Back to Journals » International Journal of Women's Health » Volume 10
Increased proportions of HIV-infected women met cervical cancer screening guideline in 2016
Authors Mohammed DY , Shukla P, Babayants Y, Sison R, Slim J
Received 1 October 2017
Accepted for publication 22 November 2017
Published 16 February 2018 Volume 2018:10 Pages 83—87
DOI https://doi.org/10.2147/IJWH.S153003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Debbie Y Mohammed,1,2 Prerak Shukla,2 Yuriy Babayants,3 Raymund Sison,2 Jihad Slim2
1Department of Nursing, College of Science and Health, William Paterson University, Wayne, NJ, 2Division of Infectious Disease, Department of Medicine, Saint Michael’s Medical Center, Newark, NJ, 3School of Osteopathic Medicine, Rowan University, Stratford, NJ, USA
Background: HIV-infected women are five times more likely to develop invasive cervical cancer. Routine screening can detect early signs of cancer and provide an opportunity for treatment. However, suboptimal screening rates are reported in this population. This retrospective study examined the rates of cervical cancer screening in HIV-positive women, conducted according to the current guidelines, from 2014 to 2016 in an inner-city clinic.
Materials and methods: We implemented focused scheduling for eligible women by a designated medical assistant. Testing was conducted using Thin Prep™ and Cervista HPV HR™. Chi-square tests and logistic regression models were used to assess predictors of cervical cancer screening in 2016.
Results: A total of 360 adult HIV-infected women were active in medical care, as of December 31, 2016. Most were African American (77%) and aged 51–60 years (38%). In 2016, 75% of women met the guidelines for cervical cancer screening, compared to 48% in 2014. There was a significant association between receipt of cervical cancer screening in the prior 3 years and screening in 2016. In an adjusted model, those with a prior screening were 6.88 times (95% CI, 3.47–13.67) more likely to be screened in 2016, compared to those who were never previously screened.
Conclusion: Focused scheduling and implementation of the updated cervical cancer screening guideline extending the period of rescreening, after 3 yearly negative results or negative Papanicolaou/human papilloma virus testing, resulted in an increased proportion of women meeting the current guideline.
Keywords: cervical cancer screening guidelines 2015, HIV-infected women, Pap smear, HPV
Introduction
Cervical cancer remains prevalent in the United States despite increased rates of screening. In 2014, 12,578 new cases and 4,115 deaths were reported among all women.1 From 2008 to 2012, at least 90% of cases were associated with human papilloma virus (HPV), and a higher incidence of cervical cancer was noted among Black and Hispanic women (9.2 and 9.7/100,000, respectively) compared to whites (7.1/100,000).2 Invasive cervical cancer (ICC) is regarded as an opportunistic infection in HIV-infected women with incidence rates 4–10 times higher compared to those who are HIV negative.3–5 The increased incidence of ICC in HIV-infected women is influenced by the persistence of HPV infection and lower CD4+ count.6
Screening guidelines
The previous guideline recommended cervical cancer screening for women at the time of HIV diagnosis or within a year of first sexual activity.7 If the result was normal, they were rescreened in 6 months and then at yearly intervals. According to the 2015 guidelines, a Papanicolaou test (Pap test) is recommended for women aged 30 years or younger.8 If the initial result is negative, future Pap testing is repeated at yearly intervals; if three consecutive test results are negative, then screening can be repeated every 3 years. This is similar for women aged 30 years or older who can have HPV co-testing, if available, in the outpatient clinical setting. If both tests are negative, the next screening can be repeated in 3 years.
Screening rates
Even though routine screening may prevent the development of ICC, there are differences among population groups. The reported rate of cervical cancer screening in 2013, among all women aged 21–65 years, was 80.7%.9 Even lower rates were reported in HIV-infected women with only 63% receiving screening in 2011.10 Older age, African American race/ethnicity, lack of insurance, and drug use were identified as barriers to cervical cancer screening among these women.11,12 The cervical cancer screening rate was 39% in 2011, at Peter Ho Clinic (PHC).13 The purpose of this study is to evaluate the cervical cancer screening rate after implementation of the updated 2015 guidelines among HIV-infected women in PHC.
Materials and methods
Study design
This is a retrospective chart review assessing cervical cancer screening rates, from January 1, 2014, to December 31, 2016, among HIV-infected women, engaged in medical care at the PHC, as of December 31, 2016. Engagement in medical care was defined as having at least one medical visit in the last 6 months of 2016. The PHC served an estimated 1,130 patients in 2016, of whom 360 (32%) were women. The criteria of eligible women were as follows: age ≥18 years, alive, and in medical care as of December 31, 2016. Data were downloaded from the electronic medical record and included Pap and HPV test results.
As a part of an ongoing quality assurance process, electronic chart review was conducted every 2 months to identify women eligible for cervical cancer screening and a designated medical assistant made appointments. In 2014–2015, cervical cancer screening was done at the initial medical visit and repeated in 6 months. If both results were negative, then screening was repeated at yearly intervals. In contrast, for 2016, we evaluated all women actively in care for successful completion of cervical cancer screening in the prior 3 years. If the Pap test was negative for 3 consecutive years, the next screening was scheduled in 3 years. Similarly, if the results of both the Pap and HPV test were negative, the next scheduled screening was 3 years later. In scheduling, we focused on those who did not meet the 2015 guideline for cervical cancer screening.
We used the Thin Prep™ and Cervista™ (Hologic Inc., Marlborough, MA, USA) HPV HR as screening tests. When compared with conventional Pap smears, Thin Prep is more sensitive at detecting high-grade squamous intraepithelial lesions (HGSIL; 100% vs 92.9%) and cancer (90.9% vs 77.8%; P<0.001).14 In two recent studies, the sensitivity of Cervista HPV HR test in detecting cervical cancers ranged from 91.9% to 94.9% and specificity ranged from 89.7% to 93.0%.15,16
Outcome
Screening of cervical cancer (yes or no) was determined by available Pap results. Results were reported as negative, atypical squamous cell of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSILs), HGSILs, and cervical intraepithelial neoplasia (CIN). HPV results are reported as either negative or positive.
Covariates
Covariates included age; race/ethnicity (black, Hispanic, and others); transmission risk (heterosexual and injection drug use); housing: private (renting, living in own home, and living with friends or family) and public (living in any publicly funded site: shelter, subsidized housing, and nursing home); income (≤ federal poverty limit [FPL] for 2016 for one person [$11,770], > FPL);17 insurance (Medicare, Medicaid, and others: private and none); history of drug use (heroin, cocaine, both, and none); history of mental illness (yes or no); care status in clinic (current: in medical care for the past 3 years at this clinic, reengaged: transferred care from another clinical site, or newly diagnosed); and history of prior screening (yes or no).
Data analysis
Statistical analysis was performed using Statistical Analysis Software version 9.3 (SAS Institute, Inc., Cary, NC, USA). Chi-square test was used to evaluate the association between completed cervical cancer screening in 2016 and demographic factors, with P<0.05 being statistically significant. Logistic regression was used to determine correlates of cervical cancer screening in 2016. This study was approved by the Institutional Review Boards of Saint Michael’s Medical Center and William Paterson University. We obtained a waiver of consent as any attempt to obtain signed consents would involve contact with each patient and create a paper trail. In addition, before data analysis, patient’s identifiers were deleted, and a unique study identifier was assigned to each observation to maintain confidentially of the patient’s identity.
Results
A total of 360 women were included in this evaluation, and the characteristics were similar between those who received cervical cancer screening and those who did not (Table 1). An increased proportion of women met the guideline in 2016 (75%), compared to 2014 (48%) and 2015 (50%; Figure 1). Similar proportions had ASCUS (10%, 11%, and 8%) and LGSIL (16%, 17%, and 10%) declined slightly for 2014, 2015, and 2016, respectively. In 2016, 3% had an HGSIL/CIN result. HPV positivity was similar in 2014 and 2016 (24% and 22%, respectively).
  | Table 1 Characteristics of women by cervical cancer screening status, 2016 |
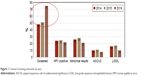  | Figure 1 Cervical screening outcomes by year. |
When compared with those who did not meet the cervical cancer screening guideline in 2016, higher proportions of women who had a prior screening met the updated guidelines in 2016 (82% vs 43%; P<0.0001). In a model adjusted for age, race, insurance, risk, housing, FPL for 2016, drug use, and mental illness, prior cervical cancer screening was associated with screening in 2016 (adjusted odds ratio, 6.88; 95% CI, 3.47–13.67; Table 2).
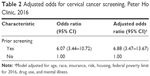  | Table 2 Adjusted odds for cervical cancer screening, Peter Ho Clinic, 2016 |
Discussion
This is the first study that evaluates implementation of the 2015 cervical cancer screening guidelines in HIV-infected women. The cervical cancer screening rate increased from 48% in 2014 to 75% in 2016, representing a 50% increase in the proportion of women meeting the current guideline. Focused scheduling for women who did not meet current guidelines increased our ability to screen those who were eligible compared to attempting screening in all women in the clinic. Despite this increase, the screening rate at PHC was still below the national targets, in 2016 of in HIV-infected women (90%) and the 83% in 2015 among all women in the United States.18,19
In this study, women receiving or not receiving cervical cancer screening were similar based on demographic characteristics, transmission risks, insurance status, history of mental health, and substance use, suggesting an elimination of disparities in these population groups. Previous studies identified differences among population groups receiving cervical cancer screening, whereas there are other studies supporting our findings of no differences by age, race, and insurance status.9–13,20,21 We attribute this to the systematic manner used to retrieve data every 2 months, allowing us to identify those women who needed to be scheduled for a Pap test. For future screening, we will consider other barriers that we did not measure in this study. For example, pain and discomfort associated with receiving Pap smears and subsequent procedures; lack of awareness of cervical cancer as a preventable disease; limited transportation access; and systemic issues as it relates to scheduling gynecological appointments.22
When comparing the results of cervical cancer screening from 2014 to 2016, similar proportions of HPV positivity and abnormal Pap results were noted. This suggests that by implementing the 2015 guidelines and focusing on those who did not meet the guidelines, we screened women likely to have abnormal results and reduced screening in those less likely to have abnormal results, based on findings from the prior 3 years including co-testing for HPV. This strategy is recommended in the current guideline and supported in recent studies reporting that a negative HPV test result predicts a low risk for the development of cancer and that Pap/HPV co-testing or 3 yearly negative Pap results can extend the time to subsequent cervical cancer screening.7,23,24
There are limitations to this study. First, despite attempts to locate the medical records of women who received cervical cancer screening at their primary care provider or gynecologist, we were not able to obtain many of these records. Therefore, those women may be differentially categorized as not having screening done according to the current guideline. However, we expect that this will have minimal effects on the results of our findings. Second, this study was conducted in an urban teaching hospital with a large, well-established clinic, so results of this study may not be generalizable to other settings.
Conclusion
Implementing the 2015 cervical cancer screening guideline and focusing scheduling on eligible women led to an increased proportion of women meeting the cervical cancer screening guideline in 2016. Conversely, women at low risk for developing cervical cell changes who met the current guideline for screening were not subjected to an unnecessary procedure and the associated discomfort. We did not detect any differences among women who received screening compared to those who did not, an indication of elimination of barriers and disparities in cervical cancer screening at this clinic. However, we did not meet the national target for screening in HIV-infected women and will need to reassess our clinic practices and patient barriers for nonscreening.
Acknowledgments
We would like to thank all the staff of PHC who contributed to improving cervical cancer screening, especially Sonia Hayes for scheduling patients. This work did not receive any external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
U.S. Cancer Statistics Working Group [webpage on the Internet]. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-Based Report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017. Available from: http://www.cdc.gov/uscs. Accessed on March 17, 2017. | ||
Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus associated cancers, United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661–666. | ||
Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR–17):1–19. | ||
Abraham AG, Strickler HD, Jing Y, et al; North American AIDS Cohort Collaboration on Research and Design of IeDEA. Invasive cervical cancer risk among HIV-infected women: a North American multi-cohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62(4):405–413. | ||
D’Souza G, Jing Y, Strickler HD, et al. Cervical cancer epidemiology among HIV-infected women in North America. Infect Agent Cancer. 2010;5(suppl 1):A9. | ||
Fontaine J, Hankins C, Money D, et al; Canadian Women’s HIV Study Group. Human papillomavirus type 16 (HPV-16) viral load and persistence of HPV-16 infection in women infected or at risk for HIV. J Clin Virol. 2008;43(3):307–312. | ||
Kaplan JE, Benson C, Holmes KK, et al; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents recommendations from CDC, the National Institutes of Health, and the HIV medicine association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR–4):1–207; quiz CE1–CE4. Available from: https://www.cdc.gov/mmwr/pdf/rr/rr5804.pdf. Accessed on November 15, 2017. | ||
Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents [webpage on the Internet]. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available from: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0. Accessed March 17, 2017. | ||
Sabatino SA, White MC, Thompson TD, Klabunde CN; Centers for Disease Control and Prevention (CDC). Cancer screening test use – United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):464–468. | ||
National Quality Center. e-HIVQUAL. Annual Data Report. 2011. Available from: https://www.ehivqual.org/scripts/eHIVQUAL%202011%20Report%20-%20National.pdf. Accessed January 29, 2018. | ||
Baranoski A, Horsburgh C, Cupples L, Aschengrau A, Stier E. Risk factors for nonadherence with Pap testing in HIV-infected women. J Womens Health. 2011;20(11):1635–1643. | ||
Daley E, Allo A, Anstey E, Chandler R, Dyer K, Helmy H. Examining barriers to cervical cancer screening and treatment in Florida through a socio-ecological lens. J Community Health. 2011;36(1):121–131. | ||
Mohammed D, Kokkola M, Garcia S, et al. Cervical cancer screening as part of routine medical care in HIV positive women. Poster Presented at: 4th International Workshop on HIV & Women. Washington, DC: 2014. | ||
Hutchinson ML, Zahniser DJ, Sherman ME, et al. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer. 1999;87(2):48–55. | ||
Boers A, Wang R, Slagter-Menkema L, van Hemel BM, Ghyssaert H, van der Zee AG. Clinical validation of the Cervista HPV HR according to the international guidelines for human papillomavirus test requirements for cervical cancer screening. J Clin Microbiol. 2014;52(12):4391–4393. | ||
Alameda F, Garrote L, Mojal S, et al. Cervista HPV HR test for cervical cancer screening: a comparative study in the catalonian population. Arch Pathol Lab Med. 2015;139(2):241–244. | ||
United States Department of Health and Human Services. Office of the Assistant Secretary for Planning and Evaluation [webpage on the Internet]. Computations for the 2016 Poverty Guidelines. Available from: https://aspe.hhs.gov/computations-2016-poverty-guidelines. Accessed March 17, 2017. | ||
Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC: US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2017. Available from: https://www.healthypeople.gov. Accessed March 17, 2017. | ||
White A, Thompson TD, White MC, et al. Cancer screening test use-United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. | ||
Rahangdale L, Sarnquist C, Yavari A, Blumenthal P, Israelski D. Frequency of cervical cancer and breast cancer screening in HIV-infected women in a county-based HIV clinic in the western United States. J Womens Health. 2010;19(4):709–712. | ||
Stein M, Cunningham W, Nakazono T, et al; HCSUS Consortium. Screening for cervical cancer in HIV-infected women receiving care in the United States. J Acquir Immune Defic Syndr. 2001;27(5):463–466. | ||
Fletcher FE, Buchberg M, Schover L, et al. Perceptions of barriers and facilitators to cervical cancer screening among low-income, HIV-infected women from an integrated HIV clinic. AIDS Care. 2014;26(10):1229–1235. | ||
Keller MJ, Burk RD, Xie X, et al. Risk of cervical pre-cancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012;308(4):362–369. | ||
Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293(12):1471–1476. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
