Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Increased Plasma Kynurenic Acid Levels are Associated with Impaired Attention/Vigilance and Social Cognition in Patients with Schizophrenia
Authors Huang X, Ding W, Wu F , Zhou S, Deng S, Ning Y
Received 23 November 2019
Accepted for publication 13 January 2020
Published 23 January 2020 Volume 2020:16 Pages 263—271
DOI https://doi.org/10.2147/NDT.S239763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Xingbing Huang, 1, 2,* Wenhua Ding, 2,* Fengchun Wu, 2 Sumiao Zhou, 2 Shuhua Deng, 2 Yuping Ning 1– 3
1First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong, People’s Republic of China; 3Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuping Ning
36 Mingxin Road, Liwan District, Guangzhou, People’s Republic of China
Tel +86 20 8189 1425
Fax +86 20 8189 1391
Email [email protected]
Objective: Preclinical studies have reported that abnormal kynurenic acid (KYNA) may play a role in cognitive deficits. Schizophrenia (SCZ) is characterized by a wide range of cognitive deficits that may evolve from abnormal KYNA. This study aimed to explore the relationship between KYNA and cognitive impairment in SCZ, which has not yet been reported.
Methods: We recruited 30 SCZ patients and 34 healthy controls, measured clinical symptoms by using the Positive and Negative Syndrome Scale and performed cognitive tests using the MATRICS Consensus Cognitive Battery (MCCB). Plasma levels of tryptophan, kynurenine, and KYNA were determined by high-performance liquid chromatography–tandem mass spectrometry.
Results: We found that plasma KYNA levels were significantly higher in patients than in healthy controls (p=0.009). The cognitive performance of patients in the total MCCB scores and the scores of all subscales were significantly lower than those in healthy controls (all P < 0.01). Correlation analysis showed that KYNA levels were negatively correlated with attention/vigilance (r=– 0.457, p=0.019) and social cognition (r=– 0.481, p=0.013) only in SCZ patients.
Conclusion: Our results indicate that elevated plasma KYNA levels may serve as a biomarker of cognitive impairment in SCZ patients.
Keywords: schizophrenia, kynurenic acid, cognitive impairment, symptom
Introduction
Cognitive deficit is the key feature of schizophrenia (SCZ), and includes a range of domain deficits such as executive function, learning, memory, attention, and social cognition.1–4 These may emerge long before illness onset and remain unchanged with improvement of clinical symptoms in SCZ patients.5 Cognitive impairments are correlated with poor clinical outcome and rehabilitation of social function.6,7 However, the pathophysiological mechanisms underlying the cognitive deficits in SCZ patients are currently unknown.
Kynurenic acid (KYNA) is an exogenous product of the kynurenine (KYN) pathway of tryptophan degradation, which antagonizes the α7 nicotinic acetylcholine receptor (α7nAChR) and ionotropic glutamate receptors.8,9 Moreover, among the ionotropic glutamate receptors, KYNA preferentially inhibits the N-methyl-D-aspartate receptor (NMDAR).10–12 Some studies have found that KYNA affects several neurotransmitters. For example, studies have reported that increased KYNA resulted in decreased glutamate levels, whereas decreased KYNA leads to increased glutamate.13,14 Also, KYNA levels are negatively correlated with dopamine, gamma-aminobutyric acid and acetyl choline.15–17 All of these neurotransmitter abnormalities are thought to play important roles in the pathogenesis of SCZ.18,19
In recent years, KYNA has also been reported to be associated with cognitive function. In animal studies, increased KYNA levels were found to induce cognitive impairments, such as impaired spatial working memory, and broad monitoring deficits.20–22 In human studies, the relationship between KYNA and cognition has been reported to be inconsistent. For example, Fazio and colleagues reported that KYNA levels were negatively correlated with speed of processing.23 However, another study found that KYNA levels were not correlated with processing speed or working memory.24 The inconsistent results may be explained by the relationship between peripheral and central KYNA levels. Sellgren and colleagues found no association between central and peripheral KYNA levels in bipolar disorder patients and healthy controls.25
Interestingly, KYNA levels were reported to be increased in cerebrospinal fluid and post-mortem brain tissue of SCZ patients.26,27 Moreover, KYNA was found to be associated with psychopathological symptoms. For example, Fazio and colleagues reported a negative association between KYNA levels and positive symptom score as measured by the Positive and Negative Syndrome Scale (PANSS).23 However, whether there is a significant correlation between KYNA and cognitive deficits in SCZ patients has not been explored yet.
In this study, we compared the cognitive function between SCZ patients and healthy controls using the MATRICS Consensus Cognitive Battery (MCCB), a widely used tool for cognition approved by the US Food and Drug Administration.28 More importantly, we examined the correlation between plasma KYNA and MCCB performance in patients with SCZ. We hypothesized that SCZ patients would have greater KYNA levels, which may be implicated in their cognitive deficits.
Materials and Methods
Participants
Thirty patients with SCZ (15 males, 15 females) were recruited from the inpatients at Guangzhou Huiai hospital, a public psychiatric hospital in Guangzhou city. Inclusion criteria included: (1) fulfilling the DSM-IV criteria for SCZ; (2) aged 17 to 50 years; (3) at least 6 years of education, and (4) a score of more than 60 on PANSS. Meanwhile, exclusion criteria were: (1) history of brain injury or mental retardation, and (2) history of electric shock treatment within the past 6 months.
Thirty-four healthy individuals (13 males, 21 females) were enrolled from the local community, who were matched for age, sex, and education to the patients. All participants were Han Chinese, who entered this study at the same period. None of them had serious physical diseases, or alcohol or other substance abuse/dependence, except for nicotine.
This study was approved by the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital). All participants provided written informed consent and this study was conducted in accordance with the Declaration of Helsinki.
Clinical Assessment
Symptom severity of patients was measured using the PANSS. PANSS was evaluated by four raters, who undertook training before this study. Their inter- observer correlation coefficient reached more than 0.80 for the PANSS total score at repeated measurement after training.
Neuropsychological Assessment
All participants were tested using the MCCB for cognition. The MCCB consists of 10 tests: Trail Making Test A (TMT-A), Symbol Coding, Hopkins Verbal Learning Test (HVLT), Wechsler Memory Scale–Spatial Span, the University of Maryland Letter Number Span Test (LNS), Mazes, Brief Visuospatial Memory Test (BVMT), Category Fluency, Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), and the Continuous Performance Test (CPT). These 10 tests cover the following seven cognitive domains: Speed of Processing, Attention/Vigilance, Working Memory, Verbal Learning, Visual Learning, Reasoning/Problem Solving, and Social Cognition.
The MCCB was translated into Chinese, and the psychometric properties of Chinese MCCB were good (Cohen’s d from 0.02 to 0.49). For test-retest reliability, the reliability was good (interclass correlation coefficient from 0.73 to 0.94).29
KYN Metabolite Measurement
All blood samples were collected in the morning after fasting overnight. Peripheral blood samples were collected in EDTA-containing anticoagulant tubes and then centrifuged at 3000 r/min for 10 min. The supernatant was transferred into polypropylene tubes and immediately frozen at –80 ºC until further use. Plasma TRP, KYN, and KYNA levels were measured using high-performance liquid chromatography–tandem mass spectrometry (LC-MS/MS), as described in our previous study.30 The time intervals between blood collection and clinical/cognitive assessments were not more than 2 days.
Statistical Analysis
Statistical analyses were performed using SPSS version 22.0. The χ2 test was used for categorical variables. For those variables without normal distribution and those with unequal variances, we used the Mann–Whitney U-test to compare group differences between patients and controls. For those variables with normal distribution, we used analysis of variance (ANOVA). Whenever the ANOVA result was significant, we tested the effects of age, sex, and education by adopting them as the covariates. Correlation analyses were performed for relationships between KYN metabolites and clinical characteristics or cognitive domain scores in patients and healthy controls, respectively. Bonferroni corrections were utilized to control for multiple tests. Lastly, stepwise multiple regression analysis was performed to investigate associations of KYN metabolites and cognitive tests by adjusting for various confounders. Two-tailed significance values were used, and statistical significance was set at p<0.05.
Results
Demographic Data
Table 1 shows no significant differences in age, sex, and years of education between the patients and healthy controls (all p>0.05). Correlation analysis showed that age, sex and years of education were not associated with KYNA levels in either SCZ patient group or healthy control group (all p>0.05).
 |
Table 1 Demographic and Clinical Data of Patients with SCZ and Controls |
Plasma KYN Metabolite Levels in Patients with SCZ and Controls
Table 2 shows that patients with SCZ had higher plasma KYNA levels than controls (F=10.211, p=0.002). Further, after controlling for age, sex and education as covariates, we still found significantly higher blood KYNA levels in patients than in controls (F=−2.617, p=0.009). In addition, we found significantly higher KYN/TRP ratios in patients than in controls (F=8.739, p=0.004). However, we did not find significant differences in TRP or KYN between the two groups (all p>0.05).
 |
Table 2 Plasma KYN Metabolite Levels in Patients with SCZ and Controls |
Cognition Domain Scores in Patients with SCZ and Controls
Table 3 shows significantly lower scores for the MCCB total and its domains in SCZ patients than in controls (all p≤0.01).
 |
Table 3 MCCB Scores in Patients with SCZ and Controls |
Correlation Between KYN Metabolite Levels and PANSS Scores or Cognition
Table 4 shows no significant association between KYN metabolite levels and PANSS scores. Pearson correlation analysis revealed a significantly negative association between KYNA levels and attention/vigilance (r=–0.477, p=0.008), and social cognition (r=–0.624, p=0.010).
 |
Table 4 Correlation Between Plasma KYN Metabolite Levels and PANSS Scores in Patients with SCZ |
Stepwise multiple regression analysis identified KYNA levels (β=–0.477, t=–2.871, p=0.008) as the influencing factor for attention/vigilance in patients with SCZ. Similarly, stepwise multiple regression analysis identified KYNA levels (β=–0.462, t=–2.760, p=0.010) as the influencing factor for social cognition in patients with SCZ (Figure 1).
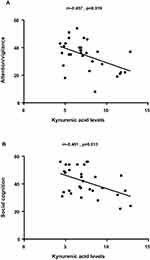 |
Figure 1 Correlation analysis revealed a significantly negative association between kynurenic acid levels and attention/vigilance (A) and social cognition (B). |
In addition, we did not find any association between KYNY levels and cognitive tests in healthy controls (all p>0.05).
Discussion
This study had three major findings. (1) Plasma KYNA levels and KYN/TRP ratios were significantly higher in SCZ patients than in healthy controls. (2) Patients showed significantly impaired cognitive performances, as assessed by the MCCB, compared with healthy controls. (3) Plasma KYNA level was negatively correlated with attention/vigilance and social cognition only in patients with SCZ. To our best knowledge, this is the first study to find an association between KYNA and attention/vigilance or social cognition in SCZ patients.
In this study, we found that plasma KYNA levels were significantly higher in SCZ patients than in healthy controls, which is consistent with three previous studies.23,31,32 In contrast, two studies reported a decrease in blood KYNA levels in untreated SCZ patients,33,34 and two other studies reported no significant differences in blood KYNA levels between medicated SCZ patients and controls.35,36 Several factors, such as differences in demographic data (sex, age), technical differences in measuring KYN metabolite levels, and differences in tested materials or antipsychotic treatments, may have an impact on blood KYNA levels, and lead to differences in study results. Taking age into consideration, some studies have reported a positive correlation between age and KYNA in SCZ patients.37,38 However, in our study, we did not find that age had a significant effect on plasma KYNA level in both patients and healthy controls (both p>0.05). Next, with regard to sex, a previous study found that the KYNA level was higher in the female than that in the male healthy controls.39 However, we did not find a sex difference in KYNA levels in either patients or healthy controls (both p>0.05). Consistent with some previous studies, the KYN/TRP ratio was also increased in the plasma of individuals with SCZ in our study, and an elevated KYN/TRP ratio is associated with immune activation in individuals with SCZ.40,41 In the brain, immune activation can activate astrocytes, where KYN can be transaminated to KYNA through KYN aminotransferases (KATs).42,43 Thus, we speculate that elevated plasma KYNA levels and KYN/TRP ratio may indicate higher KYNA levels in the brain.
Cognitive impairment is a key feature in SCZ patients. In this study, SCZ patients had significantly lower MCCB scores than healthy controls, which are consistent with previous studies.4,44 Evidence concerning the role of KYNA in cognitive dysfunctions in SCZ has mainly arisen from preclinical studies. In animal studies, animals that overexpressed KYNA showed cognitive impairments similar to those observed in SCZ patients, including impaired sensorimotor gating, working memory, and cognitive flexibility.22,45–47 Nevertheless, human studies have shown inconsistent results. One study found a negative correlation between KYNA levels and the processing speed in first-episode SCZ,23 while another study found no correlation between KYNA levels and processing speed or working memory.24 Compared with simple cognitive assessment, the MCCB assessment covers more cognitive domains. To our best knowledge, this study is the first to investigate the correlation between blood KYNA levels and MCCB scores, and we found that plasma KYNA levels were negatively correlated with attention/vigilance or social cognition in SCZ patients.
Sustained attention is the ability to maintain a consistent focus on certain continuous stimuli or activity. Attention deficit is one of the most common cognitive impairments in SCZ patients.48,49 It appears to be a major type of cognitive deficit in SCZ and is separable from other neurocognitive factors. Sustained attention is frequently measured by the Continuous Performance Test (CPT), a subtest of the MCCB. In this study, we found that patients with SCZ had significantly lower CPT scores than healthy controls. In addition, our study is the first to find a correlation between plasma KYNA levels and attention/vigilance in SCZ patients. Previous studies have reported the role of NMDAR in attention. A preclinical study found that an NMDAR antagonist–induced robust decrease in synaptic responses was correlated with attention deficits.50 In an animal study, Kazak and colleagues found that reduced brain KYNA levels improved performance in a sustained attention task.51 Furthermore, a clinical study found that anti-NMDAR encephalitis-induced white matter damage was correlated with attention deficits, and another human study reported that the NMDAR-glycine site obligatory co-agonist
Social cognitive impairment in SCZ has been reported in many studies.55,56 Consistent with previous studies, our study found significant lower scores of social cognition assessed by the MCCB in SCZ patients than in healthy controls. We also found a correlation between plasma KYNA levels and social cognition in SCZ patients. The decline of NMDAR function may be one of the causes of social cognition impairment. One study found that social cognition was impaired in patients with anti-NMDAR encephalitis.57 Another study reported that the NMDAR agonist DSR reversed impaired auditory emotion recognition, a critical component of social cognitive impairment in SCZ.58 Therefore, as an NMDAR antagonist, KYNA may be involved in impaired social cognition in SCZ by inhibiting the NMDAR.
Our research had some limitations. First, the sample size was comparatively small. We recruited 30 SCZ patients and 34 healthy controls in this study, which to some extent may have affected statistical ability. Second, the use of antipsychotic drugs may affect both cognitive performance and KYNA levels. Finally, elevated peripheral KYNA levels may not be representative of an increase in KYNA in the brain. Although KYN does cross the blood-brain barrier, and elevated peripheral KYN may lead to elevated brain KYNA, central and peripheral KYNA levels may be inconsistent. As previously mentioned, KYNA degenerates from KYN through KATs. We speculated that increased plasma KYNA may indicate increased levels of KATs activity in the periphery and brain. However, no studies have detected both central and peripheral KYNA levels or KATs activity in SCZ patients.
In conclusion, we found that elevated plasma KYNA levels were associated with impaired attention/vigilance and social cognition of SCZ, suggesting that KYNA seems to be a promising biomarker for cognitive investigations in SCZ. To confirm the role of KYNA in cognitive deficits in SCZ, more studies should be conducted by measuring both blood and CSF KYNA simultaneously, together with cognitive performance in a large sample of first-episode drug-naïve SCZ patients using a longitudinal and prospective design.
Acknowledgments
We would like to thank Yarong Ma and Chengyu Wang for providing consulting services for statistical analysis.
Funding
This study was supported by the National Natural Science Foundation of China (81571333), Science and Technology Department of Guangdong Province Major Science and Technology (2016B010108003), China Postdoctoral Science Foundation (2018M640769), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2015BAI13B01), National Science and Technologic Program of China (2015BAI13B02), Guangzhou Municipal Psychiatric Diseases Clinical Transformation Laboratory (201805010009), Science and Technology Program of Guangzhou (201807010064; 201704020168), Science and Technology Program of Guangdong (2016A020216004), Guangzhou medical and health science and technology project (20171A011268, 20181A011044). Science and Technology Plan Project of Guangdong Province (No. 2019B030316001).
Disclosure
All authors declare no potential conflicts of interest.
References
1. Bygrave AM, Jahans-Price T, Wolff AR, et al. Hippocampal-prefrontal coherence mediates working memory and selective attention at distinct frequency bands and provides a causal link between schizophrenia and its risk gene GRIA1. Transl Psychiatry. 2019;9:142. doi:10.1038/s41398-019-0471-0
2. Han W, Zhang T, Ni T, et al. Relationship of common variants in CHRNA5 with early-onset schizophrenia and executive function. Schizophr Res. 2019;206:407–412. doi:10.1016/j.schres.2018.10.011
3. Mak M, Tyburski E, Starkowska A, Karabanowicz E, Samochowiec A, Samochowiec J. The efficacy of computer-based cognitive training for executive dysfunction in schizophrenia. Psychiatry Res. 2019;279:62–70. doi:10.1016/j.psychres.2019.06.041
4. Strauss GP, Chapman HC, Keller WR, et al. Endogenous oxytocin levels are associated with impaired social cognition and neurocognition in schizophrenia. J Psychiatr Res. 2019;112:38–43. doi:10.1016/j.jpsychires.2019.02.017
5. Chang WC, Tang JY, Hui CL, et al. The relationship of early premorbid adjustment with negative symptoms and cognitive functions in first-episode schizophrenia: a prospective three-year follow-up study. Psychiatry Res. 2013;209:353–360. doi:10.1016/j.psychres.2013.02.014
6. Tominaga T, Tomotake M, Takeda T, et al. Relationship between social and cognitive functions in people with schizophrenia. Neuropsychiatr Dis Treat. 2018;14:2215–2224. doi:10.2147/NDT.S171207
7. Viviano JD, Buchanan RW, Calarco N, et al. Resting-state connectivity biomarkers of cognitive performance and social function in individuals with schizophrenia spectrum disorder and healthy control subjects. Biol Psychiatry. 2018;84:665–674. doi:10.1016/j.biopsych.2018.03.013
8. Elmslie KS, Yoshikami D. Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res. 1985;330:265–272. doi:10.1016/0006-8993(85)90685-7
9. Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi:10.1523/JNEUROSCI.21-19-07463.2001
10. Dobelis P, Staley KJ, Cooper DC. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE. 2012;7:e41108. doi:10.1371/journal.pone.0041108
11. Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi:10.1111/jnc.1989.52.issue-4
12. Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi:10.1016/0006-8993(82)91048-4
13. Carpenedo R, Pittaluga A, Cozzi A, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. doi:10.1046/j.0953-816x.2001.01592.x
14. Konradsson-Geuken A, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169:1848–1859. doi:10.1016/j.neuroscience.2010.05.052
15. Beggiato S, Tanganelli S, Fuxe K, Antonelli T, Schwarcz R, Ferraro L. Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology. 2014;82:11–18. doi:10.1016/j.neuropharm.2014.02.019
16. Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm (Vienna). 2007;114:33–41. doi:10.1007/s00702-006-0562-y
17. Zmarowski A, Wu HQ, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi:10.1111/j.1460-9568.2008.06594.x
18. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23:187–191. doi:10.1017/S1092852918001013
19. Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi:10.1523/JNEUROSCI.6158-09.2010
20. Hahn B, Reneski CH, Pocivavsek A, Schwarcz R. Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood. Psychopharmacology (Berl). 2018;235:651–661. doi:10.1007/s00213-017-4780-9
21. Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl). 2014;231:2799–2809. doi:10.1007/s00213-014-3452-2
22. Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi:10.1038/npp.2011.127
23. Fazio F, Lionetto L, Curto M, et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci Rep. 2015;5:17799. doi:10.1038/srep17799
24. Chiappelli J, Pocivavsek A, Nugent KL, et al. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry. 2014;71:761–768. doi:10.1001/jamapsychiatry.2014.243
25. Sellgren CM, Gracias J, Jungholm O, et al. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl Psychiatry. 2019;9:37. doi:10.1038/s41398-019-0378-9
26. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. doi:10.1093/schbul/sbq086
27. Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi:10.1016/j.brainres.2005.12.056
28. Buchanan RW, Keefe RS, Umbricht D, Green MF, Laughren T, Marder SR. The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what do we know 5 years later? Schizophr Bull. 2011;37:1209–1217. doi:10.1093/schbul/sbq038
29. Shi C, Kang L, Yao S, et al. The MATRICS Consensus Cognitive Battery (MCCB): co-norming and standardization in China. Schizophr Res. 2015;169:109–115. doi:10.1016/j.schres.2015.09.003
30. Wu Y, Zhong X, Mai N, et al. Kynurenine pathway changes in late-life depression. J Affect Disord. 2018;235:76–81. doi:10.1016/j.jad.2018.04.007
31. Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Peripheral kynurenine-3-monooxygenase deficiency as a potential risk factor for metabolic syndrome in schizophrenia patients. Integr Clin Med. 2017;1. doi:10.15761/ICM.1000105.
32. Ravikumar A, Deepadevi KV, Arun P, Manojkumar V, Kurup PA. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India. 2000;48:231–238.
33. Chiappelli J, Notarangelo FM, Pocivavsek A, et al. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology. 2018. doi:10.1038/s41386-018-0038-4
34. Szymona K, Zdzisinska B, Karakula-Juchnowicz H, et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-alpha, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26. doi:10.1007/s12640-017-9714-0
35. Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. doi:10.1177/0269881108089583
36. Fukushima T, Iizuka H, Yokota A, et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS ONE. 2014;9:e101652. doi:10.1371/journal.pone.0101652
37. Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi:10.1016/S0304-3940(01)02242-X
38. Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–322. doi:10.1016/j.schres.2005.07.013
39. Nilsson LK, Nordin C, Jonsson EG, Engberg G, Linderholm KR, Erhardt S. Cerebrospinal fluid kynurenic acid in male and female controls - correlation with monoamine metabolites and influences of confounding factors. J Psychiatr Res. 2007;41:144–151. doi:10.1016/j.jpsychires.2005.12.001
40. Kindler J, Lim CK, Weickert CS, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0401-9
41. Okusaga O, Fuchs D, Reeves G, et al. Kynurenine and tryptophan levels in patients with schizophrenia and elevated antigliadin immunoglobulin G antibodies. Psychosom Med. 2016;78:931–939. doi:10.1097/PSY.0000000000000352
42. Fukuyama K, Tanahashi S, Hoshikawa M, Shinagawa R, Okada M. Zonisamide regulates basal ganglia transmission via astroglial kynurenine pathway. Neuropharmacology. 2014;76(Pt A):137–145. doi:10.1016/j.neuropharm.2013.08.002
43. Nemeth H, Toldi J, Vecsei L. Role of kynurenines in the central and peripheral nervous systems. Curr Neurovasc Res. 2005;2:249–260. doi:10.2174/1567202054368326
44. Rodriguez-Jimenez R, Santos JL, Dompablo M, et al. MCCB cognitive profile in Spanish first episode schizophrenia patients. Schizophr Res. 2019;211:88–92. doi:10.1016/j.schres.2019.07.011
45. Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi:10.1093/schbul/sbl033
46. Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. doi:10.1016/j.biopsych.2004.06.006
47. Pershing ML, Bortz DM, Pocivavsek A, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41. doi:10.1016/j.neuropharm.2014.10.017
48. Ghanbari Jolfaei A, Moshki P, Asgharpour M, Moshki H. The relationship between attention/vigilance and symptom severity in schizophrenic patients. Iran J Psychiatry. 2012;7:22–25.
49. Nuechterlein KH, Green MF, Calkins ME, et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163:38–46. doi:10.1016/j.schres.2015.01.017
50. Guidi M, Kumar A, Foster TC. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. J Neurosci. 2015;35:3966–3977. doi:10.1523/JNEUROSCI.3523-14.2015
51. Kozak R, Campbell BM, Strick CA, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–10602. doi:10.1523/JNEUROSCI.1107-14.2014
52. Levin R, Dor-Abarbanel AE, Edelman S, et al. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: initial findings. J Psychiatr Res. 2015;61:188–195. doi:10.1016/j.jpsychires.2014.12.007
53. Phillips OR, Joshi SH, Narr KL, et al. Superficial white matter damage in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89:518–525. doi:10.1136/jnnp-2017-316822
54. Plitman E, Iwata Y, Caravaggio F, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43:764–777. doi:10.1093/schbul/sbw221
55. Dewangan RL, Singh P, Mahapatra T, Mahapatra S. Demographic and clinical correlates of social cognition in schizophrenia: observation from India. Indian J Psychol Med. 2018;40:143–155. doi:10.4103/IJPSYM.IJPSYM_156_17
56. Navarra-Ventura G, Fernandez-Gonzalo S, Turon M, et al. Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can J Psychiatry. 2017;706743717746661. doi:10.1177/0706743717746661
57. McKeon GL, Scott JG, Spooner DM, et al. Cognitive and social functioning deficits after anti-N-methyl-D-aspartate receptor encephalitis: an exploratory case series. J Int Neuropsychol Soc. 2016;22:828–838. doi:10.1017/S1355617716000679
58. Kantrowitz JT, Epstein ML, Beggel O, et al. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain. 2016;139:3281–3295. doi:10.1093/brain/aww262
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
