patients on statin use had lower incidences of all-cause mortality and HF hospitalization, no significant reduction in the risk of death and HF hospitalization was observed in the Statin group.
 |
Table 2 Association of AF Status and Statin Use with All-Cause Mortality and HF Hospitalization |
Joint Association of AF Status and Statin Use with Prognosis
In the joint analysis, CRT patients in the Non-AF/Statin group were at the lowest risk of all-cause mortality and HF hospitalization (Figure 3). With the Non-AF/Statin group as a reference, the Non-AF/Non-Statin, AF/Statin, and AF/Non-Statin groups had a significantly higher incidence of all-cause mortality and HF hospitalization (Table 3). In particular, as compared with the Non-AF/Statin group, the AF/Statin group was associated with a significantly higher risk of all-cause mortality (HR, 1.64; 95% CI, 1.02–2.72) and HF hospitalization (HR, 1.97; 95% CI, 1.23–3.14). Similarly, the AF/Non-Statin group suffered a higher risk of all-cause mortality (HR, 1.75; 95% CI, 1.04–2.93) and HF hospitalization (HR, 1.76; 95% CI, 1.08–2.86).
 |
Table 3 Joint Association of AF Status and Statin Use with All-Cause Mortality and HF Hospitalization |
Incremental Predictive Value of AF and Statin
With the addition of AF to the conventional model (Table 4), the new model yielded a significant improvement in predictive accuracy for all-cause mortality (C-statistic from 0.654 to 0.691, P<0.001) and HF hospitalization (C-statistic from 0.613 to 0.675, P<0.001), but not significantly improve with the addition of statin. Moreover, the new model with the addition of AF had a significantly improved predictive power for all-cause mortality compared to the conventional model, with an IDI was 0.044 and NRI was 0.191 (P<0.05). Similarly, the predictive power of the new model for HF hospitalization was improved considerably, with an IDI was 0.048 and NRI was 0.181 (P<0.05).
 |
Table 4 Reclassification and Discrimination Statistics for AF Status and Statin Use |
Discussion
To our knowledge, this is the first study to investigate the independent and joint associations of AF status and statin therapy with adverse outcomes of HF patients with CRT. Among these patients, more than 20% reported a history of AF, and nearly half received statin therapy, with more than one-third of CRT patients in the Non-AF/Statin group. During as many as 14 years of follow-up, AF was associated with an increased risk of all-cause mortality and HF hospitalization. However, this study failed to observe the effect of statin use on the prognosis. In the joint analysis, the combination of AF-free status and statin therapy had the lowest incidence of all-cause mortality and HF hospitalization. Further analysis showed that adding AF to the conventional risk factor model significantly improved the predictive value of the prognosis of patients with CRT.
The prevalence of AF in CRT patients is approximately 25%,23 and the presence of AF has an important impact on the prognosis of CRT patients.6 Previous studies frequently investigated AF status, with little consideration of its joint relationship with statin use. Wilton et al6 conducted a meta-analysis and found that AF was associated with a 32% increased risk of CRT non-response (relative risk [RR], 1.32; 95% CI, 1.12–1.55); and a 50% increased risk of all-cause mortality (RR, 1.50; 95% CI, 1.08–2.09). The APAF-CRT study suggested that CRT combined with atrioventricular node ablation significantly improved CRT response rates and survival benefits in HF patients with AF than pharmacotherapy.24 The above findings support that AF status adversely affects the prognosis of CRT patients and that CRT patients can derive a substantial clinical benefit from controlling the AF rhythm. Consistent with previous studies, our findings suggested that AF status was associated with a higher risk of all-cause mortality and HF hospitalization in patients with CRT compared to those without AF.
The prognostic effects of statins in cardiac patients have received increasing attention due to their multiple effects.24,25 Liakopoulos et al8 conducted a meta-analysis that perioperative statin therapy resulted in a 43% reduction in the risk of all-cause mortality (odds ratio [OR], 0.57; 95% CI, 0.49–0.67). However, in this Chinese population-based RCT cohort, perioperative statin therapy failed to prevent adverse outcomes in cardiac surgery patients.26 In addition, Goldberger et al11 found that statin use was associated with a 78% reduced risk of death in patients with ICD (HR, 0.22; 95% CI, 0.09–0.55). Buber et al12 found that statin use significantly reduced the risk of fatal ventricular tachycardia in patients with CRT (HR, 0.23; 95% CI, 0.13–0.40). Nevertheless, Bašinskas et al13 reported that statin use was associated with unfavorable outcomes in patients with CRT (HR 2.98, 95% CI, 1.15–7.77). Therefore, there is no consensus in previous studies regarding the prognostic impact of statin use in cardiac patients, which may be attributed to differences in study design, race, statin type, and other confounding factors. In this study, we found that perioperative statin use did not prevent death and HF hospitalization in patients with CRT, despite a lower incidence of adverse outcomes observed in the statin group.
Previous studies have shown that statin is helpful in reducing the risk of AF due to its antiarrhythmic effect.27,28 Fang et al29 found that statin therapy was significantly associated with a reduced risk of developing or recurring AF (OR, 0.49; 95% CI, 0.37–0.65), but the benefit of statin was limited to the atorvastatin and simvastatin groups, and was not observed in the pitavastatin and rosuvastatin groups. Liu et al30 conducted a meta-analysis of RCTs, and statin use had no significant effect on the occurrence of AF (RR, 0.76; 95% CI, 0.55–1.05); however, a summary of observational studies found that statin use significantly reduced the risk of AF (RR, 0.77; 95% CI, 0.70 −0.85). Fauchier et al31 reported that statin use was associated with a reduced risk of AF with no difference between intensive vs standard statin regimens (OR, 1.01; 95% CI, 0.77–1.32). This evidence suggests that statin’s effect on AF remains unclear and that this effect may be susceptible to the type of statin, study design, and independent of statin dose.
Given that nearly half of the CRT patients observed in our study received statin therapy. Investigating the impact of the combination of AF status and statin use on the prognosis of CRT patients not only fills a previous knowledge gap. Furthermore, the findings have potentially great clinical value due to the high prevalence of AF status and statin use in CRT patients.32,33 In this study, CRT patients in the Non-AF/Statin group had the lowest incidence of adverse outcomes. However, it needs to be noted that although statin use appears to attenuate the adverse effect of AF on the prognosis of CRT, the prognostic impact of statins on patients with CRT is limited. This study provides information on the prevalence of the combination of AF and statin use in patients with CRT and supports that CRT patients free of AF and statin use have the best prognosis. The findings suggest that AF is a major risk factor for poor prognosis in patients with CRT and that controlling the AF rhythm can provide a significant clinical benefit in the prognosis of CRT patients. However, the effect of statin therapy on improving the prognosis of CRT was less pronounced.
Prior to our study, some studies have explored the role of statins in preventing and managing AF. However, the inconsistency of this evidence makes us still confused about the specific effects of statin on the management of AF. For example, Reilly et al34 found that statins could prevent AF by inhibiting the activity of Rac1 and NOX2-NADPH oxidase in the atria but not in its management. However, Zheng et al26 found that perioperative statin use in cardiac surgery did not prevent postoperative AF. This contradictory evidence seriously interferes with clinicians’ treatment decisions. On the other hand, the current evidence regarding statins’ use in treating patients with AF is relatively scarce, especially in patients with CRT. These uncertain factors will undoubtedly bring some confusion to clinicians’ decision-making. To address gaps in previous studies, we investigated the effect of statin on the prognosis of patients treated with CRT and whether statin could alleviate the adverse impact of AF. Our findings answer the above research question definitively that statin therapy does not improve the prognosis of these patients and is ineffective in the management of AF. Therefore, additional statin therapy to improve prognosis in HF patients with CRT is not necessary, but control of AF is beneficial in reducing the risk of adverse events in this high-risk population.
The underlying mechanisms remain unclear, but there are some plausible explanations. The impact of AF status on the prognosis of CRT is mainly through two aspects. On the one hand, persistent AF causes atrial electrophysiological remodeling and enlargement of the left atrium size, and the fast ventricular rate caused by AF leads to a decrease in cardiac output; the combination of these factors can aggravate the deterioration of cardiac function.35,36 On the other hand, the rapid atrial rate and the variable atrioventricular conduction time during the onset of AF significantly reduce the BVP percentage, affecting CRT’s efficacy.37,38 Increased evidence from basic research suggests that statins have multiple effects, including the ability of statin therapy to reduce inflammation, and oxidative stress, improve endothelial function, stabilize plaque, and reduce myocardial ischemia-reperfusion injury.39 However, this study did not observe that statin therapy improved the prognosis of patients with CRT, and statin use did not significantly attenuate the adverse effect of AF status on the prognosis of patients with CRT. Unlike AF, statin use appears to have little effect on CRT; as shown in Table 1, statin use did not change BVP percentages except for the effect of AF. In addition, the benefits of statins for cardiovascular disease are susceptible to differences in study populations, study design, and disease status.40 The available research evidence is unable to fully elucidate the detailed mechanisms underlying the effects of statins and combinations of statins and AF on the prognosis of CRT. Further basic research evidence is needed to elucidate the mechanisms behind the joint relationship.
Limitations
Several limitations in this study should be considered. First, we cannot draw causal inferences from this observational cohort study.41 Second, the type and dose of statin were not recorded in this study, and this detailed information may have influenced this association. Third, statin use and AF status were assessed at baseline, and data on statin use during follow-up were not collected, and changes in statin therapy may have an impact on prognosis. Future studies employing repeated collections may be needed to assess the longitudinal impact of AF and statins on the prognosis of patients with CRT. Fourth, the population in this study was from a single center, and the generalizability of the findings was limited; future multicenter studies are needed to verify this conclusion.
Conclusions
In this retrospective cohort study of CRT patients, despite the combination of AF-free status and statin use was associated with a reduced risk of all-cause mortality and HF hospitalization. However, the effect of statin therapy on the prognosis of patients with CRT has not been observed. These findings suggest that AF status is a major risk factor for an unfavorable prognosis in CRT patients and that statin therapy is ineffective in attenuating the adverse effects of AF. Prospective studies are needed to validate this joint association.
Data Sharing Statement
None of the datasets generated for this study are publicly available but are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All participants provided written informed consent, and the Ethics Committee approved this study of Fuwai Hospital, Chinese Academy of Medical Sciences (IRB2012-BG-006).
Acknowledgments
The authors thank all the patients and researchers who participated in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427–3520. doi:10.1093/eurheartj/ehab364
2. Patel N, Viles-Gonzalez J, Agnihotri K, et al. Frequency of in-hospital adverse outcomes and cost utilization associated with cardiac resynchronization therapy defibrillator implantation in the United States. J Cardiovasc Electrophysiol. 2018;29(10):1425–1435. doi:10.1111/jce.13701
3. Khan NK, Goode KM, Cleland JG, et al. Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail. 2007;9(5):491–501. doi:10.1016/j.ejheart.2006.11.003
4. Prinzen FW, Vernooy K, Auricchio A. Cardiac resynchronization therapy: state-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation. 2013;128(22):2407–2418. doi:10.1161/CIRCULATIONAHA.112.000112
5. Daubert JC, Saxon L, Adamson PB, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Heart Rhythm. 2012;9(9):1524–1576. doi:10.1016/j.hrthm.2012.07.025
6. Wilton SB, Leung AA, Ghali WA, Faris P, Exner DV. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8(7):1088–1094. doi:10.1016/j.hrthm.2011.02.014
7. Pinho-Gomes AC, Reilly S, Brandes RP, Casadei B. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Signal. 2014;20(8):1268–1285. doi:10.1089/ars.2013.5542
8. Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J. 2008;29(12):1548–1559. doi:10.1093/eurheartj/ehn198
9. Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51(8):828–835. doi:10.1016/j.jacc.2007.09.063
10. O’Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients. Am J Med. 2008;121(1):24–33. doi:10.1016/j.amjmed.2007.06.033
11. Goldberger JJ, Subacius H, Schaechter A, et al. Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2006;48(6):1228–1233. doi:10.1016/j.jacc.2006.05.053
12. Buber J, Goldenberg I, Moss AJ, et al. Reduction in life-threatening ventricular tachyarrhythmias in statin-treated patients with nonischemic cardiomyopathy enrolled in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2012;60(8):749–755. doi:10.1016/j.jacc.2012.03.041
13. Bašinskas P, Stoškutė N, Gerulytė A, Abramavičiūtė A, Puodžiukynas A, Kazakevičius T. Prognostication of poor survival after cardiac resynchronization therapy. Medicina. 2020;56(1):19. doi:10.3390/medicina56010019
14. Hanna IR, Heeke B, Bush H, et al. Lipid-lowering drug use is associated with reduced prevalence of atrial fibrillation in patients with left ventricular systolic dysfunction. Heart Rhythm. 2006;3(8):881–886. doi:10.1016/j.hrthm.2006.05.010
15. Cameron A, Schwartz MJ, Kronmal RA, Kosinski AS. Prevalence and significance of atrial fibrillation in coronary artery disease (CASS Registry). Am J Cardiol. 1988;61(10):714–717. doi:10.1016/0002-9149(88)91053-3
16. Proietti M, Laroche C, Nyvad O, et al. Use of statins and adverse outcomes in patients with atrial fibrillation: an analysis from the EURObservational Research Programme Atrial Fibrillation (EORP-AF) general registry pilot phase. Int J Cardiol. 2017;248:166–172. doi:10.1016/j.ijcard.2017.08.055
17. Zhang N, Cai M, Hua W, et al. Prognostic effects of longitudinal changes in left ventricular ejection fraction with cardiac resynchronization therapy. ESC Heart Fail. 2021;8(1):368–379. doi:10.1002/ehf2.13082
18. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
19. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. doi:10.1161/CIR.0000000000000665
20. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi:10.1161/CIR.0000000000000678
21. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi:10.1056/NEJMoa1002358
22. Barbour SJ, Coppo R, Zhang H, et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179(7):942–952. doi:10.1001/jamainternmed.2019.0600
23. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6a):2d–8d. doi:10.1016/S0002-9149(02)03373-8
24. Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021;42(46):4731–4739. doi:10.1093/eurheartj/ehab569
25. Paraskevas KI, Athyros VG, Briana DD, Kakafika AI, Karagiannis A, Mikhailidis DP. Statins exert multiple beneficial effects on patients undergoing percutaneous revascularization procedures. Curr Drug Targets. 2007;8(8):942–951. doi:10.2174/138945007781386893
26. Zheng Z, Jayaram R, Jiang L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374(18):1744–1753. doi:10.1056/NEJMoa1507750
27. Abuissa H, O’Keefe JH, Bybee KA. Statins as anti-arrhythmics: a systematic review part II: effects on risk of ventricular arrhythmias. Clin Cardiol. 2009;32(10):549–552. doi:10.1002/clc.20435
28. Rezaei Y, Gholami-Fesharaki M, Dehghani MR, Arya A, Haghjoo M, Arjmand N. Statin antiarrhythmic effect on atrial fibrillation in statin-naive patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2016;21(2):167–176. doi:10.1177/1074248415602557
29. Fang WT, Li HJ, Zhang H, Jiang S. The role of statin therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2012;74(5):744–756. doi:10.1111/j.1365-2125.2012.04258.x
30. Liu T, Li L, Korantzopoulos P, Liu E, Li G. Statin use and development of atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008;126(2):160–170. doi:10.1016/j.ijcard.2007.07.137
31. Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr Opin Cardiol. 2013;28(1):7–18. doi:10.1097/HCO.0b013e32835b0956
32. Sumner AD, Boehmer JP, Saxon LA, et al. Statin use is associated with improved survival in patients with advanced heart failure receiving resynchronization therapy. Congest Heart Fail. 2009;15(4):159–164. doi:10.1111/j.1751-7133.2009.00057.x
33. Birnie D, Hudnall H, Lemke B, et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the Adaptive Cardiac Resynchronization Therapy Trial. Heart Rhythm. 2017;14(12):1820–1825. doi:10.1016/j.hrthm.2017.08.017
34. Reilly SN, Jayaram R, Nahar K, et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124(10):1107–1117. doi:10.1161/CIRCULATIONAHA.111.029223
35. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516–2525. doi:10.1161/CIRCULATIONAHA.108.821306
36. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019;7(6):447–456. doi:10.1016/j.jchf.2019.03.005
37. Ousdigian KT, Borek PP, Koehler JL, Heywood JT, Ziegler PD, Wilkoff BL. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7(3):370–376. doi:10.1161/CIRCEP.113.001212
38. Ruwald MH, Mittal S, Ruwald AC, et al. Association between frequency of atrial and ventricular ectopic beats and biventricular pacing percentage and outcomes in patients with cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64(10):971–981. doi:10.1016/j.jacc.2014.06.1177
39. Rauch U, Osende JI, Chesebro JH, et al. Statins and cardiovascular diseases: the multiple effects of lipid-lowering therapy by statins. Atherosclerosis. 2000;153(1):181–189. doi:10.1016/S0021-9150(00)00397-X
40. Dorsch MP, Lester CA, Ding Y, Joseph M, Brook RD. Effects of race on statin prescribing for primary prevention with high atherosclerotic cardiovascular disease risk in a large healthcare system. J Am Heart Assoc. 2019;8(22):e014709. doi:10.1161/JAHA.119.014709
41. Kanwal F, Hernaez R, Liu Y, et al. Factors associated with access to and receipt of liver transplantation in veterans with end-stage liver disease. JAMA Intern Med. 2021;181(7):949–959. doi:10.1001/jamainternmed.2021.2051
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

