Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study
Authors Greulich T , Kostikas K , Gaga M, Aalamian-Mattheis M, Lossi NS, Patalano F , Nunez X, Pagano VA, Fogel R , Vogelmeier CF, Clemens A
Received 13 December 2017
Accepted for publication 12 March 2018
Published 16 April 2018 Volume 2018:13 Pages 1229—1237
DOI https://doi.org/10.2147/COPD.S159732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Timm Greulich,1 Konstantinos Kostikas,2 Mina Gaga,3 Maryam Aalamian-Mattheis,2 Nadine S Lossi,4 Francesco Patalano,2 Xavier Nunez,5 Veronica A Pagano,5 Robert Fogel,6 Claus F Vogelmeier,1,* Andreas Clemens2,7,*
1University Medical Center Giessen and Marburg, German Center for Lung Research (DZL), Marburg, Germany; 2Novartis Pharma AG, Basel, Switzerland; 37th Respiratory Medicine Department, Athens Chest Hospital Sotiria, Athens, Greece; 4Novartis Pharma GmbH, Nürnberg, Germany; 5TFS Develop, Barcelona, Spain; 6Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 7Heart Center Freiburg University, Cardiology and Angiology I, Faculty of Medicine, Freiburg, Germany
*These authors contributed equally to this work
Purpose: COPD is a progressive disease characterized by exacerbations and a decline in health status and lung function. Clinically important deterioration (CID) is a composite endpoint used to evaluate treatment efficacy. This analysis evaluated the impact of a direct switch to once-daily indacaterol/glycopyrronium 110/50 µg (IND/GLY) from previous monotherapy with a long-acting β2-agonist (LABA) or long-acting muscarinic antagonist (LAMA) or with an LABA and an inhaled corticosteroid (LABA + ICS) on reducing CID.
Methods: CRYSTAL was a 12-week, prospective, multicenter, randomized, open-label study conducted in clinical practice settings. Three definitions of CID (D1–D3) were used, including: 1) ≥100 mL decrease in trough forced expiratory volume in 1 second (FEV1), 2) ≥1 point decrease in transition dyspnea index (TDI) and/or ≥0.4 points increase in clinical COPD questionnaire score (CCQ), or 3) an acute moderate/severe exacerbation (AECOPD). In D1 and D2, either TDI or CCQ was evaluated along with FEV1 and AECOPD, whereas in D3, all 4 parameters were included. ClinicalTrials.gov number: NCT01985334.
Results: Of the 2,159 patients analyzed, 1,622 switched to IND/GLY and 537 continued their baseline treatments. The percentage of patients with a CID was significantly lower after a direct switch to IND/GLY versus LABA or LAMA using all 3 CID definitions (D1: odds ratio [OR] 0.41 [95% CI: 0.30–0.55]; D2: OR 0.41 [95% CI: 0.31–0.55]; D3: OR 0.39 [95% CI: 0.29–0.52]). Compared with LABA + ICS, IND/GLY also reduced the risk of CID (D1: OR 0.76 [95% CI: 0.56–1.02]; D2: OR 0.75 [95% CI: 0.56–1.00]; D3: OR 0.67 [95% CI: 0.51–0.89]).
Conclusion: In this analysis, IND/GLY reduced the risk of a CID in moderate COPD patients after direct switch from LABA + ICS or LABA or LAMA in real-life clinical practice.
Keywords: clinically important deterioration/CID, direct-switch, pragmatic, open-label, clinical COPD questionnaire/CCQ, transition dyspnea index/TDI
Introduction
COPD is characterized by progressive deterioration of lung function, and worsening of symptoms and health status. The main goals of treatment and management of the disease are to improve lung function, and reduce symptoms and the risk of future exacerbations.1 Clinical trials are generally designed to evaluate improvements in lung function, symptoms, health status, quality of life, and risk of exacerbations, usually following a switch in treatment regimen. Analysis of treatment responsiveness using a composite endpoint is practiced in clinical trials of multifaceted diseases, such as cardiovascular disease.2,3 A composite endpoint typically includes both objective and subjective outcome measures, taking into account both the physicians’ clinical opinion and the patients’ perception; these endpoints are helpful in understanding the overall worsening of a disease and the effectiveness of a therapeutic intervention to reduce this. Given the generally progressive nature of COPD, treatment success can also be defined as the prevention of disease progression. Therefore, there is an increased implementation of a composite endpoint in COPD trials that considers deterioration in lung function, dyspnea, health status, and incidence of exacerbation.4
Clinically important deterioration (CID) was first evaluated as a composite endpoint for COPD trials in a pooled analysis of the fixed-dose long-acting β2-agonist/long-acting muscarinic antagonist (LABA/LAMA) combination vilanterol/umeclidinium.4 In this analysis, CID was defined based on individual outcome measures of deterioration in lung function (forced expiratory volume in 1 second [FEV1]), patient-reported outcomes (St George’s Respiratory Questionnaire [SGRQ]), and incidence of moderate or severe exacerbations.4 In a more recent analysis involving the LABA/LAMA formoterol/aclidinium, transition dyspnea index (TDI) was included in the definition of CID together with FEV1, SGRQ and exacerbations.5 Randomized, controlled, Phase III trials (ILLUMINATE, SHINE, LANTERN, and FLAME) involving the LABA/LAMA combination indacaterol/glycopyrronium (IND/GLY) demonstrated superiority in health-related quality of life and a decrease in exacerbations in patients with moderate-to-very-severe COPD, compared with monotherapies and an LABA/inhaled corticosteroid (ICS) combination.6–9 Analyses from the LANTERN, ILLUMINATE, and SHINE studies have also shown that IND/GLY effectively reduced the risk of CID versus salmeterol/fluticasone (SFC) and tiotropium using 2 different CID definitions composed of FEV1, SGRQ or TDI, and exacerbations.10
CRYSTAL was an open-label, randomized study in which patients were directly switched to IND/GLY from their baseline therapy without any washout period, mimicking routine clinical practice.11 The CRYSTAL study demonstrated superior effectiveness of IND/GLY in patients with moderately symptomatic COPD who were receiving monotherapy with LABA or LAMA or LABA + ICS dual therapy either as free or fixed-dose combination at baseline.11 The aim of this analysis was to evaluate the effectiveness of IND/GLY in reducing the risk of CID in patients with moderate COPD after direct switch to IND/GLY from previous therapies. Different CID definitions were also evaluated in order to improve our knowledge of this composite endpoint.
Methods
Study design
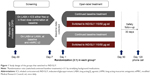
The CRYSTAL study design has been described previously (ClinicalTrials.gov number: NCT01985334).11 Briefly, this was a 12-week, prospective, multicenter, randomized, open-label trial that evaluated the efficacy and safety of IND/GLY or GLY after a direct switch from previous treatments in patients with moderate COPD and ≤1 exacerbation in the previous year, in both hospital and primary care settings. Patients were randomized (3:1) to IND/GLY or ongoing therapies stratified by previous treatment and modified Medical Research Council (mMRC) score. This post hoc analysis aimed to determine the effect of the direct switch to IND/GLY from previous treatment with LABA + ICS (in free or fixed-dose combinations) or a single bronchodilator (LABA or LAMA) to IND/GLY on reducing the risk of CID (Figure 1).
Patients
Male or female patients, aged ≥40 years were included if they had a clinical diagnosis of moderate COPD and a history of ≤1 moderate-to-severe exacerbation in the previous year, smoking history of ≥10 pack-years, mMRC score ≥1, moderate airflow limitation with FEV1 ≥50% and <80% predicted, and FEV1/forced vital capacity ratio <0.7. Patients who were switched to IND/GLY from an LABA or an LAMA had mMRC score ≥2, whereas those switched from LABA + ICS to IND/GLY had an mMRC score ≥1. All patients provided written informed consent before any study-related procedure.11 (All inclusion and exclusion criteria are provided in the Supplementary material).
Definitions and assessments of CID
Three definitions of CID were evaluated in this analysis (D1, D2, and D3), which are summarized in Table 1. Each definition comprised a combination of subjective and objective outcome measures typically associated with COPD; these were as follows: 1) deterioration in lung function (as expressed by ≥100 mL decrease in trough FEV1 from baseline); 2) deterioration in patient-reported outcomes (ie, a decrease in TDI total score of ≥1 point and/or an increase in clinical COPD questionnaire (CCQ) total score of 0.4 point); and 3) a moderate or severe exacerbation (moderate COPD exacerbation was defined as an exacerbation requiring treatment with either systemic corticosteroids and/or antibiotics, whereas a severe COPD exacerbation was defined as one that required hospitalization or an emergency room visit lasting longer than 24 hours). A patient meeting any 1 of those deterioration endpoints at Week 12 was considered as having a CID. If a patient met 2 or more of these deterioration endpoints, this was considered as a single CID.
In addition, subgroup analyses were performed based on demographic and baseline clinical characteristics. Subgroups comprised age, gender, smoking history, lung function, exacerbation history, duration of COPD, symptoms, and baseline therapy.
Statistical analysis
All statistical analyses were performed on the intention-to-treat population who switched to IND/GLY from LABA + ICS or from an LABA or an LAMA. Patients were classified into clinically deteriorating (D1, D2, and D3) or non-deteriorating groups, in accordance with the definitions in Table 1. The comparisons of CID between treatments (IND/GLY versus LABA + ICS or LABA or LAMA) were analyzed using the observed odds ratio (OR) for patients deteriorating versus non-deteriorating and the corresponding 95% CIs. A subgroup analysis assessed the effect of treatments on the risk of CID across relevant subgroups, such as: age (<65 or ≥65; <75 or ≥75 years), gender (male or female), smoking history (current or ex-smoker), number of exacerbations in the previous years (0 or ≥1), FEV1 at baseline (<60% or ≥60%), short-acting bronchodilator reversibility (<12% or ≥12%), mMRC score at baseline (≤1 or ≥2), time since diagnosis of COPD (<5 or ≥5 years), baseline therapy for patients who switched to IND/GLY from LABA + ICS (SFC; formoterol/budesonide; or other) and baseline therapy for patients who switched to IND/GLY from LABA, LAMA, or other. The optimal cut-off point for the time since diagnosis of COPD (<5 or ≥5 years) was identified by a Receiver Operating Characteristic curve analysis. P-values of <0.05 were considered as statistically significant. Statistical Analysis Software (SAS) version 9.3 was used for statistical analyses.
Ethics approval and consent to participate
Protocol and all related documents of the CRYSTAL study were reviewed by an Independent Ethics Committee (IEC) or Institutional Review Board (IRB) for each center, in each country (Austria, Belgium, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Norway, Poland, Portugal, Romania, Russian Federation, Slovakia [Slovak Republic], Slovenia, Spain, Sweden, and UK). The study was performed abiding by the ethical principles of the Declaration of Helsinki. A full list of the IEC or IRB is presented in the Supplementary material section S2.
Results
Patients and baseline characteristics
Of the 2,159 patients analyzed, 1,622 were directly switched to IND/GLY (811 from LABA or LAMA, and 811 from LABA + ICS), and 537 continued their baseline treatment with LAMA or LABA (n=268) or LABA + ICS (n=269). Patients who were receiving LABA or LAMA at baseline had a higher dyspnea score (mMRC ≥2) than those who were receiving LABA + ICS (mMRC ≥1), as per the inclusion criteria for these groups. Patients’ demographics and baseline characteristics were balanced across all groups (Table 2).
Effect of IND/GLY on CID compared with LABA or LAMA
A lower proportion of patients met the criteria for a CID with IND/GLY versus LABA or LAMA according to D1 (23.2% versus 42.5%), D2 (25.9% versus 45.9%), and D3 (30.6% versus 53.0%). Treatment with IND/GLY significantly reduced the risk of CID versus LABA or LAMA according to all definitions (D1: OR, 0.41 [95% CI: 0.30–0.55]; D2: OR, 0.41 [95% CI: 0.31–0.55]; and D3: OR, 0.39 [95% CI: 0.29–0.52]; Figure 2). Deteriorations in the individual outcome measures used to define the CID are presented in Table 3. Risk of deterioration was significantly lower for all outcome measures, except exacerbations, in patients who received IND/GLY compared with those receiving LABA or LAMA treatment. The effect of IND/GLY was most pronounced on risk reduction in FEV1 decline. The proportion of patients meeting the individual CID outcome measures in the IND/GLY-treated versus LABA- or LAMA-treated groups was lower for all measures (Table 3). Results of subgroup analyses according to baseline characteristics were consistent with the overall results – there was a general trend favoring IND/GLY compared with LABA or LAMA (Figure S1A–C).
  | Figure 2 Effect of IND/GLY on CID compared with LABA or LAMA for each CID definition. |
Effect of IND/GLY on CID compared with LABA + ICS
As with the monotherapies comparison, a lower percentage of patients met the criteria for a CID in the IND/GLY group versus the LABA + ICS group for the different definitions (D1: 27.9% versus 33.8%; D2: 32.1% versus 38.7%; and D3: 35.5% versus 45.0%), as summarized in Figure 3. Treatment with IND/GLY significantly reduced the risk of CID versus LABA + ICS according to definition D3 (OR, 0.67 [95% CI: 0.51–0.89]; P=0.0059). Risk of CID was also reduced by IND/GLY using definitions D1 and D2; however, these differences did not reach statistical significance (D1: OR, 0.76 [95% CI: 0.56–1.02]; P=0.0642; D2: OR, 0.75 [95% CI: 0.56–1.00]; P=0.0529; Figure 3). Deterioration in the individual outcome measures used to define the CID is presented in Table 4. Patients who received IND/GLY had a lower risk of deterioration in most individual outcome measures compared with those who continued to receive LABA + ICS therapy (FEV1: 15.5% versus 22.7%; TDI: 11.2% versus 14.5%; and CCQ: 13.9% versus 17.8%), except for exacerbations (7.8% versus 5.9%). The most pronounced effect of IND/GLY compared with LABA + ICS was on preventing FEV1 decline (OR, 0.66 [95% CI: 0.47–0.93]). Results of a subgroup analysis based on baseline characteristics were consistent with the overall results with trends in favor of IND/GLY and larger CIs due to the smaller patient numbers (Figure S2A–C).
  | Figure 3 Effect of IND/GLY on CID compared with LABA + ICS for each CID definition. |
Discussion
This exploratory analysis of the CRYSTAL study demonstrated that a direct switch to IND/GLY from LABA + ICS or monotherapy with LABA or LAMA reduced the risk of CID in patients with moderate COPD in routine clinical practice. The effectiveness of IND/GLY was more pronounced in patients who were switched from LABA or LAMA and may be related to the fact that these patients were more symptomatic at baseline. The results obtained from the subgroup analyses (based on patient demographics, clinical characteristics, and baseline treatments) were found to be consistent with the overall population. The complex and progressive nature of COPD can impact patients’ responsiveness to a treatment and can be influenced by demographic profile, as well as clinical and physiological characteristics. Furthermore, evaluation of disease progression can assist the physicians in choosing the most appropriate treatment strategy on a case-by-case basis.1,11 Analysis of the effectiveness of a treatment using a composite endpoint is now widely accepted in clinical trials of complex diseases, including cardiovascular diseases and neoplasia, assuming that the individual components of the composite endpoint are of clinical importance.3 The effective use of composite endpoints may increase the efficiency of clinical trials by reducing sample size, costs, and time. It may also help investigators identify outcomes that refer to disease progression and facilitate the assessment of patient-reported outcomes that provide information on multiple aspects of the patients’ perception of their health status.2,3 In the present analyses, the use of CID as a composite endpoint for COPD was based on the major drivers of worsening lung function, symptoms, health status, and exacerbations, all of which contribute to the long-term prognosis of the disease.4,5,10 Two key determinants of bronchodilator responsiveness and worsening of COPD, namely FEV1 and exacerbations, were included in all 3 CID definitions.12 These were considered in conjunction with patient-reported outcomes, namely TDI, CCQ, or both.1,12 The analyses also attempted to identify the best possible combination of individual outcome measures (accounting for lung function and exacerbation parameters) for the CID endpoint that would best assess treatment responsiveness and disease progression. Hence, TDI and CCQ were interchanged in D1 and D2 on the basis that TDI is more suitable for clinical trials while CCQ is a convenient tool in daily practice for assessment of health status. Deteriorations in TDI and CCQ had a similar performance in definitions D1 and D2, as shown by the similar CID data for these 2 definitions. Using CCQ, a simpler tool, may make the data more relevant for clinical practice than previous CID analyses of IND/GLY, vilanterol/umeclidinium, and formoterol/aclidinium, which used SGRQ as the measure of health status.4,5,10,13 Another reason for selecting the CCQ test is time for completion, as it provides information on patients’ health status much quicker than the lengthier SGRQ, although the correlation between the 2 tools is weak.14 It is important to note that the 12-week CRYSTAL study was not designed specifically to detect exacerbations, therefore, the data related to exacerbation endpoints need to be interpreted with caution. This may represent an important reason for the absence of a difference in exacerbations between IND/GLY and previous treatments, in contrast to previous randomized controlled trials specifically designed to answer this question.15,16 As expected, in this short-duration study, the major determinant of early CID prevention by IND/GLY versus previous treatments was the prevention of FEV1 reduction.
Age, gender, airflow limitation, history of exacerbations and duration of disease are all known to have an impact on treatment responsiveness of patients with COPD.1 Findings for the subgroup analysis reported here (based on demographics and baseline characteristics) showed consistency with previous efficacy data of IND/GLY in reducing the risk of CID.10 Interestingly, in all the additional analyses, we were not able to identify specific subgroups where IND/GLY would be more effective in reducing CID, suggesting that the benefit was, in general, consistent in all the subgroups evaluated (see Supplementary material).
The outcomes of this analysis support a previous report that demonstrated effectiveness of IND/GLY in reducing the risk of CID,10 where significantly delayed first occurrence of CID and delayed sustained CID were observed with IND/GLY versus tiotropium and SFC. Previous reports showed that 2 fixed-dose LABA/LAMA combinations (vilanterol/umeclidinium and formoterol/aclidinium) were significantly better than placebo and their mono-components in delaying CID in patients with moderate-to-severe COPD.4,5 However, the definition of composite endpoint used in these studies varied, comprising a combination of decrease in FEV1, increase in SGRQ, decrease in TDI, and occurrence of exacerbations.4,5 Furthermore, time-to-first and sustained CID was analyzed, whereas in this analysis, we have determined proportion of patients with CID and risk of CID, which provide similar clinically meaningful information on treatment response.10 The potential mechanisms for the increased efficacy of dual bronchodilators versus previous treatments on the reduction of CID risk are associated with their mechanism of action and may include the effective lung deflation, a potential improvement of mucociliary clearance, and the reduction of symptoms and COPD exacerbations.17,18 However, the present analysis was not designed to assess these potential mechanisms of action. Outcomes of the present analysis, along with previous reports, showed that IND/GLY can prevent CID in patients with COPD of all severities (moderate-to-very severe).8,10 In this analysis, the results were consistent across the different definitions used, which indicated that any of the definitions can be adapted to examine CID in a patient and aid a clinical decision. It further justified the selection of individual parameters by showing stability of the components and precision of the process. These analyses add to the existing repertoire of CID definitions applied to COPD trials, which may aid in determining the most applicable definition of CID endpoint for COPD to be used in routine clinical settings. In addition, the open-label design with a direct switch without a washout period mimics routine clinical practice settings, and thus enhances its applicability/generalizability, unlike previous studies,4,5,13,19
The present analysis has some limitations. First, the relatively short duration of the CRYSTAL study may not allow for appropriate evaluation of exacerbations and patients were evaluated at 2 time points only, at baseline and Week 12 and not in between. However, the CID analysis of 2 long-term trials (TORCH and ECLIPSE) showed that early CID in COPD patients was associated with long-term worsening of the disease,20 and a pooled analysis showed that earlier introduction of dual-bronchodilator therapy (vilanterol/umeclidinium) in symptomatic patients with COPD provides better protection against deterioration compared with tiotropium monotherapy.19 Therefore, the clinical importance of performing this analysis in the CRYSTAL trial is that it enriches the available evidence from other trials by adding information from a direct switch study mimicking clinical practice.
A second limitation might be that the aspect of the statistical analysis of a composite endpoint assumes that a treatment has equal impact on all individual components of the endpoint; in reality, a treatment impacts each individual parameter differently. This adds to the complexity of interpreting the results of an analysis that uses a composite endpoint, and the results derived from such analyses should be interpreted with caution.2,3 Finally, the concept of CID needs to be validated in prospective trials in order to identify the potential clinical value of this composite endpoint and, specifically, long-term studies are needed to substantiate the real-life effectiveness of IND/GLY in reducing the risk of CID.
In conclusion, this analysis – using data from the open-label CRYSTAL study – demonstrated that in real-life clinical practice, a direct switch to IND/GLY from LABA + ICS, or LABA or LAMA therapies significantly reduces CID in patients with moderate COPD, leading to prevention of disease progression. These results support the use of LABA/LAMA as a preferred treatment option in symptomatic patients with moderate COPD and up to 1 exacerbation in the previous year, complementing similar data from other randomized controlled trials.
Acknowledgments
Writing and editorial assistance for this manuscript was provided by Santanu Bhadra and Vivek Khanna (Novartis Healthcare Pvt. Ltd., India). The study was supported and funded by Novartis. None of the authors received any compensation for the development of the manuscript.
Author contributions
All authors have contributed substantially in data interpretation, development and revision of manuscript draft, and have provided their consent and approval for publishing this manuscript.
Disclosure
TG has received compensation from Novartis during the conduct of the study. He has also received lecture fees from AstraZeneca, Chiesi, CSL-Behring, GlaxoSmithKline, Grifols, Mundipharma, and Novartis, and received compensation for organizing or participating in advisory boards from AstraZeneca, CSL-Behring, Novartis, Boehringer Ingelheim and Grifols, and received a grant to support an AATD-Lab from Grifols. KK has previously received honoraria for speeches and consulting services from AstraZeneca, Chiesi, ELPEN, and Takeda, and received honoraria for speeches from Boehringer Ingelheim outside the submitted work. MG has received a grant and personal fees from Novartis, Pharmaten and Menarini, outside the submitted work. She has also received personal fees from Chiesi, Boehringer Ingelheim and Teva, and received compensation for organizing or participating in advisory boards from GlaxoSmithKline, and received a grant from AstraZeneca. XN and VAP were statisticians for this subgroup analysis of the CRYSTAL study and work at a Novartis contracted CRO. CFV has received personal fees from Novartis during the conduct of the study. Outside the submitted work, he has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma and Teva, and grants from GlaxoSmithKline and Grifols.
KK is an employee and shareholder of Novartis Pharma AG. MA-M and NL are employees of Novartis Pharma AG. AC and FP are employees and shareholders of Novartis Pharma AG. RF is an employee and shareholder of Novartis Pharmaceutical Corporation. The authors report no other conflicts of interest in this work.
References
Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. Available form http://www.goldcopd.org. Accessed February 2, 2018. | ||
Ferreira-Gonzalez I, Permanyer-Miralda G, Busse JW, et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60(7):651–657. | ||
Tomlinson G, Detsky AS. Composite end points in randomized trials: there is no free lunch. JAMA. 2010;303(3):267–268. | ||
Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. | ||
Singh D, D’Urzo AD, Chuecos F, Munoz A, Garcia Gil E. Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol. Respir Res. 2017;18(1):106. | ||
Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. | ||
Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. | ||
Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. | ||
Zhong N, Wang C, Zhou X, et al; LANTERN Investigators. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. | ||
Anzueto AR, Vogelmeier CF, Kostikas K, et al. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1325–1337. | ||
Vogelmeier CF, Gaga M, Aalamian-Mattheis M, et al; CRYSTAL study investigators. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140. | ||
Vestbo J, Edwards LD, Scanlon PD, et al; ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. | ||
Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. | ||
Tsiligianni IG, Alma HJ, de Jong C, et al. Investigating sensitivity, specificity, and area under the curve of the Clinical COPD Questionnaire, COPD Assessment Test, and Modified Medical Research Council scale according to GOLD using St George’s Respiratory Questionnaire cutoff 25 (and 20) as reference. Int J Chron Obstruct Pulmon Dis. 2016;11:1045–1052. | ||
Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. | ||
Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. | ||
Beeh KM, Burgel PR, Franssen FME, et al. How do dual long-acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2017;196(2):139–149. | ||
Kostikas K, Siafakas NM. Does the term “Deflators” reflect more accurately the beneficial effects of long-acting bronchodilators in COPD? COPD. 2016;13(5):537–539. | ||
Maleki-Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short-term deterioration in maintenance-naive patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three tandomized trials. Adv Ther. 2017;33(12):2188–2199. | ||
Naya I, Tombs L, Mullerova H, Compton C, Jone P. Long-term outcome following first clinically important deterioration in COPD. Eur Respir J. 2016;48:PA304. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.