Back to Journals » Cancer Management and Research » Volume 12
Increased Expression of TAP Is Predictive of Poor Prognosis in Patients with Non-Small Cell Lung Cancer
Authors Cheng Y, Chen Y , Zang G, Chen B, Yao J, Zhang W, Wang H, Yu L, He P, Zhang Y, Wu H
Received 21 November 2019
Accepted for publication 20 February 2020
Published 13 March 2020 Volume 2020:12 Pages 1941—1946
DOI https://doi.org/10.2147/CMAR.S239593
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Yuanjun Cheng, 1 Yongbing Chen, 2 Guohui Zang, 1 Bin Chen, 1 Jie Yao, 1 Wenguang Zhang, 1 Haibing Wang, 1 Liu Yu, 1 Pinghai He, 1 Youming Zhang, 1 Hanqing Wu 1
1Department of Cardiothoracic Surgery, People’s Hospital of Chizhou, Chizhou, People’s Republic of China; 2Department of Thoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
Correspondence: Yuanjun Cheng
3 Baiya Road, Guichi District, Chizhou 247000, Anhui Province, People’s Republic of China
Email [email protected]
Background: The most common cancer among humans is lung cancer. Non-small cell lung cancer (NSCLC) comprises the majority of these cases. In the development and progression of cancers across the spectrum, tumor abnormal protein (TAP) plays crucial roles. Additionally, in the advancement of the bladder and colorectal cancers, the involvement of glycoproteins like TAP is present. However, it is worth noting that current literature has yet to clarify the clinical significance of the TAP in NSCLC.
Methods: In the present study, to evaluate the relative level of TAP, we utilized a TAP detection agent in 154 cases of NSCLC and normal patients who underwent surgical resection anytime from March 2013 to January 2019 at the People’s Hospital of Chizhou.
Results: Our results demonstrated that in NSCLC patients, the expression level of TAP was significantly higher than in normal patients. Moreover, after surgery, TAP expression was significantly downregulated in NSCLC patients. TAP expression is associated with an array of factors, which include the patient’s sex, history of smoking use, tumor size, pTNM, distant cancer, metastasis of lymph nodes, invasive and aggressive indicator pleural invasion, and differentiation degree of NSCLC. Additionally, TAP has no association with the patient’s age, history of drinking, location of the tumor, hypertension, and diabetes. In NSCLC patients, a poor overall survival rate within 5 years is significantly correlated with the increased TAP expression. For NSCLC patients, an independent prognostic factor is the TAP, which is confirmed using the multivariate survival analysis.
Conclusion: In the malignant progression of NSCLC, our results demonstrate how the promoting role of the upregulated TAP expression takes place. Hence, a therapeutic aim for NSCLC and a potential biomarker for NSCLC progress is a TAP.
Keywords: tumor abnormal protein, TAP, lung, cancer, prognosis, predictive
Introduction
Currently, the most common new type of cancer is lung cancer, which is also the leading cause of cancer-related deaths. Majority of these cases are classified as non-small cell lung cancer (NSCLC).1,2 The NSCLC mortality and recurrence rates have not significantly declined amid the developments in oncology and multidisciplinary treatments.
Unfortunately, one of the main reasons is at the time of the diagnosis, patients are already at the advanced stage. As a result, it is vital to generate effective and efficient approaches to predict tumor genesis and development. Recent literature has brought light to numerous proteins that have a close association to TAP’s progression and occurrence but (USSR) named Kostyantin, A. and Galakhin, discovered the tumor abnormal protein (TAP).3,4 During the metabolism of cancer cells, they emit complex abnormal glycoproteins and calcium-histone proteins that comprise the TAP.5 Essentially, TAP is the result of changes in the glycosylation of cancer cells. The presence of TAP indirectly indicates the number and extent of cell cancerization. After reaching a certain volume, these substances are discharged into the blood while more of them continue to exist in the peripheral blood. In early cancer detection, an important indicator is the increased TAP expression in the blood. However, there exists a crucial need for the biological function of TAP to be further investigated. The mutations in oncogenes and tumor-suppressor genes in cells produce TAP. Additionally, tumor growth contributes to TAP upregulation. In numerous types of carcinomas, such as breast, colon, ovarian, endometrial, stomach and lung cancers, a biomarker like TAP is upregulated.6 In the development, progression, and metastasis of the tumor, the involvement of TAP is evident. As a result, it is considered to be an important indicator in tumor prognosis. Additionally, it is important to note that in the context of tumorigenesis and progression of NSCLC, there is still a need to further investigate the role of TAP. In this study, we aim to evaluate TAP expression levels between NSCLC and normal patients. Additionally, our study covers the analysis of the correlation between the expression of TAP and clinicopathological characteristics of patients with NSCLC. These characteristics include age, sex, history of smoking use and drinking, hypertension, diabetes, tumor size and location, pTNM, Lymph node metastasis, distant metastasis, pleural invasion, differentiation degree, and 5-year overall survival.
Materials and Methods
Patients and Samples Collection
The Ethical Committee of ChiZhou People’s Hospital in Anhui Province, China approved this study. This study was conducted in accordance with the Declaration of Helsinki. We also obtained signed informed consents from all the patients. For the control group of the study, we included healthy patients. We collected NSCLC and non-tumor blood samples from patients who underwent surgical resection anytime from March 2013 to January 2019 at the People’s Hospital of Chizhou. The inclusion criteria required that all the NSCLC patients did not undergo any preoperative radiotherapy and/or chemotherapy. The histopathological evaluation confirmed the results (8th edition of the TNM Classification for Lung Cancer). We collected and retained the follow-up data of all 154 NSCLC patients. Our definition for the overall survival (OS) is the period between the diagnosis and the date that the patient was last known to be alive or the patient’s date of death. Table 1 provides a summary of all the clinicopathological characteristics.
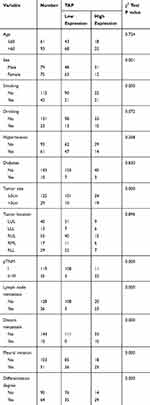 |
Table 1 Clinical Association Between TAP Expression and Clinicopathological Variables in NSCLC Patients |
TAP Detection
Detection Methods
From the first and second drops of a patient’s blood from his/her fingertips, we collected whole blood samples and prepared the specimen on glass slides accordingly. After the samples have naturally dried, we used them for TAP detection along with the TAP detection reagent (Biosharp Biotech, Hefei, China). Then, we performed the condensation staining reaction. Upon the completion of the reaction and drying of the stain, we executed a microscopic examination to observe the results.
Determination of TAP Detection Results
The existence of TAP in the blood is demonstrated by its reaction with the reagent that produces a condensation product that is crystal-like. On one hand, the condensation particle area for TAP-positive is ≥225 µm2. On the other hand, the condensation particle area for TAP weakly positive or critical type is between 121 and 225 µm2. For TAP-negative, there was no evident observation of crystal-like condensation product. In the study, the condensation particle area was>225 µm2 in the high, and<225 µm2 was not observed in the low expression group.
Statistical Analysis
We repeatedly ran the experiments in this study for at least three times. We expressed all the statistical data as the mean ± standard error of the mean (SEM). To perform our statistical analysis, we utilized the SPSS 18.0 software package (SPSS, Chicago, IL, USA). Using the independent-samples t-test, we compared the differences in the TAP expression between the two groups of patients. Concerning the mean value, the classification of the TAP expression levels in the NSCLC blood samples was the low expression and high expression. Furthermore, to evaluate the relationship between the TAP expression and the clinicopathological parameter of NSCLC, our study used the chi-square test. Then, to examine and compare the prognoses, we worked with the Kaplan-Meier method and Log rank test. Finally, to study the independent prognostic factors for survival among the NSCLC patients, we utilized the analytical tools, such as the univariate and multivariate Cox proportional hazards. Our statistical significance is set at P-value 0.05.
0.05.
Results
In NSCLC Blood, TAP Is Upregulated – After Surgery, TAP Is Downregulated
Initially, we used 154 cases of blood samples extracted from NSCLC and non-tumor patients to compare TAP expression between the two groups. Then, we used a TAP detection reagent to further evaluate these TAP expression levels. The results suggest that in NSCLC blood, TAP expression significantly increased than in non-tumor blood (Figure 1A and B). We also conducted examinations on TAP’s expression in NSCLC patients who underwent surgery. Figure 1B illustrates how there was a significant decrease in the TAP expression level in NSCLC patients after surgery, as indicated by the tap detection reagent. Therefore, after surgery, the upregulation of TAP is evident in NSCLC patients.
TAP Expression Is Associated with the Patient’s Sex, History of Smoking Use, Tumor Size, pTNM, Distant Cancer, Metastasis of Lymph Nodes, Invasive and Aggressive Indicator Pleural Invasion, and Differentiation Degree of NSCLC
We assessed the relationship between TAP expression and the clinicopathological characteristics of NSCLC to broaden the understanding of the role of TAP in NSCLC. Concerning the mean value, the classification of the TAP expression levels in the NSCLC blood samples was the low expression and high expression. Table 1 highlights the significant association of high TAP expression levels to clinicopathological factors, such as sex, tumor size, pTNM, distant cancer, metastasis of lymph nodes, invasive and aggressive indicator pleural invasion, and differentiation degree of NSCLC. Additionally, as seen in Table 1, TAP has no association with the patient’s age, history of drinking, location of the tumor, hypertension, and diabetes. These outcomes underline the involvement of increased TAP expression levels in the progression of the NSCLC malignancy.
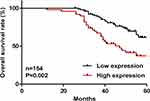
Among NSCLC Patients, High TAP Expression Levels are Predictive of Their Poor Prognosis
Our in-depth analysis covered the assessment of the association between the TAP expression and survival period of NSCLC patients, which obtained results that showed a worse prognosis for NSCLC patients with high TAP expression levels than those with low TAP levels (Figure 2). To study the independent prognostic factors for survival among the NSCLC patients, we utilized the analytical tools, such as the univariate and multivariate Cox proportional hazards. The significant association of the overall survival of NSCLC patients with the tumor size, pTNM, lymph node metastasis, distant metastasis, pleural invasion, differentiation degree, and TAP expression reflected in the univariate analysis data (Table 2). For the prognosis of NSCLC patients, another independent prognostic factor is the TAP expression (Table 3). For the overall survival (OS) of NSCLC patients, these are not independent prognostic factors: the tumor size, pTNM, lymph node metastasis, distant metastasis, pleural invasion, and differentiation degree (Table 3). Furthermore, our results confirm that high TAP expression levels can predict the poor prognosis of NSCLC patients accordingly.
 |
Table 2 Univariate Analysis of Prognostic Factors of NSCLC |
 |
Table 3 Multivariate Analysis of Independent Prognostic Factors of NSCLC |
Discussion
Recent studies have collected evidence on the critical role that TAP plays in the development and progression of cancer, as well as the regulation of cell proliferation, apoptosis, differentiation, and development.7–9 In gastric cancer patients, the upregulation of TAP was significant compared to healthy volunteers. Also, progression-free survival (PFS) is evident among patients with high TAP levels.10 By combining the clinical signs and symptoms, TAP examination could be utilized in the diagnosis of urothelial carcinoma of the bladder. Additionally, it could also detect urothelial carcinoma of the bladder.11 In colorectal cancer (CRC) patients, the detection of TAP has high levels of sensitivity and specificity. Also, in the course of chemotherapy and clinical monitoring of CRC patients, TAP detection is used as a new independent indicator.12 In the advancements of the diagnostics and therapeutics of cancers in humans, it is crucial to study the precise roles of specific TAP.
In this research, we utilized 154 peripheral blood samples of NSCLC patients and evaluated the TAP expression accordingly. In NSCLC and healthy patients, the remarkable upregulation of TAP was evident in the TAP detection reagent data. After the surgery of NSCLC patients, their TAP expression levels also decreased. These outcomes suggest that in the development of NSCLC, TAP is released in the blood. Furthermore, our in-depth analysis found that high TAP expression levels were significantly related to the patient’s sex, history of smoking use, tumor size, pTNM, distant cancer, metastasis of lymph nodes, pleural invasion, and differentiation degree of NSCLC. However, we found that TAP had no association with the patient’s age, history of drinking, location of the tumor, hypertension, and diabetes. Men have higher TAP than women, which may have something to do with the high proportion of men who smoke. Our speculation includes the possible impact of TAP on the prognosis of NSCLC patients given the general association of invasive and metastatic tumor cells with poorer prognosis of cancer patients.
We evaluated the association between the expression of TAP and the overall survival (OS) of NSCLC patients to evaluate the legitimacy of our speculation. In contrast with patients that have low TAP expression, our data confirm the worse prognosis of NSCLC patients with high TAP expression. Also, an independent prognostic factor is the TAP expression among NSCLC patients. Similarly, a higher TAP level was found in breast cancer patients than patients with a benign diagnosis (P<0.001). On one hand, TAP was not associated with estrogen receptor, progesterone receptor, her-2 expression, tumor size, and pathological degree. On the other hand, TAP was significantly associated with the patient’s age, metastasis of lymph nodes, and TNM stage.13 The outcome of recent studies suggests that for the diagnosis of lung cancer and evaluation of progression among lung cancer patients, TAP could be utilized as an indicator.14 We could sensitively distinguish abnormal sugar chains associated with malignant tumors in the digestive tract using TAP detection. As a result, numerous signals related to the tumor could be obtained. In malignant tumors of the subclinical stage, we could detect TAP.15–17 As verified by previous studies, the detection of TAP expression could be up to 2 years earlier than the revelation of clinical signs, symptoms, and malignant lumps.18 Our recommendation for future research is in-depth coverage on the exact regulatory role and mechanism of TAP in the proliferation, migration, and invasion of NSCLC cells. The significance will revolve around the rising need of patients that are TAP-positive for therapeutic interventions, as well as for cancer prevention and treatment. Encouraging results have been found in cancer vaccines that are based on glycans.19,20 Apart from lung cancer, the existence of TAP expression is evident in the stomach, colorectal, thyroid, and bladder tumors.9–12,21 Our research findings add to the general overview of TAP’s role in a tumor’s development and progression.
In conclusion, our data suggest the significant upregulation of the TAP expression in the NSCLC blood and downregulation following a surgery. The TAP expression has a significant relationship with the patient’s sex, history of smoking use, tumor size, pTNM, lymph node metastasis, distant metastasis, pleural invasion, and differentiation degree of NSCLC patients. More importantly, a predictive factor of a worse prognosis in NSCLC patients is an increased TAP expression. Taking all these data into consideration, in the malignant progression of NSCLC, we would like to shed light on the promoting role of TAP and its potential to become an NSCLC prognostic biomarker.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Joanna D, Urszula W, Mańczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med Epidemiol. 2016;4(8):150. doi:10.21037/atm.2016.03.11
3. Moniaux N, Andrianifahanana M, Brand RE, et al. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91(9):1633–1638. doi:10.1038/sj.bjc.6602163
4. Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99(16):10231–10233. doi:10.1073/pnas.172380699
5. Dube DH, Bertozzi CR. Glycans in cancer and inflammation — potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–488. doi:10.1038/nrd1751
6. Liu J, Huang XE. Clinical application of serum tumor abnormal protein from patients with gastric cancer. Asian Pac J Cancer Prev. 2015;16(9):4041. doi:10.7314/APJCP.2015.16.9.4041
7. Sun C, Deng F, Meng L, et al. Correlation between TAP detection and common digestive tract precancerous lesions. Oncol Lett. 2018;15(2):1616–1620. doi:10.3892/ol.2017.7496
8. Ma A, Fan D, Yan F. A study of the application of TAP combined with transvaginal ultrasound in the diagnosis of early-stage endometrial cancer. Oncol Lett. 2018. doi:10.3892/ol
9. Liu Z, Cai J, Yu Y, et al. Tumor abnormal protein as a novel biomarker in papillary thyroid carcinoma. Clin Lab. 2017;63(3):479–485.
10. Lan F, Zhu M, Qi Q, et al. Prognostic value of serum tumor abnormal protein in gastric cancer patients. Mol Clin Oncol. 2016;5(1):216–220. doi:10.3892/mco.2016.877
11. Zhang L, Guo X, Min Y, et al. Tumor abnormal protein (TAP) examination contributes to primary diagnosis of bladder cancer. Int J Clin Exp Med. 2014;8(10):18528–18532.
12. Wu XY, Huang XE. Clinical application of Serum Tumor Abnormal Protein (TAP) in colorectal cancer patients. Asian Pac J Cancer Prev. 2015;16(8):3425–3428. doi:10.7314/APJCP.2015.16.8.3425
13. Chen WX, Yang LG, Cheng L, Zhu Y-L. Tumor abnormal protein in the diagnosis of breast cancer in patients with a palpable mass. Ann Clin Lab Sci. 2019;49(3):297–301.
14. Qiuli C, Lianzi W, Xian W. The diagnostic value of serum tumor abnormal protein in lung cancer. Acta Universitatis Medicinalis Anhui. 2018.
15. Kamalapuram SK, Kanwar RK, Kanwar JR. Nanotheranostic based iron oxide (Fe3O4) saturated lactoferrin nanocapsules for colonic adenocarcinoma. J Biomed Nanotechnol. 2016;12(9):1758. doi:10.1166/jbn.2016.2295
16. Sun L, Xu S, Liang L, et al. Analysis of ROC: the value of HPV16 E6 protein in the diagnosis of early stage cervical carcinoma and precancerous lesions. Oncol Lett. 2016;12(3):1769–1772. doi:10.3892/ol.2016.4844
17. Halkias C, Sloane J, Ben-Gashir M, Bashir G. Synchronous metastatic omental melanoma and colonic adenocarcinoma: a case report. BMC Res Notes. 2015;8(1):125. doi:10.1186/s13104-015-1099-7
18. Jia A, Lv Y, Guo X, et al. Ectopic expression of p33ING1b suppresses proliferation and induces apoptosis in colonic adenocarcinoma cells. Oncol Lett. 2015;10(3):1517–1522. doi:10.3892/ol.2015.3385
19. Krug LM, Govind R, Ng KK, et al. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10(3):916. doi:10.1158/1078-0432.CCR-03-0101
20. Holmberg LA, Sandmaier BM. Vaccination with theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2005;3(6):655–663. doi:10.1586/14760584.3.6.655
21. Carta F, Sionis S, Cocco D, et al. Enhanced contact endoscopy for the assessment of the neoangiogenetic changes in precancerous and cancerous lesions of the oral cavity and oropharynx. Eur Arch Otorhinolaryngol Suppl. 2016;273(7):1895–1903. doi:10.1007/s00405-015-3698-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.