Back to Journals » Clinical Epidemiology » Volume 10
Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease
Authors Wang J, Wang B, Liang M, Wang G, Li J , Zhang Y, Huo Y , Cui Y, Xu X, Qin X
Received 2 September 2017
Accepted for publication 23 November 2017
Published 15 January 2018 Volume 2018:10 Pages 121—132
DOI https://doi.org/10.2147/CLEP.S150687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Irene Petersen
Jiancheng Wang,1,* Binyan Wang,1,2,* Min Liang,1 Guobao Wang,1 Jianping Li,3 Yan Zhang,3 Yong Huo,3 Yimin Cui,4 Xiping Xu,1,5 Xianhui Qin1
1National Clinical Research Center for Kidney Disease, State Key Laboratory for Organ Failure Research, Renal Division, Nanfang Hospital, Southern Medical University, Guangzhou, 2Institute for Biomedicine, Anhui Medical University, Hefei, 3Department of Cardiology, 4Department of Pharmacy, Peking University First Hospital, Beijing, 5Beijing Advanced Innovation Center for Food Nutrition and Human Health, Key Laboratory of Functional Dairy, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing, China
*These authors contributed equally to this work
Objective: Whether serum bilirubin and cigarette smoking affect the risk of renal function decline remains inconclusive. We aimed to test the independent and combined effects of bilirubin and cigarette smoking on the progression of chronic kidney disease (CKD) in hypertensive adults.
Methods: The study population consisted of 12,633 patients in the renal sub-study of the China Stroke Primary Prevention Trial. The primary outcome was progression of CKD, defined as a decrease in estimated glomerular filtration rate (eGFR) of ≥30% and to a level of <60 mL/min/1.73 m2 if baseline eGFR was ≥60 mL/min/1.73 m2, or a decrease in eGFR of ≥50% if baseline eGFR was <60 mL/min/1.73 m2, or end-stage renal disease. The secondary outcomes included 1) rapid decline in renal function and 2) annual rate of eGFR decline.
Results: The median follow-up duration was 4.4 years. Cigarette smoking had no significant effect on the progression of CKD (odds ratio [OR]: 1.11, 95% confidence interval [95% CI]: 0.78–1.57). However, a significantly lower risk of the primary event (OR: 0.72, 95% CI: 0.55–0.95) was found in participants in tertile 3 compared to those in tertiles 1–2 for total bilirubin (TBiL) levels. More importantly, there was an interaction between TBiL and smoking status on the primary outcome (P for interaction =0.013). Among ever smokers, TBiL levels had no significant effect on the primary outcome. However, among never smokers, higher TBiL levels were significantly associated with a lower risk of the primary outcome (tertile 3 vs 1–2; OR: 0.53, 95% CI: 0.36–0.78). Similar trends were observed for direct bilirubin and secondary outcomes.
Conclusion: Among hypertensive patients, bilirubin was inversely associated with the progression of CKD in never smokers, but not in ever smokers.
Keywords: serum bilirubin, cigarette smoking, chronic kidney disease, hypertensive adults, rapid renal function decline, annual rate of eGFR decline
Plain language summary
Whether serum bilirubin and cigarette smoking affect the risk of renal function decline remains inconclusive. We aimed to test the independent and combined effects of bilirubin and cigarette smoking on the progression of chronic kidney disease (CKD) in hypertensive adults. A total of 12,633 hypertensive adults were included in the current study. The primary outcome was progression of CKD, defined as a decrease in eGFR of ≥30% and to a level of <60 mL/min/1.73 m2 if baseline eGFR was ≥60 mL/min/1.73 m2, or a decrease in eGFR of ≥50% if baseline eGFR was <60 mL/min/1.73 m2, or end-stage renal disease. The median follow-up duration was 4.4 years. Our study suggested that there was a significant, inverse association between serum bilirubin concentrations and the progression of CKD in never smokers among hypertensive patients. However, the inverse association was attenuated and became insignificant in ever smokers. Our findings indicate that modulating bilirubin levels could be a potential therapeutic target for CKD among hypertensive patients. Furthermore, our results may provide an adjuvant strategy for smoking cessation programs aimed at the prevention of CKD in smokers.
Introduction
Chronic kidney disease (CKD) is one of the major complications of hypertension and substantially increases the risk of cardiovascular disease and progression to end-stage kidney disease.1,2 Furthermore, even a mild decline in renal function is associated with an increased risk of cardiovascular morbidity and mortality.3 CKD has become a major health issue worldwide.4 A 2012 national survey found the overall prevalence of CKD in China to be 10.8% (95% confidence interval [95% CI]: 10.2–11.3).5 A better understanding of the modifiable risk factors of renal function decline would be helpful for clinical decision-making and timely management, for it would lead to early detection and prevention, alleviating the future burden of CKD and its associated complications.
It has been reported that oxidative stress and inflammation play a key role in both the initiation and promotion of CKD.6 Bilirubin is a metabolic end product of heme degradation by heme oxygenase (HO) and the reduction of biliverdin by the enzyme biliverdin reductase.7 Bilirubin has been suggested to be a potential antioxidant and anti-inflammatory agent under physiological conditions.7–9 Indeed, previous studies reported an inverse association between serum bilirubin and risk of stroke, cardiovascular disease, and metabolic syndrome.10–12 At the same time, several studies also suggest cigarette smoking as an independent risk factor for cardiovascular disease and incident CKD.13,14 Recent research has found that cigarette smoke extract can sequentially increase the production of reactive oxygen species (ROS) and inflammation.15,16 However, although lower serum bilirubin level or cigarette smoking seemed to have similar mechanisms regarding renal damage, whether serum bilirubin alone, or in combination with cigarette smoking, may affect the risk of renal function decline remains inconclusive.
Hypertension is significantly associated with cardiovascular disease, peripheral arterial disease, and CKD.17,18 The China Stroke Primary Prevention Trial (CSPPT) reported that the combined use of enalapril and folic acid, compared with enalapril alone, significantly reduced the risk of first stroke by 21% among hypertensive Chinese adults.19 Furthermore, in the renal sub-study of the CSPPT, enalapril–folic acid therapy, compared with enalapril alone, delayed the progression of CKD among patients with mild to moderate CKD (odds ratio [OR]: 0.44, 95% CI: 0.26–0.75).20 The current study, a post hoc analysis of the renal sub-study of the CSPPT, aimed to examine the independent and combined effects of bilirubin and cigarette smoking on the progression of CKD in hypertensive adults.
Methods
Study participants and design
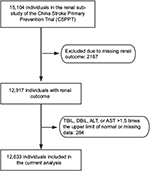
The rationale and study design for the CSPPT and the renal sub-study of the CSPPT have been described previously.19,20 Briefly, the CSPPT was a randomized, double-blind, actively controlled trial conducted from May 2008 to August 2013 in 32 communities in Anhui and Jiangsu provinces of China, and the study was registered with ClinicalTrials.gov, NCT00794885. The study enrolled a total of 20,702 hypertensive adults aged 45–75 years and without a history of major cardiovascular diseases. Participants were randomized to receive treatment with either a combination of enalapril and folic acid or enalapril alone and were followed up for a median of 4.5 years. The renal sub-study enrolled CSPPT participants from the 20 communities in Jiangsu Province, excluding those with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 or a missing eGFR at baseline.
The present study was a post hoc analysis of the renal sub-study of the CSPPT. Participants with missing data on renal outcome and liver enzymes (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) or with a serum bilirubin (total, direct) >1.5 times the upper limit of normal were excluded from the study (Figure 1). The study was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (EC) (FWA assurance number: FWA00001263). Participant’s written informed consent was waived by the EC because this study was a post hoc analysis. The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding author (Xianhui Qin or Xiping Xu) upon request, after the request is submitted and formally reviewed and approved by the EC.
Intervention and follow-up
Eligible participants, stratified by methylenetetrahydrofolate reductase C677T genotypes (CC, CT, or TT), were randomly assigned, in a 1:1 ratio, to one of two treatment groups: a daily oral dose of one tablet containing 10 mg enalapril and 0.8 mg folic acid (single tablet combination: enalapril–folic acid group) or a daily oral dose of one tablet containing 10 mg enalapril alone (enalapril-only group). All study investigators and participants were blinded to the randomization procedure and the treatment assignments. During the trial period, concomitant use of other anti-hypertensive drugs (mainly calcium channel blockers or diuretics) was allowed, but not B vitamins. Participants were scheduled for follow-up every 3 months.
Laboratory assays
Serum and spot urine samples from the participants were collected at both the baseline and the exit visit. Serum creatinine, total bilirubin (TBiL), direct bilirubin (DBiL), ALT, AST, lipids, and fasting glucose were measured using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Guangzhou, China. Specifically, serum creatinine was measured using an enzymatic assay, calibrated to be isotope dilution mass spectrometry traceable. The coefficient of variation for the assay was 1.4%. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Proteinuria was determined using a dipstick test (Dirui-H100).
Outcomes
The primary outcome was progression of CKD, defined as a decrease in eGFR of ≥30% and to a level <60 mL/min/1.73 m2 at the exit visit if baseline eGFR was ≥60 mL/min/1.73 m2, or a decrease in eGFR of ≥50% at the exit visit if baseline eGFR was <60 mL/min/1.73 m2, or end-stage renal disease (eGFR<15 mL/min/1.73 m2 or need for dialysis).
The secondary outcomes included the following: 1) rapid decline in renal function, defined as an average decline in eGFR of 5 mL/min/1.73 m2 or more per year and 2) annual rate of relative decline in eGFR, estimated as 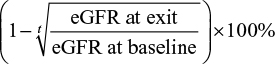 , where t is the time length in years from baseline to exit visit.
, where t is the time length in years from baseline to exit visit.
Statistical analyses
Means (standard deviation, SD) or proportions were calculated for population characteristics by bilirubin tertiles and smoking status (never, former, and current smokers). The differences in population characteristics were compared using ANOVA tests, signed rank tests, or chi-square tests, accordingly.
Generalized linear models with a logit link were used to test the independent and combined effects of bilirubin and cigarette smoking on the progression of CKD or rapid decline in renal function (binary variables) with crude or full model adjusted for age, sex, treatment group, body mass index (BMI), eGFR, proteinuria, alcohol intake, systolic blood pressure (SBP), fasting glucose, ALT, AST and total cholesterol, as well as mean SBP during the treatment period. Generalized linear models with an identity link were used to test the independent and combined effects of bilirubin and cigarette smoking on the annual rate of eGFR decline (continuous variable) with crude or full model adjusted for the aforementioned variables.
In additional stratified analyses, possible modifications of the association between TBiL and the progression of CKD were also assessed for the following variables: age (<60 vs ≥60 years), sex, treatment group (enalapril vs enalapril + folic acid), eGFR levels (<90 vs ≥90 mL/min/1.73 m2), and proteinuria (yes vs no).
A two-tailed P<0.05 was considered to be statistically significant in all analyses. R software (http://www.R-project.org) and Empower (R) (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA, USA) were used for all statistical analyses.
Results
Study participants and baseline characteristics
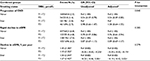
A total of 12,633 participants were included in the final analysis (Figure 1). Participants were divided according to tertiles of baseline TBiL concentrations. General characteristics of study participants are presented by TBiL tertiles in Table 1. Baseline TBiL concentrations were directly associated with male gender, alcohol intake, baseline diastolic blood pressure, timed-averaged SBP, and total cholesterol and liver enzymes levels, and were inversely associated with BMI and eGFR.
Those participants excluded from the analyses due to missing data did not differ substantially in characteristics from those included in the final analyses (Table S1).
Independent effect of serum bilirubin and cigarette smoking on primary and secondary outcomes
During a median treatment duration of 4.4 years, the primary event and the rapid decline in renal function occurred in 294 (2.3%) and 803 (6.4%) participants, respectively. The mean annual rate of eGFR decline in the total participants was 1.37% per year.
Overall, there was a significant inverse association between TBiL concentrations and the primary and secondary outcomes (Figure 2). An SD increase in TBiL levels was associated with a 20% reduction in the adjusted risk of the primary event (OR: 0.80, 95% CI: 0.70–0.92; P=0.002), a 14% lower risk of rapid decline in renal function (OR: 0.86, 95% CI: 0.79–0.94; P<0.001), and a significantly slower rate of eGFR decline (regression coefficient: −0.13; 95% CI: −0.19, −0.06; P<0.001). Consistently, when TBiL was assessed as tertiles, a significantly lower risk of the primary event (OR: 0.72, 95% CI: 0.55–0.95), rapid decline in renal function (OR: 0.80, 95% CI: 0.68–0.95), and a slower rate of eGFR decline (1.16% vs 1.47% per year; P<0.001) were found in those in tertile 3 compared with participants in tertiles 1–2 (Tables 2 and S2).
Due to a similar incidence in primary events, we combined former smokers and current smokers into an ever smoker group. Compared to never smokers, ever smokers had a significantly increased risk of rapid renal function decline (OR: 1.29, 95% CI: 1.02–1.63). However, smoking status did not have a significant effect on the primary event nor on the annual rate of eGFR decline (Table 2).
Similar results were observed for the association between DBiL and the primary and secondary outcomes (Table S3).
Stratified analyses were performed to further assess the association between TBiL and the primary outcome in various subgroups. None of the variables, including age (<60 vs ≥60 years; P for interaction=0.907), sex (P for interaction=0.116), treatment group (enalapril vs enalapril + folic acid; P for interaction=0.902), eGFR levels (<90 vs ≥90 mL/min/1.73 m2; P for interaction=0.827), or proteinuria (yes vs no; P for interaction=0.688), significantly modified the association between TBiL and the primary outcome (Figure 3).
Interaction between serum bilirubin and cigarette smoking on study outcomes
We observed an interaction between TBiL levels and smoking status on the primary outcome (P for interaction=0.013). Among ever smokers, TBiL levels had no significant effect on any of the study outcomes. Among never smokers, however, higher TBiL levels were significantly associated with a lower risk of the primary outcome (OR: 0.53, 95% CI: 0.36–0.78) (Table 3).
Similar trends were observed for the secondary outcomes (Table 3) and for DBiL levels (Table S4).
Discussion
To our knowledge, this is the first large-scale prospective analysis to examine the independent and combined effects of serum bilirubin and cigarette smoking on the risk of progression of CKD among hypertensive adults. We found a significant, inverse association between serum bilirubin (TBiL or DBiL) concentrations and progression of CKD in never smokers. In contrast, this inverse association was attenuated and became insignificant in ever smokers.
Previous research findings on the association between bilirubin and renal function were inconsistent. Fukui et al21 showed an inverse correlation of bilirubin with logarithmic urinary albumin excretion and a positive correlation with eGFR in patients with type 2 diabetes. Shin et al22 observed similar results in both non-diabetic and diabetic adults. However, Targher et al23 found that serum bilirubin levels were negatively associated with eGFR in a US adult population. Moreover, a study by Ryu et al24 demonstrated that only DBiL, but not TBiL and indirect bilirubin (IBiL), was associated with a lower risk of incident CKD. The reasons for these contradictory findings might include differences in study methodology, sample size, area, and population. More importantly, potential effect modifiers were not thoroughly investigated in these previous studies. Our results suggest that the association between bilirubin and the progression of CKD could be significantly influenced by smoking status.
Oxidative stress and inflammation were believed to be major causal mechanisms of renal dysfunction.6 Previous research has indicated that antioxidant treatments in CKD patients result in a reduction in oxidative stress and many show improved renal function.25 HO-1 is a microsomal enzyme, which degrades free heme into biliverdin, iron, and carbon monoxide. Biliverdin is then reduced into bilirubin by the cytosolic enzyme biliverdin reductase.7 Bilirubin has been reported to be an effective antioxidant that effectively scavenges peroxyl radicals and suppresses the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.7,26,27 Mancuso et al and Barone et al found that bilirubin interacts with nitric oxide and scavenges reactive nitrogen species (RNS).28–31 Furthermore, high bilirubin had also been associated with decreased C-reactive protein, tumor necrosis factor α, and interleukin-1β levels.8,9 Therefore, the potential renoprotective role of bilirubin could be related to its antioxidant and anti-inflammatory properties.
In our study, we found a significant inverse association between bilirubin and the progression of CKD in never smokers. However, this inverse association was not seen among current and former smokers. Cigarette smoking has long been recognized as an oxidative stressor by inducing the generation of ROS and RNS.15,32 Overproduction of ROS and RNS can lead to renal vasoconstriction, sodium retention, and kidney damage.33,34 In addition, prior studies have shown that cigarette smoking increases levels of inflammatory cytokines.35 Furthermore, a recent study suggested that cigarette smoking is able to suppress HO-1 gene and protein with the involvement of the repressor transcription factor Bach1 in rat fibroblasts and lung cells.36 Based on these existing literatures and our data, we infer that it may be the overwhelming oxidative stress and inflammation introduced by smoking that offsets the beneficial effect of bilirubin on renal function. However, the exact mechanisms regarding the relationship of bilirubin and cigarette smoking with the risk of CKD remain to be further investigated, and our findings and hypotheses also warrant further confirmation.
Our study has several unique strengths. First, previous studies mainly focused on the effects of TBiL on renal function,21–23 while the current study examined the effects of both TBiL and DBiL in the same cohort. Of note, TBiL comprised both IBiL and DBiL, but is mainly composed of IBiL and there are some differences between the two. IBiL has extremely poor water solubility and bonds strongly to albumin in plasma, whereas DBiL is soluble in water.37 Both IBiL and DBiL are reported to be effective antioxidants.38 Accordingly, our study suggests that both DBiL and TBiL have a protective effect against the progression of CKD. Second, more than half of the Chinese males smoke. A novel and important finding was that smoking significantly modified the association between TBiL and the progression of CKD. Finally, we examined different renal outcomes, including the progression of CKD, rapid decline in renal function, and annual rate of relative decline in eGFR, and yielded consistent results.
Several potential concerns or limitations are worth mentioning. First, although we used the data from a large clinical trial, our results were based on post hoc analyses. Despite extensive adjustments for known factors, we cannot exclude the possibility that unrecorded risk factors may explain some of our results. Second, the bilirubin and smoking status were not assessed in the follow-up periods. More frequent measurements of bilirubin or smoking status would allow a more accurate assessment of its effect. Third, although smoking has been reported as one of the major risk factors for incident CKD, especially in studies with durations of follow-up >10 years,14 our current study found that cigarette smoking only significantly increased the risk of rapid renal function decline and did not significantly affect the progression of CKD and the annual rate of eGFR decline. The possible explanation was that the follow-up period (4.4 years) in our study might be too short to demonstrate the long-term effect of cigarette smoking. Fourth, the renal sub-study of the CSPPT only included participants with an eGFR of ≥30 mL/min/1.73m2. We could not examine participants with an eGFR <30 mL/min/1.73m2. Fifth, hypertension is significantly associated with the development of CKD. We have adjusted the blood pressure at baseline and during the treatment period in our analyses. However, the CSPPT did not collect detailed information about the white coat hypertension among the participants, which may possibly influence the results of our present study. Finally, our study was conducted in Chinese hypertensive patients. Whether the results can be extrapolated to other populations requires further verification. Due to these limitations, further confirmation of our findings in an independent study is greatly needed.
Conclusion and implications
In this prospective analysis of Chinese adults with hypertension, there was a significant, inverse association between serum bilirubin concentrations and the progression of CKD in never smokers, but not in ever smokers.
Cigarette smoking is a worldwide public health problem. In particular, China is the world’s largest cigarette consuming country and hence is the most vulnerable to smoking health hazards, especially among hypertensive patients.39,40 Therefore, if confirmed by future studies, our findings may provide an adjuvant strategy for smoking cessation programs aimed at the prevention of CKD in smokers.
Our findings raised a possibility of bilirubin as an intervention target for CKD. Previous study found that patients with diabetic nephropathy were under condition of systemic oxidative stress, and the induction of HO-1 plays an important role in counteracting the intracellular prooxidant status.41 Future studies are needed to further evaluate whether modulating bilirubin levels, perhaps by inducing HO-1,42 could be a potential therapeutic target for CKD.
Finally, we would like to underscore that our study findings be regarded as hypothesis generating. Hopefully, our study will stimulate future clinical and mechanistic studies to confirm our findings and to better understand the protective mechanism of TBiL and DBiL in CKD.
Acknowledgments
This work was supported by the Projects of the National Natural Science Foundation of China (81402735, 81473052, 81670669); the Science and Technology Project of Guangdong Province (2014B090904040); the Science and Technology Planning Project of Guangzhou, China (201606211534575); the Science, Technology and Innovation Committee of Shenzhen (JSGG20160229173428526, KC2014JSCX0071A); and the National Key Research and Development Program (2016YFC0903101, 2016YFC0904900). The funders had no role in the design and conduct of the study. The authors would like to thank the following for assistance in the concept and design of this study: Xianglin Zhang, Yaren Yu, Yun Song, Youbao Li, Liling Xie, and Chongfei Jiang from the Department of Nephrology at Nanfang Hospital and Lulu Chen from the Institute of Biomedicine at Anhui Medical University.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
Dr Xiping Xu reports grants from the Projects of the National Natural Science Foundation of China (81473052); the Science and Technology Project of Guangdong Province (2014B090904040); the Science and Technology Planning Project of Guangzhou, China (201606211534575); and the Science, Technology and Innovation Committee of Shenzhen (JSGG20160229173428526, KC2014JSCX0071A).
Dr Xianhui Qin reports grants from the Projects of the National Science Foundation (81402735).
Dr Binyan Wang reports grants from the National Key Research and Development Program (2016YFC0903101, 2016YFC0904900).
Dr Guobao Wang reports grants from the Projects of the National Science Foundation (81670669).
The authors report no other conflicts of interest in this work.
References
Matsushita K, van der Velde M, Astor BC, et al; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. | ||
Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension. 2017;70(4):687–694. | ||
Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–2511. | ||
Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives – a position statement from kidney disease improving global outcomes. Kidney Int. 2007;72(3):247–259. | ||
Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. | ||
Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009;114:S3–S11. | ||
Mancuso C. Bilirubin and brain: a pharmacological approach. Neuropharmacology. 2017;118:113–123. | ||
Zhou ZX, Chen JK, Hong YY, et al. Relationship between the serum total bilirubin and inflammation in patients with psoriasis vulgaris. J Clin Lab Anal. 2016;30(5):768–775. | ||
Zelenka J, Dvořák A, Alán L, Zadinová M, Haluzík M, Vítek L. Hyperbilirubinemia protects against aging-associated inflammation and metabolic deterioration. Oxid Med Cell Longev. 2016;2016:6190609. | ||
Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med. 2008;121(9):781–788.e1. | ||
Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298(12):1398–1400. | ||
Huang SS, Chan WL, Leu HB, Huang PH, Lin SJ, Chen JW. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged nonsmoking men. Am J Med. 2015;128(10):1138.e35–e41. | ||
Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014–1021. | ||
Xia J, Wang L, Ma Z, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017;32(3):475–487. | ||
Edirisinghe I, Arunachalam G, Wong C, et al. Cigarette-smoke-induced oxidative/nitrosative stress impairs VEGF- and fluid-shear-stress-mediated signaling in endothelial cells. Antioxid Redox Signal. 2010;12(12):1355–1369. | ||
Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294(6):H2721–H2735. | ||
Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. | ||
He M, Qin X, Cui Y, et al. Prevalence of unrecognized lower extremity peripheral arterial disease and the associated factors in Chinese hypertensive adults. Am J Cardiol. 2012;110(11):1692–1698. | ||
Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015; 313(13):1325–1335. | ||
Xu X, Qin X, Li Y, et al; investigators of the Renal Substudy of the China Stroke Primary Prevention Trial (CSPPT). Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016;176(10):1443–1450. | ||
Fukui M, Tanaka M, Shiraishi E, et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int. 2008;74(9):1197–1201. | ||
Shin HS, Jung YS, Rim H. Relationship of serum bilirubin concentration to kidney function and 24-hour urine protein in Korean adults. BMC Nephrol. 2011;12:29. | ||
Targher G, Bosworth C, Kendrick J, Smits G, Lippi G, Chonchol M. Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001–2006. Clin Chem Lab Med. 2009;47(9):1055–1062. | ||
Ryu S, Chang Y, Zhang Y, et al. Higher serum direct bilirubin levels were associated with a lower risk of incident chronic kidney disease in middle aged Korean men. PLoS One. 2014;9(2):e75178. | ||
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton). 2012;17(4):311–321. | ||
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. | ||
Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50(4):636–642. | ||
Mancuso C, Bonsignore A, Capone C, Di Stasio E, Pani G. Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid Redox Signal. 2006;8(3–4):487–494. | ||
Mancuso C, Capone C, Ranieri SC, et al. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J Neurosci Res. 2008;86(10):2235–2249. | ||
Mancuso C, Barone E, Guido P, et al. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci Lett. 2012;518(2):101–105. | ||
Barone E, Trombino S, Cassano R, et al. Characterization of the S-denitrosylating activity of bilirubin. J Cell Mol Med. 2009;13(8B):2365–2375. | ||
Rom O, Kaisari S, Aizenbud D, Reznick AZ. Identification of possible cigarette smoke constituents responsible for muscle catabolism. J Muscle Res Cell Motil. 2012;33(3–4):199–208. | ||
Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol. 2004;24(4):354–365. | ||
Hua P, Feng W, Ji S, Raij L, Jaimes EA. Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol. 2010;299(4): F732–F739. | ||
McEvoy JW, Nasir K, DeFilippis AP, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–1010. | ||
Palozza P, Serini S, Currò D, Calviello G, Igarashi K, Mancuso C. Beta-carotene and cigarette smoke condensate regulate heme oxygenase-1 and its repressor factor Bach1: relationship with cell growth. Antioxid Redox Signal. 2006;8(5–6):1069–1080. | ||
Kramer P. Present concepts of the nature of direct and indirect bilirubin. Am J Dig Dis. 1957;2(6):330–334. | ||
Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51(6):859–862. | ||
Reitsma MB, Fullman N, Ng M, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389(10082):1885–1906. | ||
Wang Y, Li X, Qin X, et al. Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45–75 years. Br J Nutr. 2013;109(7):1284–1293. | ||
Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12(4):299–306. | ||
Mccarty MF. Serum bilirubin may serve as a marker for increased heme oxygenase activity and inducibility in tissues – a rationale for the versatile health protection associated with elevated plasma bilirubin. Med Hypotheses. 2013;81(4):607–610. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.