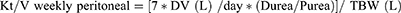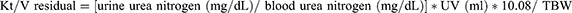Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Incremental Hemodialysis: What We Know so Far
Authors Soi V , Faber MD, Paul R
Received 19 January 2022
Accepted for publication 29 March 2022
Published 29 April 2022 Volume 2022:15 Pages 161—172
DOI https://doi.org/10.2147/IJNRD.S286947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Pravin Singhal
Vivek Soi,1,2 Mark D Faber,1,2 Ritika Paul1
1Division of Nephrology and Hypertension, Henry Ford Hospital, Detroit, MI, USA; 2Wayne State University School of Medicine, Detroit, MI, USA
Correspondence: Vivek Soi, Email [email protected]
Abstract: Traditionally, patients that develop progressive chronic kidney disease in need of kidney replacement therapy are prescribed thrice weekly in-center hemodialysis sessions at the beginning of therapy. This empiric prescription is based on historic trials that were comprised of mostly prevalent patients. Incremental hemodialysis is the process of performing < 3 sessions of dialysis per week or limiting dialysis dose by duration at the initial onset of treatment to provide a more gradual transition, mimicking the progressive nature of kidney disease. Adding clearance contributions from residual kidney function is the standard of care with peritoneal dialysis but has not routinely been employed with hemodialysis. Accounting for residual kidney function accompanied by improvement in adjuvant pharmacotherapy, such as newer potassium binding agents and dietary modification, can augment dialytic clearances and allow for an incremental approach. Utilizing incremental dialysis has been associated with both preserving residual kidney function as well as improving patient quality of life. Barriers to this approach include concerns regarding patient acceptance of dialysis prescription changes, adherence to therapy, and provider factors that would require a restructuring of the current thrice weekly hemodialysis rubric. Candidacy for incremental therapy has shown the best outcomes when urea clearances exceed 3 mL/min and urine volumes are > 500 mL/day, although these measures have been deemed conservative. A significant amount of retrospective and registry data has been supportive of initiating incremental hemodialysis and several pilot studies have shown the feasibility of implementing such an approach. Larger, randomized control trials are needed to fully evaluate safety and efficacy to allow for more widespread acceptance of this patient-centered approach to chronic kidney disease.
Keywords: hemodialysis, incremental dialysis, twice-weekly
Introduction
Initiating kidney replacement therapy is a life altering process that requires joint decision-making to ensure optimal outcomes. Most patients with end-stage kidney disease (ESKD) are treated with a conventional regimen of thrice weekly in-center hemodialysis to receive a minimum dialysis single pool Kt/V of >1.2.1 Incremental hemodialysis involves altering the initial hemodialysis prescription to either less than the traditional thrice weekly model or decreasing the prescribed dose of dialysis in selected patients with significant residual kidney function (RKF) with intentions to increase dialysis intensity as time progresses.2 In many areas of the world, limiting frequency and duration of dialysis to twice a week or reducing dialysis time continues to be the initial standard prescription as a matter of necessity due to constraints on medical resources and personnel shortages, conditions that have been magnified during the COVID-19 pandemic.3–5 Precedence for an incremental method can be found in patients undergoing peritoneal dialysis and has been associated with preservation of RKF. This approach offers an integrated, targeted therapeutic approach to advanced chronic kidney disease (CKD) in selected patients who begin hemodialysis.
Historically, the thrice weekly hemodialysis paradigm was empirically derived following the invention of the Scribner shunt in 1960 that allowed for repeated hemodialysis sessions to be performed in a practical manner. Initially, patients underwent dialysis every 5 to 7 days with recurrence of uremic symptoms prompting further therapy; however, intensification of treatments from longer twice weekly schedules to shorter, 6- to 8-hour, thrice weekly overnight treatments was adopted based on the nocturnal hemodialysis experience.6 With the advent of the Medicare End-Stage Renal Disease program in 1973, thrice weekly dialysis became the usual practice providing a compromise between providing adequate therapy and utilizing limited medical resources. Both the US National Cooperative Dialysis Study, which illustrated reduction in urea was associated with clinical benefits, and the HEMO study, which established a floor adequacy of single pool Kt/V of 1.25, utilized thrice weekly schedules to avoid the complexity of dealing with potential confounding variables.7,8 It should be noted that cohorts from both the US National Cooperative Dialysis Study and HEMO studies were restricted to prevalent patients with limited to no RKF with creatinine clearances <3 mL/min and urea clearances <1.5 mL/min making it difficult to generalize results to those with higher RKF. The Frequent Hemodialysis Network trial, which compared 6 times per week vs 3 times per week dialysis, showed improvement in left ventricular mass as well as hypertension and phosphorus control in the former group with fewer access complications in the latter, but it was not designed to evaluate an early transition from stage 5 CKD to ESKD.4,9
The United States Renal Data Systems reports 133,405 incident in-center hemodialysis patients for 2019, of which the Dialysis Outcomes Practice Patterns Study reports only 2.3% (reported February 2019) and 3.5% (reported August 2019) were started with 2 or less dialysis sessions weekly. During those same time frames, 1.2% and 0.1% were started on 4 or more weekly sessions. The majority, 96.4%, for both February and August were started on the standard regimen of thrice weekly dialysis. Data from the Dialysis Outcomes Practice Patterns Study reports from 2012 to 2021 show nearly 95% of incident patients were started on the standard regimen in this period (Figure 1).10
 |
Figure 1 Prescribed dialysis sessions per week in the United States from 1st quarter 2012–2nd quarter 2019 by incidence and prevalence shows that the overwhelming majority of patients initiate hemodialysis on a thrice weekly schedule. (<90d)/prevalent (90 +d). Adapted from DOPPS practice monitor – hemodialysis; 2021. Available from: https://www.dopps.org/DPM-HD/DPMSlideBrowser.aspx?type=ComGrp&id=18. Accessed October 11, 2021. Modifying DOPPS Practice Monitor data, tables, or graphics in any form is not permitted without prior approval from the DOPPS coordinating center staff at Arbor Research. You may contact us at [email protected].10 Abbreviation: d, days. |
The Economic Impact of Incremental Dialysis
When Medicare eligibility first extended to persons with “irreversible kidney failure” in the early 1970s, the national expenditures for healthcare were approximately $75 billion (approximately $450 billion in 2018 dollars based on estimates of adjustments for inflation). Analysts of the Assistant Secretary for Health in the Department of Health, Education and Welfare predicted that once the End-Stage Renal Disease program was in “steady-state”, approximately 20,000–30,000 patients would require maintenance hemodialysis with projected total expenditure of approximately $1 billion ($6 billion in 2018 dollars). However, by 2018, there were over 500,000 patients receiving maintenance hemodialysis, accounting for approximately 7.2% of Medicare fee-for-service spending. In inflation unadjusted terms, this accounted for $36.6 billion or 30.7% of Medicare fee-for service spending. Of all the modalities, hemodialysis remains the most expensive treatment. In 2018 for ESKD, the cost to treat one patient with ESKD on hemodialysis was on average $93,191 annually.
According to Trish et al in 2021, 80% of US patients receiving dialysis have Medicare as their primary payor, incurring average costs of $3364 monthly ($200-300 per session). This is even higher for non-Medicare spending, which is often greater by three-fold.11 For every 2 patients on a standard thrice weekly schedule, 3 incremental patients on twice weekly dialysis could use the same chair in any given week (Figure 2). According to a study by Obi et al,45 if incremental hemodialysis regimens could be used for the first several months of therapy while patients still have sufficient RKF, $250 million to $500 million could be saved every year based on Medicare spending alone. This number could potentially triple when taking private payors into account. With this in mind, incremental dialysis was anticipated to become the preferred treatment for ESKD Seamless Care Organizations. While the US Presidential Executive Order on Advancing American Kidney Health Initiative signed in 2019 was expected to replace the original plan, the emphasis on increasing affordable alternative treatment plans was emphasized and is inclusive of incremental therapy.12
 |
Figure 2 Proposed twice weekly vs traditional thrice weekly schedules for a single dialysis chair over 1 week. With incremental dialysis, there is potential for one station to be used by 3 patients instead of the 2 that would be able to be managed in the traditional schedule. Note that once weekly dialysis (not pictured) has also been proposed as an option in incremental initiation. Adapted from Kalantar-Zadeh K, Crowley ST, Beddhu S, et al. Renal Replacement Therapy and Incremental Hemodialysis for Veterans with Advanced Chronic Kidney Disease. Semin Dial. 2017;30(3): 251-261. © 2017 Wiley Periodicals, Inc.53 |
Adaptation of RKF with Declining Disease
A physiologic justification for maintaining RKF as an adaptive response to advanced CKD has been discussed by Golper and Mehrotra and termed “the intact nephron hypothesis in reverse.”13 As kidney disease progresses, the nephrons that remain, progressively increase their rates of excretion to compensate for damaged nephrons and retain some essential functional integrity. The diseased kidney’s adaptation to nephron loss is potentially compromised with the initiation of dialysis. Volume expansion is thought to be a stimulus for the functioning nephrons to continue activity and its loss by dialysis plays a role in the decline of RKF. The diseased kidney’s adaptation may remain stimulated when dialysis is gently and incrementally introduced. Intense dialysis, particularly where significant ultrafiltration occurs, removes these stimuli for adaptation. Furthermore, decreasing exposure to the extracorporeal circuit can mitigate compounded intradialytic hypotensive episodes affecting kidney blood flow. Even a moderately low RKF may be adequate to increase clearance of the larger middle molecular weight molecules, which is difficult for even modern dialyzers to selectively accomplish.14
Adapting Experiences with Peritoneal Dialysis and RKF
Precedence for implementing an incremental start to kidney replacement therapy exists in the peritoneal dialysis literature. The “Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes” study was an observational, prospective study that reported a close correlation between the total sum of peritoneal and renal weekly urea Kt/V and creatinine clearance with mortality in incident peritoneal dialysis patients.15 Clinical practice guidelines formulated in its aftermath promoted a version of incremental dialysis, under the assumption that optimal patient outcomes could be maintained by substitution of diminishing RKF with increasing doses of peritoneal dialysis. However, it was impractical to maintain the specified targets (Kt/V urea = 2/week and creatinine clearance of 60 L/1.73 m2/week) for many patients without RKF. Moreover, it became clear subsequently when the study was reanalyzed in 2001 that the favorable impact of higher clearances was completely explained by higher RKF, and that almost no titration of dialysis dose was implemented during the study period.16 Incremental dialysis is still widely practiced when prescribing peritoneal dialysis but using a more realistic target (RKF plus peritoneal Kt/V urea = 1.7/week) based on the findings of the randomized, controlled ADEMEX trial of peritoneal dialysis dose.17
The seemingly disparate doses empirically associated with peritoneal dialysis and thrice weekly hemodialysis (1.7 per week and 1.2 thrice per week) long begged for mathematical clarification. While several theories have been postulated, the most widely accepted explanation has been the standard Kt/V concept of Gotch, which mathematically equates weekly urea removal across therapies delivered continuously or intermittently at differing times per week.18 The mathematics of standard hemodialysis Kt/V have recently been reviewed, including a list of available online and mobile application-based calculators to facilitate utility in prescription writing.19,20 The principal clinical implication of standard Kt/V is that the more intermittent a therapy, the higher the required clearance to achieve the same mass of solute removal. In testimony to its clinical utility, the standard Kt/V for the above-mentioned empirically derived peritoneal and hemodialysis targets are approximately equal. In 2015, the KDOQI opinion based guidelines confirmed the original targets for thrice weekly hemodialysis (per treatment Kt/V 1.4 prescribed, 1.2 delivered), which equates to a prescribed and delivered standard Kt/V of 2.3 and 2.1 per week. This included the recommendation to include RKF in the calculation, thus emphasizing its importance by eliminating a previous multiplier that decreased the contribution of residual clearance by a fractional amount.21 The practical importance of this approach is that it enables the setting of individual per treatment Kt/V targets for patients dialyzing 2, 3, 4, 5, 6, or 7 times per week (see Table 1). This has been critical in the development of targets for short daily home dialysis in particular.
 |
Table 1 Values for Modeling Included 6–8 L Ultrafiltration per Week and Watson TBW 42.9 L |
In continuous therapies, including peritoneal dialysis, standard Kt/V and directly measured Kt/V are the same. Thus, in peritoneal dialysis weekly standard Kt/V can be calculated as follows:
where DV is peritoneal effluent volume, Durea is dialysate urea concentration, Purea is plasma urea concentration, and TBW is total body water. Residual Kt/V is obtained by the following:
where UV is urine volume in milliliters/24 hours and the constant 10.08 converts milliliters/minute to liters/week.
The standard total weekly Kt/V is thus the sum of the Kt/V peritoneal + Kt/V residual. Analogous calculations allowing for the incorporation of Kt/V residual can be applied to incremental hemodialysis after calculation of Kt/V standard with this modality. There are tremendous benefits to RKF in patients undergoing hemodialysis beyond urea clearance, including improved volume control, better electrolyte balance, and lower erythropoietin-stimulating agent requirements.22 This was further illustrated in the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease study, where mortality was lower in patients undergoing hemodialysis with RKF.23 Urine output at 1 year, indicating preserved RKF, was independently associated with lower all-cause mortality (hazard ratio [HR] 0.70; 95% CI 0.52–0.93; P = 0.02) and a trend toward lower cardiovascular mortality (HR 0.69; 95% CI 0.45–1.05; P = 0.09). Participants with urine output at baseline reported better quality of life and had lower C-reactive protein (P = 0.02) and interleukin 6 (P = 0.03) levels. Importantly, erythropoietin dose was 12,000 U/week lower in those with urine output at the end of 1 year, compared to those without RKF (P = 0.001). Note that typically a 1:1 equivalence is given to both dialytic and residual Kt/V with a fixed target, but given the importance of residual function, a variable target model has been proposed by Casino and Basile that gives more weight to residual urea clearance (Kru).24 They describe titration of dialysis dose based on successive decreases in Kru with high intensity monitoring. Further prospective studies would be needed to examine the feasibility of this model prior to widespread use.
Incorporating RKF into total dialysis dose assumes that regularly scheduled urine collections will be performed as a decline portents prescription adjustment. Measuring urine collections can be a tedious process that requires significant dedication to preserve accuracy. Surrogate formulas that extrapolate Kru utilizing beta trace protein and beta 2 microglobulin have been proposed but have lacked validation and are not currently in widespread clinical use.25 Note that the use of race within this model is also difficult to justify given efforts to remove this variable in conventional estimated glomerular filtration rate (GFR) equations.
Diuretic Therapy and Volume Status
Diuretic therapy is a staple means of facilitating volume management by diminishing interdialytic weight gain and improving hypertensive control in patients with CKD. In an effort to diminish polypharmacy or due to underestimating their effects in advanced disease, these medications are often discontinued when patients are initiated on hemodialysis. This practice pattern contrasts to peritoneal dialysis where these medications continue to play a role in care. The feasibility of incremental twice weekly hemodialysis in patients with RKF was evaluated by Chin et al. They evaluated a 410 patient cohort who initiated dialysis after at least 6 months of traditional thrice weekly treatment and underwent serial timed urine collections. By assessing Kru, ultrafiltration rate and hemodynamic stability during treatment, 27% of patients had optimal characteristics that portended conversion to a twice weekly regimen. Furthermore, an additional 26% of this cohort had appropriate urea clearance and would have met the KDIGO standard Kt/V target > 2.3; however, the predicted ultrafiltration rate would have exceeded 13 mL/kg/hour. The authors evaluated diuretic use via electronic medical record medication listing and deemed that diuretics were an underutilized medication in this group and could have decreased interdialytic weight gains affecting weekly combined ultrafiltration needs.26
Evaluating volume status in patients undergoing dialysis has been a difficult task, particularly in those undergoing less frequent therapy, leading to the utilization of several diagnostic therapies such as hematocrit sensors, lung ultrasound, and bioimpedance measurements.27 A study evaluating the use of B-type natriuretic peptide and body composition analysis with bioimpedance spectroscopy showed that vintage on dialysis was a significant risk factor for mortality. Patients undergoing twice weekly dialysis versus thrice weekly dialysis were more likely to have increased signs of hypervolemia on body composition analysis, but when subgroup analysis was applied there was a similar overhydration to extracellular water ratio in patients that had been undergoing dialysis < 6 years regardless of dialysis frequency. B-type natriuretic peptide levels were a predictor of mortality in this study, and patients with B-type natriuretic peptide > 500 pg/mL had significantly worse survival compared to those with lower values.28
Concerns regarding excessive hourly ultrafiltration have permeated the hemodialysis literature with multiple studies showing adverse cardiovascular and all-cause mortality outcomes in patients with ultrafiltration rates of > 13 mL/kg body weight/hour.29 With reduced frequency of dialysis, this concern becomes magnified. A retrospective cohort trial evaluating 1524 patients who initiated maintenance hemodialysis with a frequency of twice or less per week for at least 6 weeks showed an increased HR for all-cause mortality of 1.43 (95% CI 1.09–1.88), 1.51 (95% CI 1.08–2.1) and 1.76 (95% CI 1.23–2.53) for ultrafiltration rate of 6 to < 10, 10–13, and 13 mL/kg/hour, respectively, mimicking data seen in thrice weekly dialysis.30 This association of worse outcome to higher ultrafiltration rate was attenuated by increased RKF and urea clearances that exceeded 5 mL/min. Higher ultrafiltration rate led to a graded association with rapid decline in RKF with ultimate loss of this protective factor.
Adjunctive Medical and Dietary Therapies
Additional medical and dietary therapies are often needed to complement the dialytic circuit when an incremental approach is undertaken. The potassium binding agents patiromer and sodium zirconium cyclosilicate have significant efficacy in patients with CKD and have a more favorable side effect profile when compared to previous agents, such as sodium polystyrene.31 Controlling potassium with these newer agents allows for the use of anti-renin-angiotensin aldosterone agents that can further preserve RKF. Supplemental bicarbonate is often necessary when treating these patients for metabolic acidosis to maintain arterial pH at acceptable levels and preserve bone health given decreased exposure to dialysate bicarbonate and decreased endogenous bicarbonate generation.
Incorporation of a low protein diet has been effective in attenuating uremic symptoms or complications and has had modest effects in slowing the rate of CKD progression before transitioning to dialysis. Adapting this nutritional change to patients who have transitioned to the initial phase of dialysis therapy has been suggested by Bolasco et al via a combined diet and dialysis program as a means of preserving RKF and decreasing frequency of renal replacement therapy.32 A once weekly hemodialysis treatment combined with low-protein (0.6 g/kg/day), low-phosphorus, and normal-to-high-calorie diet (30–40 kcal/kg/day) especially on non-dialysis days has also been described. This adaptation included supplementation of essential amino acids, modification of calorie intake and correction of metabolic acidosis. A study by Caria et al showed favorable outcomes in 38 patients assigned to combined diet and dialysis program when compared to 30 patients who started thrice weekly dialysis over a period of 24 months.2 This adaptive dietary regimen may elicit more favorable outcomes including better preserved RKF, lower β2-microglobulin levels, improved phosphorus control, lower hospitalization rates and lower doses of erythropoietin-stimulating agents. Note that adherence and adequate protein and energy intake should be ensured to avoid protein-energy wasting and that individualized care with the guidance of a registered dietician should be performed in the care of this patient population.
Quality of Life
The need for maintenance hemodialysis due to advanced kidney disease affects multiple aspects of patient well-being including quality of life. Prolonged transit time to and from dialysis treatment facilities, intradialytic symptoms, and a recovery time post-dialysis are commonly cited concerns that may be mitigated through a less frequent dialysis schedule when appropriate. Tools that quantify both physical and psychiatric symptoms through surveys, such as the Kidney Disease Quality of Life, and formalized depression screening have been standard practice to ensure that this aspect of patient care is adequately assessed. Twice weekly dialysis is common in China, particularly among patients that started dialysis more recently, have a lower comorbidity burden and have financial constraints. Quality of life scores do not differ between the twice and thrice weekly groups.33 In a study of 312 patients in China, the health related quality of life tended to be better in the incremental group for the majority of domains of the Kidney Disease Quality of Life Short Form and Beck’s Depression Inventory; however, only the symptoms and problems domain was significantly better in the incremental group at 3 months after hemodialysis. At 12 months after hemodialysis, there were no differences between the groups.34 These studies illustrate an improvement in quality of life during the earlier initiation phases of maintenance hemodialysis with no significant deleterious effects with further follow-up.
Limiting Access Complications
Limiting the need for venipuncture of arteriovenous access has potential benefits for access longevity. The Frequent Hemodialysis Network trial was one of the largest randomized controlled trials to examine whether 6 times a week dialysis would show advantages when compared to more traditional dialysis and prespecified vascular access as a safety outcome. Investigators found that daily hemodialysis significantly increased the risk of vascular access complications with time to first intervention, loss, or access-related hospitalization (HR 1.76, 95% CI 1.11–2.79).35 Permanent access loss was not shown to be different between groups; however, the need for arteriovenous repair was higher in the daily dialysis group. While increased frequency of cannulation may result in direct damage to access, stenosis has been postulated to occur from profibrotic cytokine production and neointimal hyperplasia requiring more frequent access evaluations and procedures. The above seems to indicate that fewer cannulations would lead to less access complications; however, there is no robust study comparing access outcomes with incremental hemodialysis versus thrice weekly treatments, making this an interesting topic for future trials.
Outcomes Data
As interest in this field has increased, several clinical trials have been performed to further evaluate outcomes in patients undergoing incremental dialysis (Table 2).2,5,28,34,36–50 Preservation of RKF has been demonstrated in several observational cohort studies. In 2014, a cohort of 85 patients from Shanghai was evaluated where 30 patients initiated twice weekly hemodialysis for 6 months or longer, and 55 patients were started and maintained on thrice weekly hemodialysis treatment.43 A subcohort study was subsequently identified with 48 incident maintenance hemodialysis patients to assess the independent risk factors responsible for RKF decline during the first year of hemodialysis therapy. Multivariate logistic regression analysis showed similar clinical outcome between both groups; however, the patients undergoing twice weekly dialysis had a significant decrease in the loss of RKF, particularly in the first year. In this study, the odds ratio for loss of RKF increased by 7.2 for each additional session of hemodialysis per week leading to the conclusion that twice weekly hemodialysis during the first year of dialysis therapy appeared to be associated with better RKF preservation.
 |
Table 2 List of Studies Comparing Incremental Dialysis Regimens with Traditional Thrice Weekly Dialysis Regimens |
A prospective multicenter observational cohort trial by Hwang et al evaluated cardiovascular outcomes and all-cause mortality over a 3-year period in 3 different cohorts: patients with RKF undergoing twice weekly dialysis (113), those with RKF undergoing thrice weekly dialysis (137) as well as controls without RKF (435).44 In patients with RKF, those undergoing twice weekly dialysis maintained greater than or equal RKF than those undergoing thrice weekly treatment. While there was a decrease in mortality comparing twice weekly dialysis patients with RKF (11.8%) compared to thrice weekly patients without RKF (16%), patients with RKF undergoing thrice weekly dialysis had the least mortality (6.8%). An independent association with a greater risk of mortality compared with patients with RKF undergoing thrice weekly dialysis (HR 4.2, 95% CI 1.02–17.32, p = 0.04) was seen. Several critiques of these results have been made. There was a decrease in the normalized protein catabolic rate in the twice weekly group over a 24-month follow-up. The use of high flux membranes was limited to 28% in the incremental group and was near 45% for both conventional groups. Additionally, there was a statistically significant difference in the number of dialysis catheters, 16.8% in the twice weekly hemodialysis with RKF versus 3.6% in the thrice weekly hemodialysis with RKF. The authors concluded that RKF alone should not be the only determinant of dialysis frequency and that other factors such as access type, normalized protein catabolic rate, and the use of high flux membranes should influence decision making.
A longitudinal cohort study compared 351 patients with incremental regimens to 8068 patients with conventional hemodialysis prescriptions matched based on baseline kidney urea clearance, urine volume, age, sex, diabetes, and central venous catheter use. The incremental group had 16% (95% CI 5–28%) more preserved urea clearance and 15% (95% CI 2–30%) greater urine volume at the second quarter, which persisted across the following quarters.45 Incremental regimens showed higher mortality risk in patients with inadequate baseline kidney urea clearance (≤ 3.0 mL/min/1.73 m2; HR 1.61, 95% CI 1.07–2.44), but not in those with higher baseline kidney urea clearance (HR 0.99, 95% CI 0.76–1.28). Results were similar in a subgroup defined by baseline urine volume of 600 mL/day. Note that this study was limited based on potential selection bias and was noted to have wide confidence intervals. These authors concluded that among incident hemodialysis patients with substantial RKF, incremental hemodialysis may be a safe treatment regimen associated with greater preservation of RKF, whereas higher mortality is observed after the first year of dialysis in those with the lowest RKF.
Supportive data for an incremental approach to kidney replacement has also been shown in a large meta-analysis of 22 studies including 15 hemodialysis and 7 peritoneal dialysis trials.51 This meta-analysis included over 75,292 patients and revealed that conventional thrice weekly dialysis could be delayed by 12.1 months. Furthermore, it suggested that incremental dialysis did not decrease survival. The authors concluded that incremental dialysis allowed for a longer preservation of RKF deferring full dose dialysis for approximately 1 year in patients undergoing both hemodialysis and peritoneal dialysis without increased risk for mortality.
Several pilot studies have been conducted in an effort to compile more robust data on the safety and efficacy of less frequent dialysis. Recently, a randomized controlled trial compared 25 patients, who were initiated on twice weekly hemodialysis with adjuvant medical therapy including patiromer for 6 weeks prior to transitioning to thrice weekly dialysis to 23 patients that had a conventional thrice weekly start.50 All patients were adults who had GFR of > 5 mL/min and urine output > 500 mL/day. At 24 weeks the need for hospitalization as shown via incidence rate ratio was 0.31 (95% CI 0.08–1.17) and 7 deaths were recorded (1 in the incremental and 6 in the conventional group). At the end of 24 weeks, the incremental hemodialysis group had a 51.0% lower decline in urine volume (95% CI −0.7–102.8) and 57.9% lower decline in the averaged urea and creatinine clearances (95% CI −22.6–138.4). Despite being limited in size and follow-up, this pilot study showed that it was feasible to enroll patients who were adherent to treatment protocols including frequent urine collections and serves as the basis to develop and execute larger multicenter clinical trials to determine the efficacy and safety of incremental hemodialysis with longer twice weekly hemodialysis periods.
Choosing Optimal Candidates for Incremental Dialysis
One challenge that affects prescribing kidney replacement therapy via an incremental approach is determining the actual onset of dialysis. The Initiating Dialysis Early and Late (IDEAL) study52 sought to determine the association of dialysis initiation timing with survival in subjects with ESRD. IDEAL included patients that were randomized to commence dialysis at a GFR that was defined as either early (10–14 mL/min/1.73m2) or late (5–7 mL/min/1.73 m2). There was no association or improvement in survival or clinical outcomes with starting dialysis at an earlier GFR. Unfortunately, a comparison of outcomes in patients who have begun incremental dialysis with those who were not started on dialysis is lacking in the literature and would be a beneficial control group in future studies. Initiation of incremental dialysis should continue to follow guidance from the IDEAL study, relying on symptoms and sequelae of ESKD to guide timing of dialysis initiation rather than a numerical estimated GFR assessment that crosses a certain threshold alone.
The process of choosing ideal candidates for incremental hemodialysis should include several factors with a precision medicine mindset and patient-centered approach to best incorporate an individual’s ESKD life plan. Preparation for incremental treatment should start prior to transitioning to dialytic therapy to set expectations that will allow for acceptance of gradual escalation of dialysis time or frequency when RKF diminishes. While perceived patient reluctance to alter therapy is a concern for care providers, pilot studies have shown feasibility of this method with patient acceptance best demonstrated when they are given notice of future prescription changes. Patient understanding of adherence to regular urine collections to assess urea clearance and urine volumes is essential to the process and will yield valuable data that will allow titration of the dialysis prescription. Current literature supports initial urea clearance > 3 mL/min and urine volume of > 600 mL/day. A note should be made that these guidelines are deemed conservative in nature, and in Southeast Asia lower urine volumes and clearances have been incorporated into the standardized care model. The timing of urine collections should occur between every 1 to 3 months with increased frequency of collections based on urine volume. Minimizing interdialytic weight gain by restricting fluid and sodium intake with corresponding interdialytic weight gains that are < 2.5 kg between successive dialysis treatments done 3 to 4 days apart is recommended to avoid excessive intradialytic ultrafiltration rates and large changes in hemodynamic factors that could impair RKF. The role of the patient’s dietician is vital to modifying dietary intake and limiting fluid gains. This may be facilitated through written diet journals or the use of computerized applications to track the above parameters.26 Active participation from both providers, patients and the entirety of the interdisciplinary team is vital to the success of an incremental start to hemodialysis.
Conclusion
Incremental hemodialysis represents a significant paradigm shift from the traditional thrice weekly model that has been in existence for the past 5 decades. Peritoneal dialysis has routinely incorporated RKF into dialysis dose and clearance assessments, and it makes sense to do so in hemodialysis as well. Given the potential benefits to RKF, quality of life, and vascular access, considerations should be made to include this option when generating the initial dialysis prescription. Incremental start hemodialysis is not contradictory to the notion that longer or more frequent hemodialysis may benefit certain groups of patients, particularly those with diuretic resistant volume concerns or in those with anuria that have had nonprogressive decline in kidney function, nor should it preclude home options as the location of therapy. In fact, these two treatment plans could encompass both ends of a continuum of precision medicine options that best individualizes the dialysis regimen for a given patient’s evolving clinical status and needs. Data from retrospective, registry and smaller pilot studies have shown the feasibility of incremental hemodialysis. To further general acceptance, larger prospective clinical trials are needed to facilitate adoption of this treatment alternative.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hemodialysis Adequacy Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–S90. doi:10.1053/j.ajkd.2006.03.051
2. Caria S, Cupisti A, Sau G, Bolasco P. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014;15:172. doi:10.1186/1471-2369-15-172
3. Mendonca S, Bhardwaj S, Sreenivasan S, Gupta D. Is twice-weekly maintenance hemodialysis justified? Indian J Nephrol. 2021;31(1):27–32. doi:10.4103/ijn.IJN_338_19
4. Rhee CM, Unruh M, Chen J, Kovesdy CP, Zager P, Kalantar-Zadeh K. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26(6):720–727. doi:10.1111/sdi.12133
5. Yan Y, Wang M, Zee J, et al. Twice-weekly hemodialysis and clinical outcomes in the China dialysis outcomes and practice patterns study. Kidney Int Rep. 2018;3(4):889–896. doi:10.1016/j.ekir.2018.03.008
6. Blagg CR. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am J Kidney Dis. 2007;49(3):482–496. doi:10.1053/j.ajkd.2007.01.017
7. Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi:10.1056/NEJMoa021583
8. Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int. 1985;28(3):526–534. doi:10.1038/ki.1985.160
9. Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–2300.
10. DOPPS practice monitor - hemodialysis; 2021. Available from: https://www.dopps.org/DPM-HD/DPMSlideBrowser.aspx?type=ComGrp&id=18.
11. Trish E, Fiedler M, Ning N, Gascue L, Adler L, Lin E. Payment for dialysis services in the individual market. JAMA Intern Med. 2021;181(5):698–699. doi:10.1001/jamainternmed.2020.7372
12. Murea M, Moossavi S, Fletcher AJ, et al. Renal replacement treatment initiation with twice-weekly versus thrice-weekly haemodialysis in patients with incident dialysis-dependent kidney disease: rationale and design of the TWOPLUS pilot clinical trial. BMJ Open. 2021;11(5):e047596. doi:10.1136/bmjopen-2020-047596
13. Golper TA, Mehrotra R. The intact nephron hypothesis in reverse: an argument to support incremental dialysis. Nephrol Dial Transplant. 2015;30(10):1602–1604. doi:10.1093/ndt/gfv271
14. Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24(5):487–494. doi:10.1111/j.1525-139X.2011.00968.x
15. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7(2):198–207. doi:10.1681/ASN.V72198
16. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–2162. doi:10.1681/ASN.V12102158
17. Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–1320. doi:10.1681/ASN.V1351307
18. Gotch FA. The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transplant. 1998;13(Suppl 6):10–14. doi:10.1093/ndt/13.suppl_6.10
19. Churchill BM, Patri P. The nitty-gritties of Kt/V (urea) calculations in hemodialysis and peritoneal dialysis. Indian J Nephrol. 2021;31(2):97–110. doi:10.4103/ijn.IJN_245_19
20. Meyer TW, Blanco IJ, Grimm JC, Leypoldt JK, Sirich TL. Barriers to reducing hemodialysis time and frequency in patients with residual kidney function. J Am Soc Nephrol. 2021;32(9):2112–2116. doi:10.1681/ASN.2021030361
21. Daugirdas JT, Depner TA, Inrig J; National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi:10.1053/j.ajkd.2015.07.015
22. Penne EL, van der Weerd NC, Grooteman MP, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(2):281–289. doi:10.2215/CJN.04480510
23. Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–358. doi:10.1053/j.ajkd.2010.03.020
24. Casino FG, Basile C. The variable target model: a paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant. 2017;32(1):182–190. doi:10.1093/ndt/gfw339
25. Wong J, Sridharan S, Berdeprado J, et al. Predicting residual kidney function in hemodialysis patients using serum β-trace protein and β2-microglobulin. Kidney Int. 2016;89(5):1090–1098. doi:10.1016/j.kint.2015.12.042
26. Chin AI, Appasamy S, Carey RJ, Madan N. Feasibility of incremental 2-times weekly hemodialysis in incident patients with residual kidney function. Kidney Int Rep. 2017;2(5):933–942. doi:10.1016/j.ekir.2017.06.005
27. Keber G, Hojs R, Dvorsak B, et al. Assessment of volume status with bioimpendance prior to hemodialysis and its importance for predicting survival in hemodialysis patients. Clin Nephrol. 2021;96(1):68–73. doi:10.5414/CNP96S12
28. Fang N, Che M, Shi L, et al. B-type natriuretic peptide levels and volume status in twice-weekly hemodialysis patients. Ren Fail. 2021;43(1):1259–1265. doi:10.1080/0886022X.2021.1971091
29. Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–257. doi:10.1038/ki.2010.383
30. Lee YJ, Okuda Y, Sy J, et al. Ultrafiltration rate, residual kidney function, and survival among patients treated with reduced-frequency hemodialysis. Am J Kidney Dis. 2020;75(3):342–350. doi:10.1053/j.ajkd.2019.08.019
31. Chaitman M, Dixit D, Bridgeman MB. Potassium-binding agents for the clinical management of hyperkalemia. Pharm Ther. 2016;41(1):43–50.
32. Bolasco P, Cupisti A, Locatelli F, Caria S, Kalantar-Zadeh K. Dietary management of incremental transition to dialysis therapy: once-weekly hemodialysis combined with low-protein diet. J Ren Nutr. 2016;26(6):352–359. doi:10.1053/j.jrn.2016.01.015
33. Bieber B, Qian J, Anand S, et al. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics and quality of life in the China Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant. 2014;29(9):1770–1777. doi:10.1093/ndt/gft472
34. Park JI, Park JT, Kim YL, et al. Comparison of outcomes between the incremental and thrice-weekly initiation of hemodialysis: a propensity-matched study of a prospective cohort in Korea. Nephrol Dial Transplant. 2017;32(2):355–363. doi:10.1093/ndt/gfw332
35. Suri RS, Larive B, Sherer S, et al. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol. 2013;24(3):498–505. doi:10.1681/ASN.2012060595
36. Hanson JA, Hulbert-Shearon TE, Ojo AO, et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625–633. doi:10.1159/000013533
37. Lin YF, Huang JW, Wu MS, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology. 2009;14(1):59–64. doi:10.1111/j.1440-1797.2008.01016.x
38. Stankuvienė A, Ziginskienė E, Kuzminskis V, Bumblytė IA. Impact of hemodialysis dose and frequency on survival of patients on chronic hemodialysis in Lithuania during 1998–2005. Medicina. 2010;46(8):516–521. doi:10.3390/medicina46080074
39. Fernández-Lucas M, Teruel-Briones JL, Gomis-Couto A, Villacorta-Pérez J, Quereda-Rodríguez-Navarro C. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32(6):767–776. doi:10.3265/Nefrologia.pre2012.Jul.11517
40. Elamin S, Abu-Aisha H. Reaching target hemoglobin level and having a functioning arteriovenous fistula significantly improve one year survival in twice weekly hemodialysis. Arab J Nephrol Transplant. 2012;5(2):81–86.
41. Lin X, Yan Y, Ni Z, et al. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33(1–3):66–72. doi:10.1159/000334634
42. Panaput T, Thinkhamrop B, Domrongkitchaiporn S, et al. Dialysis dose and risk factors for death among ESRD patients treated with twice-weekly hemodialysis: a prospective cohort study. Blood Purif. 2014;38(3–4):253–262. doi:10.1159/000368885
43. Zhang M, Wang M, Li H, et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40(2):140–150. doi:10.1159/000365819
44. Hwang HS, Hong YA, Yoon HE, et al. Comparison of clinical outcome between twice-weekly and thrice-weekly hemodialysis in patients with residual kidney function. Medicine. 2016;95(7):e2767. doi:10.1097/MD.0000000000002767
45. Obi Y, Streja E, Rhee CM, et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68(2):256–265. doi:10.1053/j.ajkd.2016.01.008
46. Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int. 2016;90(2):262–271. doi:10.1016/j.kint.2016.02.037
47. Mukherjee T, Devi G, Geetha S, Anchan NJ, Sankarasubbaiyan S. A comparison of practice pattern and outcome of twice-weekly and thrice-weekly hemodialysis patients. Indian J Nephrol. 2017;27(3):185–189. doi:10.4103/0971-4065.202844
48. Dai L, Lu C, Liu J, et al. Impact of twice- or three-times-weekly maintenance hemodialysis on patient outcomes: a multicenter randomized trial. Medicine. 2020;99(20):e20202. doi:10.1097/MD.0000000000020202
49. Vilar E, Kaja Kamal RM, Fotheringham J, et al. A multicenter feasibility randomized controlled trial to assess the impact of incremental versus conventional initiation of hemodialysis on residual kidney function. Kidney Int. 2021;101(3):615–625. doi:10.1016/j.kint.2021.07.025
50. Murea M, Patel A, Highland BR, et al. Twice-weekly hemodialysis with adjuvant pharmacotherapy and transition to thrice-weekly hemodialysis: a pilot study. Am J Kidney Dis. 2021. doi:10.1053/j.ajkd.2021.12.001
51. Garofalo C, Borrelli S, De Stefano T, et al. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol. 2019;32(5):823–836. doi:10.1007/s40620-018-00577-9
52. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. doi:10.1056/NEJMoa1000552
53. Kalantar-Zadeh K, Crowley ST, Beddhu S, et al. Renal Replacement Therapy and Incremental Hemodialysis for Veterans with Advanced Chronic Kidney Disease. Semin Dial. 2017;30(3):251–261. doi:10.1111/sdi.12601
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.