Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Increases in Autoantibody Level Associated with Degenerative Changes in the Intestinal Mucosa in Liver Cirrhosis
Authors Karpova RV , Russkova KS , Lavrentieva YN
Received 28 May 2020
Accepted for publication 12 August 2020
Published 26 August 2020 Volume 2020:13 Pages 315—320
DOI https://doi.org/10.2147/CEG.S263176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Radmila V Karpova,1 Ksenia S Russkova,2 Yuliya N Lavrentieva1
1Department of Faculty Surgery No.1, University Clinical Hospital No.1, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia; 2Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Correspondence: Ksenia S Russkova
I.M. Sechenov First Moscow State Medical University, 8-2 Trubetskaya St., Moscow 119991, Russia
Tel +7 9050164054
Email [email protected]
Background: Portal hypertensive colonopathy is a consequence of portal hypertension that develops in hepatic cirrhosis. Pathological processes occurring in intestinal epithelium cells can be revealed by changes in the autoantibody titers to intestinal antigens. It is important both in diagnosis and in the evaluation of the treatment effectiveness.
Purpose: To investigate the effect of portal hypertension in patients with liver cirrhosis on degenerative processes in the walls of the small and large intestines after stimulation of liver regeneration with cryoprecipitate.
Methods: Thirty-six patients with liver cirrhosis underwent a procedure for percutaneous introduction of cryoprecipitate into the liver tissue. Before and 1 year after it, all the patients were measured for portal blood flow parameters, performed colonoscopy with biopsy, and determined autoantibodies to intestinal antigens (ItM, SCM) in the blood. Comparative analyses of changes in the large intestine mucosa before and after reducing portal hypertension by stimulating liver regeneration with cryoprecipitate were conducted.
Results: Here, we show that the degenerative process in the mucosa of the intestines increases, despite the positive dynamics of portal blood flow parameters after the liver regeneration. Autoantibodies values for the intestine antigens were higher than normal in 22 of the 36 patients after 1 year of introduction of cryoprecipitate into the liver. Morphological analysis of the intestinal wall showed the presence of edema/mucosal atrophy and neutrophilic/lymphocytic-histiocytic infiltration in 28 of the 36 patients.
Conclusion: Changes in autoantibodies to intestinal antigens are an informative method for diagnosing colonopathy and enteropathy at early stages, providing the possibility to administer proper timely treatment. Patients with hepatic cirrhosis are recommended to have their intestinal microflora tested and be administered drugs that improve their composition.
Keywords: liver cirrhosis, portal hypertension, portal hypertensive colonopathy, intestinal autoantibodies, liver regeneration
Introduction
Autoantibodies are known to be highly sensitive markers of an early pathological process that has not yet been clinically manifested. During the active process of cell damage, autoantibody number in the peripheral blood increases, directly proportional to the volume of damage, which is of great importance both in diagnosis and in the evaluation of the treatment effectiveness.1
In such disease as hepatic cirrhosis, in which the death of hepatocytes occurs with the formation of fibrosis sites and regeneration nodes, it leads to the transformation of liver tissue and the development of portal hypertension syndrome. Increased pressure in the portal vein system, including the upper and lower mesenteric veins, leads to degenerative changes in the walls of the small and large intestines.2,3 At the same time, the earliest changes in enterocytes will manifest through the increase in autoantibodies to intestinal antigens.4
It has been proven that the percutaneous introduction of cryoprecipitate to the liver tissue stimulates liver regeneration, due to local and general anti-inflammatory and immunomodulatory effects. This arranged regeneration, with the formation of sinusoids, decreases portal hypertension.5 However, there are no studies described in the literature devoted to degenerative processes in the small and large intestine walls after stimulation of liver regeneration with cryoprecipitate.
The aim of this study was to investigate the effect of portal hypertension in patients with liver cirrhosis on degenerative processes in the walls of the small and large intestines after stimulation of liver regeneration with cryoprecipitate.
Patients and Methods
Patient Information
Thirty-six patients with liver cirrhosis at the Department of Faculty Surgery №1 of the First Moscow State Medical University (Sechenov University) from May 2017 to September 2019 were included in this study. The inclusion criteria were age more than 18 years, consent of patient to be included in the research, diagnosed liver cirrhosis (class A, B, C according to Child-Pugh score) of toxic (alcohol, drugs, etc.) and viral (hepatitis B and C) etiology. The exclusion criteria were rejection of patient to be included in the research, active gastrointestinal bleeding, primary biliary cirrhosis, liver tumors, terminal state of patient, mental disorders that prevented the research performing. Each patient gave written informed consent. This study has been conducted in accordance with the conforms to the provisions in accordance with the Helsinki Declaration as revised in 2013. This study was approved by the Ethics Committee of the University Clinical Hospital No.1 of the Sechenov University.
Eight patients were assigned to Child-Pugh class A, 13 patients class B, and 15 patients class C. Sixteen of the 36 patients had mixed cirrhosis (viral and toxic) and 20 were alcoholics. There were 19 men and 17 women. The age of the patients ranged from 25 to 60 years, with an average age of 46.5 (±9) years.
Introduction of Cryoprecipitate
Cryoprecipitate was injected into the liver tissue of all patients percutaneously. It includes pro-and anti-inflammatory cytokines (IL 6, tumor necrosis factor, hepatocyte activation factor, fibrinogen fibrin stabilizing factor XIII, etc.) as well as immunomodulators (IL 2, IL1, IL 8, IL 4, spont. interferon, etc.). Cryoprecipitate has a regenerative effect on the functionally preserved liver parenchyma, forming areas of hepatic tissue with the correct beam structure of hepatocytes and the formation of sinusoids that improve portal blood flow. Cryoprecipitate was injected percutaneously under the ultrasound control (ultrasound) using a Cook 25 Gauge needle of 1.5–2 mL into each segment of the liver. This operation was performed under local anesthesia (novocaine, 0.5%, 2–5 mL).5
Ultrasound
Ultrasound of abdominal organs with the evaluation of portal blood flow parameters (using the Acuson Sequoia (USA) 3.5 MHz sensor). The diameters of the portal and spleen veins were measured before and after cryoprecipitate administration, and the linear velocity of blood flow was calculated. Furthermore, both the stagnation index (SI) and splenoportal index (SPI) were calculated. SI was calculated using the formula: SI = πR2/LBV, where R is the radius of the vessel (cm), and LBV is the maximum linear blood flow velocity in the portal vein averaged over time (cm/s). An SI value greater than 0.1 indicates a high risk of bleeding from EPV. SPI was calculated using the formula: SPI = Qsv/Qpv × 100%, where Qpv is the volume velocity of blood flow in the portal vein (mL/min), and Qsv is the volume velocity of blood flow in the splenic vein (mL/min). The volume velocity of blood flow was calculated using the formula: Q = LBV × πR (mL/min), where LBV is the linear blood flow velocity in the vessel (cm), and R is the radius of the vessel (cm). SPI indicates a redistribution of blood flow in the direction of the splenic vein and also indicates the risk of bleeding from EPV.
EGD
EGD was performed using the “Olympus GIF-XQ40 device” (Japan), evaluating the degree of esophageal varicose veins dilatation according to the A. K. Eramishantsev classification (1998).
Colonoscopy with Biopsy
Colonoscopy with biopsy was performed on the Olympus CF-40L device (Japan). The condition of the intestinal mucosa was evaluated according to the requirements of the second minimum standard terminology developed by the OMED Committee (World Organization of Digestion Endoscopy, 1998). Colonoscopy is considered the focal or diffuse nature of the intestinal mucosa lesion, its hyperemia and edema, erosion, telangiectasia, as sings of portal hypertensive colonopathy.
Diagnostic ultrasound, EGD, and colonoscopy with biopsy were performed before and following 1 year of the introduction of cryoprecipitate into the liver tissue.
ELI-GIT-TEST
In addition, all patients underwent the “ELI-GIT-TEST” determination of autoantibodies in the blood serum for dynamic evaluation of pathological processes in the gastrointestinal tract. This test was performed before and after the introduction of cryoprecipitate into the liver tissue under ultrasound control (after 3 and 12 months).
The ELI-GIT-TEST was performed using a solid-phase EIA, the same immunoreagent kits (MRC “Immunkulus”, Moscow, Russia) used to perform a comparative semi-quantitative determination of antibody markers in the blood serum. The changes in the EIA indicated a pathological process in the gastrointestinal tract, particularly in the small and large intestine. The content of autoantibodies to intestinal antigens (ItM-antigen of the small intestine wall and SCM-antigen of the large intestine wall) was evaluated. Normal IgG values varied in the range of −20 to +10. Values beyond this range indicated structural and functional disorders as well as inflammatory and degenerative processes.4
Statistical Analysis
Statistical analyses were conducted with SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). Two types of statistics were done: 1. Descriptive statistics: quantitative data have been shown as mean, and SD, SM. 2. Analytical statistics: where Chi-square test has been used to measure the association between qualitative variables, and Mann–Whitney test has been used to compare differences between two independent groups regarding variables with quantitative not normally distributed data. The p-value was considered statistically significant when it was <0.05.
Results
To identify the correlation between portal hypertension and portal colonopathy, ultrasound data were evaluated by calculating the indicators of the SI and SPI, esophagogastroduodenoscopy the degree of esophageal protuberant varicosities (EPV), colonoscopy (signs of portal colonopathy), and morphological examination of the intestinal mucosa, as well as the level of autoantibodies to intestinal antigens, were also evaluated. All examinations were performed before and following 1 year of the introduction of cryoprecipitate into the liver tissue.
Ultrasound signs of portal hypertension included portal and splenic vein dilatation, a decrease in the linear blood flow velocity, an increase in SI and SPI, and the presence of portocaval anastomoses.
According to this study, before the introduction of cryoprecipitate, 9 of the 36 patients had severe portal hypertension with a high risk of bleeding from EPV, while the remaining 27 had no risk of bleeding (Tables 1 and 2). After the introduction of cryoprecipitate, the parameters of portal blood flow improved in most patients (Table 3). Six of the nine patients had a reduced risk of bleeding from EPV, and seven had a reduced degree of EPV (Tables 1 and 2).
 |
Table 1 The Number of Patients with EPV Before and After Cryoprecipitate Introduction |
 |
Table 2 The Number of Patients the Risk of Bleeding from EPV Before and After Cryoprecipitate Introduction |
 |
Table 3 Hepatic Blood Flow Rate Changes in Patients with Liver Cirrhosis Before and After Cryoprecipitate Introduction |
Analyzing the colonoscopy data before stimulating liver regeneration, half (18 of 36) of the patients had unchanged mucosa. The remaining 18 of the 36 patients had edema or mucosal atrophy, telangiectasia, and 4 had signs of chronic colitis with isolated erosions.
A year later, 10 of the 18 patients with unchanged mucosa were found to have inflammatory changes in the form of edema or mucosal atrophy. In 11 of the 18 patients with existing inflammatory changes in the mucosa before treatment, no improvements were detected during colonoscopy, and an increase in inflammatory and degenerative changes were noted. The remaining 7 of the 18 patients showed no negative dynamics (the number of erosions and telangiectasias did not increase) due to a decrease in portal blood flow parameters.
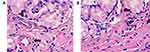
Examination of the morphological images of the intestinal mucosa before and after the introduction of cryoprecipitate showed an increase in inflammatory changes in the mucosa in 12 of the 36 patients, without increasing the number or diameter, and thickening of the walls of blood vessels (Table 4) (Figure 1).
 |
Table 4 The results of Morphological Examination of the Intestinal Wall in Patients with Liver Cirrhosis Before and After Cryoprecipitate Introduction |
Before the introduction of cryoprecipitate into the liver tissue, serum autoantibody values for the small intestine (ItM) and large intestine (SCM) antigens were higher than normal only in 6 of the 36 patients. A year later, growth was noted in 22 of the 36 patients (Figure 2).
 |
Figure 2 Autoantibody values for the small intestine and large intestine antigens in patients with liver cirrhosis. |
Thus, despite the positive dynamics of portal blood flow parameters and a reduction in the risk of bleeding from EPV after stimulation of liver regeneration with cryoprecipitate, the progression of degenerative changes in the mucous wall of the small and large intestines was noted.
Discussion
Previous studies have stated that there is a close relationship between the inflammatory process in the intestines and the liver, known as the “gut-liver axis”.6–8 Changes in physiological processes in the liver can cause the development of intestinal dysfunction with microbiota disturbance, which stimulates the secretion of pro-inflammatory cytokines, and can lead to increased enterocyte penetration.9 On the other hand, the intestinal barrier disturbance exposes the liver to toxic factors.10
Studies devoted to the investigation of chronic inflammatory processes in the intestines in patients with cirrhosis show that portal hypertension is the basis of the pathogenesis of enteropathies and colonopathies.11,12 When studying this pathology, the authors used the terms “portal hypertension duodeno-enteropathy and colonopathy”.12–15 They observed clinically significant portal colonopathy in 42% of patients with hepatic cirrhosis in the form of edema and atrophy of the intestinal mucosa (in 95% of patients), vasodilatation, and thickening of the walls (in 100%).16 In addition, it has been proven that severe portal hypertension led to microbiota disturbance, resulting in oxidative stress, which is the cause of anemia, inflammation of the abdominal aorta, spontaneous bacterial peritonitis, and hepatic encephalopathy.17,18
A study of colonopathy 2 weeks after transjugular intrahepatic portosystemic shunt (TIPS) stenting in patients with portal hypertension in hepatic cirrhosis showed that the inflammatory process decreased in the small intestine mucosa in 53% of patients, and angiodysplasia decreased by 29%.19 However, the follow-up period was not described in this article.
Our study of autoantibodies to intestinal antigens (ItM antigen of the small intestine wall and SCM antigen of the large intestine wall) showed degenerative changes in the small and large intestine mucosa in 22 of the 36 subjects, despite the improvement in the parameters of portal blood flow and the absence of increase signs of portal colonopathy. A year later, morphological analysis of the intestinal wall also showed the presence of edema or mucosal atrophy, neutrophilic or lymphocytic-histiocytic infiltration in 28 of the 36 patients. This suggests that the consequence of these morphological changes is due to the disturbance of the composition of the intestinal microbiota. It should be noted that in the complex treatment of patients with cirrhosis, we did not include drugs normalizing the intestinal microbiota.
Experimental studies on animals confirmed that normalization of the composition of the intestinal microbiota had a positive effect on degenerative changes in the intestines as well as improved hemodynamic parameters in the portal system and liver function.20 The studies investigating the microbiota in patients with cirrhosis proved its disturbance in 82.4% of the patients in a decompensated condition and recommend the administration of the drugs normalizing microflora.21,22
Conclusion
This study of changes of autoantibodies to intestinal antigens is an informative method for diagnosing colonopathy and enteropathy at early stages, providing the possibility to administer proper timely treatment. Introduction of cryoprecipitate in the liver tissue stimulates the liver regeneration, decreasing the portal blood flow parameters. Disturbance of the composition of the intestinal microbiota in patients with liver cirrhosis promotes the increasing of the degenerative changes in the mucosa of large and small intestine, enteropathy and colonopathy grow, despite the decrease of portal hypertension. Patients with hepatic cirrhosis are recommended to have their intestinal microflora tested and administered drugs that improve their composition.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sh ZA, Poletaev AB, Churilov LP. Natural autoantibodies, immunological theories and preventive medicine. Vestnik Saint Petersburg Univ. 2013;11(2):3–16. doi:10.13140/2.1.4460.5440
2. Egea-Valenzuela J, Fernández-Llamas T, García-Marín AV, Alberca-de-las-Parras F, Carballo-Álvarez F. Diagnostic and therapeutic features of small bowel involvement in portal hypertension. Rev Esp Enferm Dig. 2017;109(12):856–862. doi:10.17235/reed.2017.4596/2016
3. Misra V, Dhingra V, Misra SP, Dwivedi M. Colonoscopy in Portal hypertensive colopathy (ed. Miskovitz, P.). Intech Open. 2011;171–176. doi:10.5772/21904
4. Poletaev AB Novye podkhody k rannemu vyyavleniyu patologicheskikh izmenenii v organizme cheloveka in Metodicheskie rekomendacii dlja vrachej. Izd. 2, pererabotannoe i dopolnennoe. (M.: MRC “Immunkulus”; 2011.
5. Chernousov AF, Khorobrykh TV, Karpova RV, Zenkova KI. Effect of cryoprecipitate on liver regeneration in cirrhosis. Novosti Khirurgii. 2017;25(4):350–358. doi:10.18484/2305-0047.2017.4.350
6. Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12(1):24–33. doi:10.1007/s12072-017-9798-x
7. Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67(5):1084–1103. doi:10.1016/j.jhep.2017.05.007
8. Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi:10.1038/s41575-018-0011-z
9. Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25(39):5897–5917. doi:10.3748/wjg.v25.i39.5897
10. Daniel B, Reiner W. Gut microbiome and intestinal barrier failure - the “achilles heel” in hepatology? J Hepatol. 2012;56(6):1221–1223. doi:10.1016/j.jhep.2012.03.003
11. De Palma GD, Rega M, Masone S, et al. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62(4):529–534. doi:10.1016/s0016-5107(05)01588-9
12. Urrunaga NH, Rockey DC. Portal hypertensive gastropathy and colopathy. Clin Liver Dis. 2014;18(2):389–406. doi:10.1016/j.cld.2014.01.008
13. Rohini K, Igbinedion SO, Diaz R, Hussain N, Boktor M. Gastrointestinal bleeding secondary to portal hypertensive duodenopathy in a patient with decompensated liver cirrhosis. Case Rep Gastrointest Med. 2018. doi:10.1155/2018/9430701
14. Mekaroonkamol P, Cohen R, Chawla S. Portal hypertensive enteropathy. World J Hepatol. 2015;7(2):127–138. doi:10.4254/wjh.v7.i2.127
15. Guimarães RAP, Perazzo H, Machado L, et al. Prevalence, variability, and outcomes in portal hypertensive colopathy: a study in patients with cirrhosis and paired controls. Gastrointest Endosc. 2015;82(3):469–476. doi:10.1016/j.gie.2015.01.036
16. Kagramanova AV, Yakovenko AV, Balitskiy EV, Gioeva IZ. Portal hypertension colonopathy in patients with hepatic cirrhosis. Clin Morphological Res Sci J. 2010;3:116–119.
17. Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiota-related complications in cirrhosis. World J Gastroenterol. 2014;20(42):15624–15631. doi:10.3748/wjg.v20.i42.15624
18. Aller MA, de las Heras N, Nava MP, Regadera J, Arias J, Lahera V. Splanchnic-aortic inflammatory axis in experimental portal hypertension. World J Gastroenterol. 2013;19(44):7992–7999. doi:10.3748/wjg.v19.i44.7992
19. Matsushita Y, Narahara Y, Fujimori S, et al. Effects of transjugular intrahepatic portosystemic shunt on changes in the small bowel mucosa of cirrhotic patients with portal hypertension. J Gastroenterol. 2013;48(5):633–639. doi:10.1007/s00535-012-0660-6
20. García-Lezana T, Raurell I, Bravo M, et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology. 2018;67(4):1485–1498. doi:10.1002/hep.29646
21. Kovtun AV, Anikina EV, Jakovenko AV, Gioeva IZ, Jakovenko JP. Microflora of the gastrointestinal tract in the pathogenesis of liver cirrhosis and complications of portal hypertension. Bull Pirogov National Med Surg Cent. 2014;9(4):54–57.
22. Ulyanin AI, Poluektova YA, Pavlov CS, Ivashkin VT. Potential of nutraceutical products for irritable bowel syndrome remission maintenance. J Gastroenterol Hepatol Colorectal. 2018;28(2):101–108. doi:10.22416/1382-4376-2018-28-2-101-108
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.