Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Increased Level of Systolic Blood Pressure in Hepatocellular Carcinoma Patients with Diabetes Mellitus
Authors Zhang WS , Li XO, Zhang H , Gao C , Fang L, Yang HY
Received 29 February 2020
Accepted for publication 12 May 2020
Published 15 June 2020 Volume 2020:13 Pages 1979—1988
DOI https://doi.org/10.2147/DMSO.S251943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wei-Shuo Zhang,1 Xiao-Ou Li,1 Hui Zhang,2 Chun Gao,1 Long Fang,1 Hua-Yuan Yang1
1Department of Gastroenterology, China-Japan Friendship Hospital, Ministry of Health, Beijing 100029, People’s Republic of China; 2Department of Gastroenterology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, People’s Republic of China
Correspondence: Chun Gao
Department of Gastroenterology, China-Japan Friendship Hospital, Ministry of Health, Beijing 100029, People’s Republic of China
Tel/Fax +86-10-84205503
Email [email protected]
Background: More than 50% of patients with type 2 diabetes mellitus (DM) also have hypertension. Moreover, hypertension has been regarded as one paraneoplastic phenomenon of hepatocellular carcinoma (HCC). Our study was designed to determine the relationship between blood pressure and DM in HCC patients.
Patients and Methods: A total of 879 HCC patients were included and 151 (17.2%) were diagnosed with DM. Multivariable logistic regression analysis was used to determine the relationship and the results were expressed as adjusted odds ratios (AORs) and their 95% confidence intervals (CIs). Considering the effect of potential confounders, sub-group analysis was performed. We would further study the association of systolic blood pressure (SBP) with fasting glucose, and the association between DM duration/treatment and SBP level.
Results: Compared with non-diabetic patients, the diabetic patients had increased levels of SBP (133.7± 18.5 mmHg vs 128.3± 15.2 mmHg, P=0.001) and fasting blood glucose (9.13± 3.04 mmol/L vs 5.18± 1.08 mmol/L, P< 0.001), an elder age (58.5± 10.2 years vs 55.3± 11.2 years, P=0.001), a higher percentage of cirrhosis diagnosis (60.9% vs 48.2%, P=0.004), lower percentages of drinking (18.5% vs 30.8%, P=0.002) and smoking (30.5% vs 43.7%, P=0.003), and decreased levels of GGT (median/interquartile-range 88/53-177 U/L vs 117/58-248 U/L, P=0.037), platelet count (121.4± 76.6 × 109/L vs 151.2± 82.8 × 109/L, P< 0.001) and hemoglobin (124.3± 25.5 g/L vs 133.6± 24.2 g/L, P< 0.001). Multivariable analysis showed that, statistically significant differences were found for SBP ≥ 140 mmHg (AOR=2.101; 95% CI, 1.424– 3.100; P< 0.001), smoking (AOR=0.637; 95% CI, 0.415– 0.979; P=0.040), hemoglobin (AOR=0.990; 95% CI, 0.983– 0.998; P=0.010) and platelet count (AOR=0.996; 95% CI, 0.994– 0.999; P=0.009). For the relationship between SBP and DM, the positive result was supported by most (10/14) of the subgroup analyses.
Conclusion: SBP level was increased in HCC patients with diabetes mellitus.
Keywords: hepatocellular carcinoma, diabetes mellitus, systolic blood pressure
Introduction
Hepatocellular carcinoma (HCC), the most common pathological type of primary liver cancer, has become one of the most lethal varieties of solid-organ cancers.1–3 Some risk factors have been identified for HCC, and the top four risk factors included liver cirrhosis, hepatitis B virus (HBV), HCV and heavy alcoholic consumption.4,5 However, for about 15–50% of HCC patients, no specific risk factor can be found.6–8 Recently, diabetes mellitus (DM) has been confirmed as one potential risk factor for HCC.9,10 As described in one systematic review and meta-analysis, which included 25 cohort studies, the positive relationship between DM and increased risk of HCC was found in 18 studies and the summary relative risk (SRR) was 2.01.10
Diabetes mellitus (altered glucose metabolism) and high blood pressure, together with other components of metabolic syndrome, including dyslipidemia, abdominal obesity and low-grade chronic systemic inflammation, have been becoming important public health problems that seriously threaten the health and life of human beings in modern societies.11 More than 50% of patients with type 2 DM also have high blood pressure (hypertension), which could double the risk of cardiovascular disease.12 Moreover, the association of DM with systolic blood pressure (SBP) has been paid more attention than those with diastolic blood pressure (DBP) or mean artery pressure.13,14
One recent study was designed to determine the association between increasing blood pressure and the risk of developing diabetes.13 This study included 2225 participants from the Korean Genome Epidemiology Study and all of them were reported to have no histories of DM and cardiovascular disease at baseline. The authors observed that 5.0% (43/859) of the men and 3.4% (47/1366) of the women developed new-onset diabetes during the mean follow-up of 2.6 years.13 They demonstrated the positive relationship between increasing SBP level and incident DM after adjusting for those potential confounders. The adjusted odds ratio was 5.53 per 5 mmHg per year.13 Another case-controlled study was conducted to investigate the relationships between blood pressure, type 2 DM and vitamin D level in Trinidadian subjects.14 The authors also found the positive relationship between SBP and vitamin D levels in diabetics.14
Hypertension has also been associated with HCC by some studies.15–17 Some HCC patients had been found to have hypertension and the underlying pathophysiological mechanism was deduced to be associated with the abnormality of renin-angiotensin system.16 One report demonstrated that two HCC patients had increased eightfold to 10-fold concentrations of plasma angiotensinogen (renin substrate).17 Arterial hypertension has been regarded as one paraneoplastic phenomenon of HCC and may provide new insight into the potential predictors of survival for HCC patients.15–17 However, no information was available for the relationship between blood pressure and DM in HCC patients. Our study was designed to determine this relationship.
Patients and Methods
Study Patients
Our research project was approved by the Human Research Ethics Committee of China-Japan Friendship hospital (Beijing, China) and followed strictly the principles of the Declaration of Helsinki during the whole course of implementation. According to the diagnostic, inclusion and exclusion criteria, 879 patients who were diagnosed with HCC for the first time, treated and hospitalized at our hospital, China-Japan Friendship Hospital, during the period between January 2005 and December 2017, were included in our study. Inclusion criteria included: 1) these patients who were more than 18 years and less than 75 years old; 2) they were Chinese patients; and 3) they had been diagnosed with HCC for the first time and the whole data of the first diagnosis and/or hospitalization could be available for the purpose of our present research. Written informed consent was obtained from all patients.
Exclusion criteria included: 1) HCC had been diagnosed for more than half a month when those patients were included; 2) those patients who had been treated with any HCC-associated therapies; 3) those patients who had other malignancies except HCC, including lymphoma and leukemia; 4) those patients who had severe diseases of major organs, including uremia, chronic heart failure and chronic respiratory failure; and 5) those patients who had been diagnosed with hemochromatosis, Wilson’s disease, autoimmune hepatitis, Budd-Chiari syndrome, primary sclerosing cholangitis, primary biliary cirrhosis, schistosomiasis, allergic disorder or rheumatic diseases.
Diagnostic Criteria of HCC and DM
These patients were diagnosed with HCC mainly based on the typical findings of histological and/or radiological examinations, combined with the increased level of serum AFP. Patients who followed one of these criteria would be confirmed as the diagnosis of HCC: 1) those who had the typical findings of histological examination derived from needle biopsy or surgery; 2) those who had typical features of at least two radiological examinations, including ultrasound, computed tomography, magnetic resonance imaging and hepatic angiography; and 3) those who had typical features of one image examination, and an increased level of serum AFP (>400 ng/mL).18–21
Patients who followed one of these criteria would be confirmed as the diagnosis of DM: 1) those who had an increased level of fasting plasma glucose (≥126 mg/dL) on at least two occasions; 2) those who had an increased level of plasma glucose (≥200 mg/dL) at 2-hour oral glucose tolerance test (OGTT); and 3) those who had the need for hypoglycemic drugs, including insulin, to control their levels of blood glucose.22
Clinical, Laboratory and Metabolic Parameters
Some associated clinical, laboratory and metabolic parameters, which were obtained when the HCC patients were diagnosed for the first time, were included in our study to determine the relationship between blood pressure and DM in HCC patients. Those patients would be excluded from the final analysis if the statistical results may be affected by any patchier data. The blood pressures of these HCC patients were measured three times a day for three consecutive days from the day of hospitalization, and the mean values were calculated for systolic blood pressure (SBP) and diastolic blood pressure (DBP). The history of hypertension was determined based on the self-reported results.
Body weight (in kilograms) and height (in meters) were used to calculate the value of body mass index (BMI). BMI ≥25 kg/m2 was diagnosed as obesity and BMI ≥23 kg/m2 was diagnosed as overweight, according to our Chinese criteria.18,19 Five indicators, including serum albumin, international normalized ratio, total bilirubin, hepatic encephalopathy and ascites, were used to calculate the Child–Turcotte–Pugh (CTP) score. The laboratory parameters were measured when venous blood samples were obtained in the morning and the patients had been an overnight fasting of 8 hours. For the tumor-node-metastasis stage and clinical classification, all the findings of physical, pathological and radiological examinations would be re-assessed carefully by two authors independently. The 7th TNM staging system recommended by International Union against Cancer was used to determine the TNM stage. Clinical classification was determined as before.18
Statistical Analysis
The SPSS for Windows, version 21.0 (SPSS, Chicago, IL, USA) was used for our analysis. To determine the differences of diabetic compared with non-diabetic HCC patients, Student’s t-test, Chi-square test and Mann–Whitney U-test were used for univariable analysis. For multivariable analysis, unconditional multivariable logistic regression analysis was used to show the relationship between blood pressure and DM in HCC patients. According to the results of univariate analysis, eight variables were entered, including mean age, SBP, alcohol intake, smoking, liver cirrhosis, GGT, hemoglobin and platelet count. Stepwise analysis (Backward: Wald; Entry: 0.05, Removal: 0.10) was used. Because fasting blood glucose is the landmark index of DM, it was excluded. CTP classification was not included because it was used to describe the severity of cirrhosis. The results were expressed as adjusted odds ratios (AORs) and their 95% confidence intervals (CIs). Considering the effect of potential confounders, sub-group analysis was performed. Pearson correlation test was used to determine the association of SBP with fasting glucose. Chi-square test was used to determine the association between DM duration/treatment and SBP level. For all tests, P<0.05 was considered statistically significant and all P values quoted are two-sided.
Results
Baseline Characteristics of 879 HCC Patients with and Without DM
Eight hundred and seventy-nine HCC patients were included in our study according to the diagnostic, inclusion and exclusion criteria. The baseline characteristics are shown in Table 1, including demographic, clinical and laboratory features. Table 2 demonstrates their tumor-node-metastasis stage and clinical classification. Among all the 879 HCC patients, 151 (17.2%) were diagnosed with DM and the left 728 patients were non-diabetic. Their mean age was 55.8±11.1 years and 728 patients (82.8%) were male. One hundred and eight-seven (21.3%) patients had a history of hypertension, and liver cirrhosis was diagnosed for 443 (50.4%) patients. For the tumor-node-metastasis stage, 862 (98.1%) patients were diagnosed with stage T1-3, 788 (89.6%) were stage N0, and 587 (66.8%) were stage M0. Among the 151 diabetic patients, 117 (77.5%) had been diagnosed with DM for more than 1 year.
 |
Table 1 Baseline Characteristics and Comparative Analysis of 879 Hepatocellular Carcinoma (HCC) Patients with and Without Diabetes Mellitus (DM) |
 |
Table 2 Tumor-Node-Metastasis Stage and Clinical Classification of Total Study Population of 879 HCC Patients |
Blood Pressure and Other Metabolic Parameters in 151 HCC Patients with DM
The history of hypertension was determined based on the self-reported results of these patients. Their blood pressures were measured three times a day for three consecutive days from the day of hospitalization, and the mean values were calculated for SBP and DBP. The blood pressure and other metabolic parameters are shown in Table 1. For the 151 diabetic HCC patients, the mean SBP and DBP were 133.7±18.5 mmHg and 79.4±12.3 mmHg. Ninety-seven (64.2%) patients were overweight or obesity, 32 (21.2%) had a history of hypertension, 28 (18.5%) were drinking, and 46 (30.5%) patients were smoking. The mean values of fasting blood glucose, triglyceride and total cholesterol were 9.13±3.04 mmol/L, 1.03±0.49 mmol/L and 4.12±1.18 mmol/L.
Univariable Analysis: Comparison of HCC Patients with and Without DM
Chi-square test, Student’s t-test and Mann–Whitney U-test were used to compare the differences of diabetic with non-diabetic patients. Compared with non-diabetic HCC patients (Table 1), the diabetic patients had increased levels of SBP (133.7±18.5 mmHg vs 128.3±15.2 mmHg, P=0.001) and fasting blood glucose (9.13±3.04 mmol/L vs 5.18±1.08 mmol/L, P<0.001), an elder age (58.5±10.2 years vs 55.3±11.2 years, P=0.001), a higher percentage of cirrhosis diagnosis (60.9% vs 48.2%, P=0.004), lower percentages of drinking (18.5% vs 30.8%, P=0.002) and smoking (30.5% vs 43.7%, P=0.003), and decreased levels of GGT (median/interquartile-range 88/53-177 U/L vs 117/58-248 U/L, P=0.037), platelet count (121.4±76.6 ×109/L vs 151.2±82.8 ×109/L, P<0.001) and hemoglobin (124.3±25.5 g/L vs 133.6±24.2 g/L, P<0.001). For the CTP classification, more diabetic patients were diagnosed with Child C (18.5% vs 9.5%, P=0.001) and fewer patients were diagnosed with Child A (57.0% vs 65.7%, P=0.042).
Multivariable Analysis: Increased Level of SBP in HCC Patients with DM
Unconditional multivariable logistic regression analysis was performed to determine the relationship between SBP and DM in HCC patients. According to the results of univariate analysis, eight variables were entered (Table 3), including mean age, SBP, alcohol intake, smoking, liver cirrhosis, GGT, hemoglobin and platelet count. Because fasting blood glucose is the landmark index of DM, it was excluded from the final analysis. Child–Turcotte–Pugh classification was not included because it was used to describe the severity of liver cirrhosis. Multivariable analysis (Table 3) showed that, three variables were found to have significant differences, including SBP level (AOR =1.024; 95% CI, 1.012–1.035; P<0.001), hemoglobin (AOR=0.989; 95% CI, 0.981–0.997; P=0.005) and platelet count (AOR=0.997; 95% CI, 0.994–0.999; P=0.013).
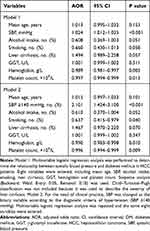 |
Table 3 Multivariable Analysis: Increased Level of Systolic Blood Pressure in HCC Patients with DM |
For the use of clinical practice, SBP was changed as the binary variable according to the diagnostic criteria of hypertension (SBP ≥140 mmHg). Multivariable analysis was repeated and the same eight variables were entered (Table 3). The results showed that, after controlling other factors, 4 variables were found to have significant differences, including SBP (AOR=2.101; 95% CI, 1.424–3.100; P<0.001), smoking (AOR=0.637; 95% CI, 0.415–0.979; P=0.040), hemoglobin (AOR=0.990; 95% CI, 0.983–0.998; P=0.010) and platelet count (AOR=0.996; 95% CI, 0.994–0.999; P=0.009).
Sub-Group Analysis: Consideration of the Effect of Potential Confounders
Considering the effect of potential confounders, sub-group analysis was performed in seven pairs of conditions (Table 4), including gender (male or female), age (elder or younger), history of hypertension (with or without), overweight or obesity (yes or no), alcohol intake (yes or no), smoking (yes or no) and liver cirrhosis (with or without). The results showed that most (10/14) of the subgroup analyses were consistent with the result of overall analysis and SBP level remained as the statistically significant difference, including the study population was restricted into the male HCC patients (AOR=2.031; 95% CI, 1.327–3.108; P=0.001), elder patients (AOR=1.783; 95% CI, 1.122–2.834; P=0.014), younger patients (AOR=2.958; 95% CI, 1.347–6.498; P= 0.007), patients without a history of hypertension (AOR=2.395; 95% CI, 1.467–3.912; P<0.001), patients who were overweight or obesity (AOR=2.819; 95% CI, 1.709–4.649; P<0.001), patients who were drinking (AOR=5.520; 95% CI, 1.881–16.199; P=0.002), patients who were not drinking (AOR=1.753; 95% CI, 1.126–2.730; P=0.013), patients who were smoking (AOR=2.435; 95% CI, 1.248–4.751; P=0.009), patients who were not smoking (AOR=1.889; 95% CI, 1.156–3.088; P=0.011), and patients with liver cirrhosis (AOR=3.360; 95% CI, 1.962–5.755; P<0.001) (Table 4).
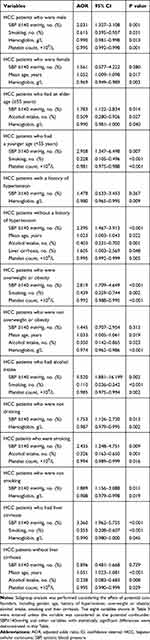 |
Table 4 Sub-Group Analysis: Consideration of the Effect of Potential Confounders |
Association of SBP Level with Fasting Blood Glucose in HCC Patients
Pearson correlation test was used to determine the association of SBP with fasting glucose. Compared with non-diabetic patients, diabetic HCC patients had increased levels of SBP (133.7±18.5 mmHg vs 128.3±15.2 mmHg, P=0.001) and fasting blood glucose (9.13±3.04 mmol/L vs 5.18±1.08 mmol/L, P<0.001). No significant correlation was found by Pearson correlation test in our patients (r=−0.059, P=0.468).
Association Between Diabetes Duration/Treatment and SBP Level
We would further study the association between DM duration/treatment and SBP level. SBP level was changed as the binary variable according to the diagnostic criteria of hypertension (SBP ≥140 mmHg) and the median of our study population (130mmHg). Among the 151 diabetic HCC patients, 90 (59.6%) patients were diagnosed with DM for more than 5 years, 29 (19.2%) received insulin use, 55 (36.4%) received oral anti-diabetic regimens, and 79 (52.3%) reported relying on diet alone to control serum glucose level (Table 5). Sixty (39.7%) patients had the level of SBP ≥140 mmHg and 98 patients had the SBP ≥130 mmHg. When 140mmHg was determined as the cutoff value, statistically significant differences were found for the association between age at diabetes diagnosis and SBP level (P=0.001), and between insulin treatment and SBP level (P=0.021). When 130mmHg was determined as the cutoff value, significant differences were found for the association between duration of diabetes and SBP level (P=0.022), and between age at diabetes diagnosis and SBP level (P=0.002) (Table 5).
 |
Table 5 Association Between Diabetes Duration/Treatment and SBP Level |
Discussion
The relationship between DM, blood pressure and HCC has been suggested by some previous studies;12–17 however, no information was available for the association of the level of blood pressure with DM in HCC patients. Our present study was designed to determine this relationship in Chinese patients diagnosed with HCC. We showed that, compared with non-diabetic HCC patients, the diabetic patients had an increased level of SBP (133.7±18.5 mmHg vs 128.3±15.2 mmHg, P=0.001), not DBP. After the confounding factors were controlled, SBP (≥140 mmHg) remained as statistically significant difference (AOR=2.101; 95% CI, 1.424–3.100; P<0.001). Moreover, most (10/14) of the subgroup analyses were consistent with the result of the overall analysis. We concluded that SBP level was increased in HCC patients with diabetes.
The major concern of our study may be the nature of design, which was designed to compare diabetic with non-diabetic HCC patients, and could not provide definite evidence for the causal association. However, our study may be regarded as one population-based case–control study because 77.5% of the 151 diabetic patients had been diagnosed with DM for more than 1 year when the HCC was diagnosed and about half of them had the need for hypoglycemic drugs, including insulin, to control their levels of blood glucose. Moreover, all patients were diagnosed for the first time and included according to the strict diagnostic, inclusion and exclusion criteria.
Considering the many very complicated factors involved in our study, we tried our best to remove the confounding factors in the research. Firstly, whenever possible, we included all the available associated clinical, laboratory and metabolic parameters, including renal function, serum lipid metabolism, anti-hypertensive treatment and diabetes duration/treatment. Secondly, based on the results of univariate analysis, multivariable logistic regression analysis was used to observe the independent risk factors, especially the SBP level. Thirdly, sub-group analysis was performed to control the effect of potential confounders. In our research, the positive result for the relationship between SBP and DM was supported by most of the subgroup analyses.
Increased arterial hypertension (hypertension) has been associated with diabetes and HCC.12,15 Hypertension is very common in diabetic patients and the incidence rate is twice that of patients without diabetes.23 Approximately 70% of diabetic patients aged 40 years or older also have hypertension and people over the age of 55 years have a 90% chance of development of hypertension.23,24 Strict control of hypertension is important for diabetic patients.25 The UK Prospective Diabetes Study (UKPDS) epidemiological study showed that each decrease of 10mmHg in mean SBP could reduce the risk of 12% for diabetes-related complication and 15% for deaths related to diabetes.25 Our study showed a similar result and found that, compared with non-diabetic HCC patients, diabetic patients had an increased SBP level.
Moreover, hypertension has been regarded as one of the paraneoplastic phenomena of HCC and may provide new insight into the potential predictors of survival for HCC patients.15–17 One study reported an old man with HCC and hypertension16 and found that his plasma concentrations of angiotensin I (>2500 pg/mL) and II (86 pg/mL) were increased. Based on the examination of the abnormality of renin-angiotensin system, the authors deduced and concluded that the development of hypertension was due to the overproduction of angiotensin I from HCC.16 Another study described three South African blacks with HCC and hypertension and found similar results that their plasma angiotensinogen concentrations were increased eightfold to 10-fold.17 The pathophysiological mechanism for HCC patients with hypertension was deduced to be due to the abnormality of renin-angiotensin system produced by HCC.
Our sub-group analysis was performed in seven pairs of conditions considering the effect of potential confounders, including gender, age, overweight or obesity, history of hypertension, alcohol intake, smoking and liver cirrhosis. Based on previous studies, they may affect the level of blood pressure.26,27 For the gender of patients, one case–control study included 10,270 participants and was designed to determine the impact of hypertension with or without diabetes on left ventricular remodeling.27 The authors found, compared with hypertension without DM, those hypertension patients with diabetes had an increased risk for left ventricular hypertrophy in the female population.27 Another cross-sectional survey described that, for patients with BMI <25 kg/m2, non-smokers had a significantly higher risk of hypertension than smokers, whereas smokers had an increased risk of hypertension than non-smokers for patients with BMI ≥25 kg/m2.26 Our results demonstrated that most (10/14) of the subgroup analyses were consistent with the result of overall analysis.
For the role of diabetes in the increasing SBP, our study could not provide definite evidence for the causal association. However, the effect of HCC could be omitted because both group patients were HCC cases. Multivariable analysis and sub-group analysis were performed to control the effect of potential confounders. Moreover, 59.6% (90/151) diabetic patients had been diagnosed with DM for more than 5 years (Table 5). Therefore, our study provided the credible result for the relationship between SBP and DM in HCC. One study was aimed to evaluate the effect of DM on the occurrence of macrovascular (total cardiovascular events [CVEs], major adverse CVEs, cardiovascular and all-cause mortality) and microvascular complications (microalbuminuria, retinopathy, renal function deterioration, peripheral neuropathy).28 The positive results may provide some insights into the potential mechanisms.
Moreover, we further studied the association between DM duration/treatment and SBP level. We found that, to some extent, SBP was associated with the age at diabetes diagnosis, duration of diabetes and insulin treatment (Table 5). Some limitations of our study should be acknowledged. The first was that for most of the patients, the diagnosis of DM was mostly dependent on their self-reported history or fasting serum glucose. The diabetic number and the role of DM may be underestimated. However, we followed the diagnostic criteria used widely in clinical practice and recommended by the authorized institutes. The second was due to the nature of the design of our study that some data could not be obtained and some possible factors could not be adjusted, for example, nonalcoholic fatty liver disease (NAFLD).
In conclusion, our study showed that compared with non-diabetic patients, diabetic HCC patients had an increased level of SBP, not diastolic blood pressure. For a better understanding of this relationship between blood pressure and diabetes mellitus, more studies are required, especially those well-designed prospective studies.
Ethics Approval and Informed Consent
The whole process of our research is in full compliance with the principles of the Declaration of Helsinki and our research project was approved by the Human Research Ethics Committee of China-Japan Friendship Hospital, Ministry of Health. Written informed consent was obtained from all patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond). 2019;39(1):22. doi:10.1186/s40880-019-0368-6
4. Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51(1):78–89. doi:10.1111/apt.15573
5. Elshaarawy O, Gomaa A, Omar H, Rewisha E, Waked I. Intermediate stage hepatocellular carcinoma: a summary review. J Hepatocell Carcinoma. 2019;6:105–117. doi:10.2147/JHC.S168682
6. Gao C. Molecular pathological epidemiology in diabetes mellitus and risk of hepatocellular carcinoma. World J Hepatol. 2016;8(27):1119–1127. doi:10.4254/wjh.v8.i27.1119
7. Gao C. Molecular pathological epidemiology: an interdisciplinary field for study of hepatocellular carcinoma. Austin J Gastroenterol. 2015;2(3):1040.
8. Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8(5):465–473.
9. Dyal HK, Aguilar M, Bartos G, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61(2):636–645. doi:10.1007/s10620-015-3983-3
10. Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi:10.1002/ijc.26165
11. O’Connor S, Chouinard-Castonguay S, Gagnon C, Rudkowska I. Prebiotics in the management of components of the metabolic syndrome. Maturitas. 2017;104:11–18. doi:10.1016/j.maturitas.2017.07.005
12. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273–1284. doi:10.2337/dci17-0026
13. Lee SW, Kim HC, Lee JM, Yun YM, Lee JY, Suh I. Association between changes in systolic blood pressure and incident diabetes in a community-based cohort study in Korea. Hypertens Res. 2017;40(7):710–716. doi:10.1038/hr.2017.21
14. Nayak SB, Ramnanansingh TG. Evaluation of vitamin D relationship with type 2 diabetes and systolic blood pressure. BMJ Open Diabetes Res Care. 2016;4(1):e000285. doi:10.1136/bmjdrc-2016-000285
15. Hu YF, Chen TC, Chau GY, et al. Baseline hypertension: new insight into the potential predictors of survival in patients with hepatocellular carcinoma. Int J Cardiol. 2013;168(3):2979–2981. doi:10.1016/j.ijcard.2013.04.106
16. Arai H, Saitoh S, Matsumoto T, et al. Hypertension as a paraneoplastic syndrome in hepatocellular carcinoma. J Gastroenterol. 1999;34(4):530–534. doi:10.1007/s005350050309
17. Kew MC, Leckie BJ, Greeff MC. Arterial hypertension as a paraneoplastic phenomenon in hepatocellular carcinoma. Arch Intern Med. 1989;149(9):2111–2113. doi:10.1001/archinte.1989.00390090135028
18. Gao C, Fang L, Zhao HC, Li JT, Yao SK. Potential role of diabetes mellitus in the progression of cirrhosis to hepatocellular carcinoma: a cross-sectional case-control study from Chinese patients with HBV infection. Hepatobiliary Pancreat Dis Int. 2013;12(4):385–393. doi:10.1016/S1499-3872(13)60060-0
19. Gao C, Zhao HC, Li JT, Yao SK. Diabetes mellitus and hepatocellular carcinoma: comparison of Chinese patients with and without HBV-related cirrhosis. World J Gastroenterol. 2010;16(35):4467–4475. doi:10.3748/wjg.v16.i35.4467
20. Zhang H, Gao C, Fang L, Yao SK. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J Gastroenterol. 2013;19(15):2395–2403. doi:10.3748/wjg.v19.i15.2395
21. Huo TI, Wu JC, Lui WY, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99(8):1479–1487. doi:10.1111/j.1572-0241.2004.30024.x
22. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi:10.2337/dc12-s064.
23. Shaikh A. A practical approach to hypertension management in diabetes. Diabetes Ther. 2017;8(5):981–989. doi:10.1007/s13300-017-0310-3
24. Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol. 2005;42(Suppl 1):S17–S25. doi:10.1007/s00592-005-0177-z
25. Arauz-Pacheco C, Parrott MA, Raskin P; American Diabetes A. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–S82.
26. Nishiyama M, Kimijima M, Muto T, Kimura K. Presence of an interaction between smoking and being overweight increases risks of hypertension, diabetes, and cardiovascular disease in outpatients with mood disorders. Environ Health Prev Med. 2012;17(4):285–291. doi:10.1007/s12199-011-0250-x
27. Li T, Chen S, Guo X, Yang J, Sun Y. Impact of hypertension with or without diabetes on left ventricular remodeling in rural Chinese population: a cross-sectional study. BMC Cardiovasc Disord. 2017;17(1):206. doi:10.1186/s12872-017-0642-y
28. Cardoso CRL, Leite NC, Salles GF. Prognostic importance of visit-to-visit blood pressure variability for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio de Janeiro type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2020;19(1):50. doi:10.1186/s12933-020-01030-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
