Back to Journals » Journal of Inflammation Research » Volume 15
Increased eEF2K Promotes Glycolysis and Aggressive Behaviors of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis
Authors Chen D, Cai X, Ouyang H, Yuan S, Wang X, Lin L, chen Z , Huang M
Received 8 September 2021
Accepted for publication 1 March 2022
Published 10 March 2022 Volume 2022:15 Pages 1729—1744
DOI https://doi.org/10.2147/JIR.S337620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Dongying Chen,1,* Xiaoyan Cai,2,* Hui Ouyang,3,* Shiwen Yuan,2 Xiaodong Wang,4 Lian Lin,5 Zhiqing Chen,5 Mingcheng Huang5
1Department of Rheumatology, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guandong, People’s Republic of China; 2Department of Rheumatology, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, Guandong, People’s Republic of China; 3Department of Digestive Medicine Center, The Seventh Affiliated Hospital, Sun Yat-Sen University, ShenZhen, Guandong, People’s Republic of China; 4Department of Ultrasound, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guandong, People’s Republic of China; 5Department of Nephrology, Kidney and Urology Center, The Seventh Affiliated Hospital of Sun Yat-Sen University, ShenZhen, Guandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingcheng Huang, Department of Nephrology, Kidney and Urology Center, The Seventh Affiliated Hospital of Sun Yat-Sen University, ShenZhen, Guandong, People’s Republic of China, Email [email protected]
Objective: Aggressive phenotype and abnormal glycolytic metabolism of fibroblast-like synoviocytes (FLSs) are essential to joint inflammation and damage in rheumatoid arthritis (RA). Eukaryotic elongation factor-2 kinase (eEF2K) is a negative regulator of protein synthesis and has been shown to play an important role in regulating various cellular processes and promoting glycolysis in tumor cells. However, the role of eEF2K in regulating the pathogenic FLS behaviors is unknown.
Methods: A specific inhibitor of eEF2K, NH125, and siRNA were used to evaluate the role of eEF2K on RA FLSs in vitro. Collagen-induced arthritis (CIA) mice were used to evaluate the in vivo effect of eEF2K. Cell migration, invasion of RA FLSs were assessed by transwell or wound healing assays. Relative changes of cytokines were analyzed by quantitative real-time PCR, western blot and ELISA.
Results: Herein, we found an increased expression of eEF2K in synovial tissues and FLSs of RA patients. eEF2K knockdown by siRNA or treatment with NH125, an inhibitor of eEF2K, significantly reduced inflammation, migration/invasion, glucose uptake and lactate productions. eEF2K knockdown suppressed TNF-α-induced activation of NF-κB and AKT pathways in RA FLSs. Lactate reversed the inhibitory effect of eEF2K knockdown on inflammation and migration of RA FLSs. Moreover, lactate was also involved in eEF2K-mediated activation of NF-κB and AKT. NH125 treatment attenuated the severity of arthritis in collagen-induced arthritis mice.
Conclusion: eEF2K inhibition suppressed glycolysis and aggressive behaviors of RA FLS, which indicated that targeting eEF2K may be a new strategy for the treatment of RA.
Keywords: rheumatoid arthritis, fibroblast-like synoviocytes, eukaryotic elongation factor-2 kinase, glycolysis, migration, invasion
Rheumatology Key Messages
The expression of eEF2K was increased in synovial tissues and FLSs of RA patients.
eEF2K inhibition suppressed glycolysis and aggressive behaviors of RA FLS in vitro.
NH125 treatment attenuated the severity of arthritis in collagen-induced arthritis mice in vivo.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease, characterized by persistent synovial, pannus formation and joints destruction.1 Fibroblast-like synoviocytes (FLSs) play an important role in the development of RA. Activated RA FLSs have “tumor-like” feathers, such as resistance to apoptosis, vigorous proliferation, increased migration and invasion ability to bone and cartilage.2 RA FLSs can also produce many cytokines that induce synovial inflammation, angiogenesis, and immune cells recruitment.3 Besides aggressive phenotype, activated glucose metabolism under pro-inflammatory conditions was described in RA FLSs.4 RA FLSs display a shift in ATP generation from oxidative phosphorylation to glycolysis, which is similar to that seen in tumor cells.5 It has been shown that enhanced glycolysis contributes to synovial inflammation and bone damage in RA.6 A number of studies including our own have indicated that inhibition of glycolysis by targeting glycolytic enzymes or glycolytic intermediate metabolites may be a therapeutic approach in RA.7,8
Eukaryotic elongation factor 2 kinase (eEF2K) is a Ca2+/calmodulin dependent protein kinase, which can phosphorylate eEF-2 on Thr-56, thereby inactivating this key elongation factor, blocking translation elongation and inhibiting global protein synthesis.9 Previous studies have observed that eEF2K overexpressed in different types of tumor cells.10–12 eEF2K plays an important role in regulating multiple cellular processes, such as promoting cell proliferation, activating autophagy, maintaining cellular energy and supporting tumor cell survival under nutrient deficiency and hypoxia conditions.13,14 More interesting, a recent study found that eEF2K could promote glycolysis, which provide an evidence that eEF2K may be a bridge connected protein synthesis and glycolysis.15
However, to date, it is unknown the effects of eEF2K in RA. Based on the aforementioned literature, we presumed that eEF2K might be involved in the pathogenic behaviors and glucose metabolism in RA FLSs. In this study, we aimed to explore the role of eEF2K in regulating disease-associated aggressive phenotype and glycolysis pathogenic behavior of RA FLSs, as well as to delineate the underlying mechanisms involved.
Materials and Methods
Patients
A total of 12 patients with active RA and 12 patients with osteoarthritis (OA) were enrolled for this study. RA patients were diagnosed based on the 1987 revised criteria of American College of Rheumatology (ACR)16 or the 2010 ACR/the European League against Rheumatology (EULAR) classification criteria.17 Active RA was defined as disease activity score in 28 joints and four variables including C-reactive protein (DAS28-CRP) ≥ 3.2. OA was diagnosed according to the 1986 ACR criteria.18
Ethical Approval
All experimental procedures in this work were carried out according to the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (SYSU) University. Informed conSent was obtained from every patient before the recruitment. Animal handling and procedures were approved by the Animal Care and Ethics Committee of SYSU (No.SYSU-FAH-2020-B1022, Feb-18-2020) and complied with the Guide for the Care and Use of Laboratory Animal published by the US National Institutes of Health (NIH publication, Eighth edition, 2011).
Synovial Tissues and FLSs Culture
Synovial tissues (STs) were obtained from patients with active RA or OA who undergoing synovectomy of the knee joint or total knee replacement. Freshly harvested STs were cut into small pieces, then digested for 3 h at 37°C with 1 mg/mL collagenase. FLSs were cultured in DMEM/F12 with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 5% CO2 and 37°C. FLSs from passages 3~6 were used in this study, during which time they were a homogeneous population of cells (<1% CD11b-positive, <1% phagocytic, and <1% FcgRII- and FcgRIII receptor–positive). The FLSs were characterized by a FLS-specific marker, vimentin and cadherin-11.
Immunofluorescence (IF) Staining for STs or FLSs
The glass coverslips with frozen STs sections were processed in 0.3% H2O2 for 20 min, then incubated with primary antibodies of anti-eEF2K (diluted 1:50) at 4° C overnight and with secondary antibodies at 37° C for 2 h. The sections were observed under a fluorescence microscope (Zeiss LSM710, Wetzlar, Germany). FLSs were seeded on glass coverslips at a density of 1×105 cells/mL. When became approximately 60% confluent, the FLSs were stimulated with TNF-α (10 ng/mL) or IL-1β (10 ng/mL) for 24 hours.Then, they were fixed with 4% paraformaldehyde for 15 minutes and permeated with PBS containing 0.1% Triton X-100 for 10 minutes, then incubated with anti-eEF2K antibody (diluted 1:50) for 1 hour. Absorb the excess liquid on the slide and add the FITC-conjugated secondary antibody (Thermo Fisher Scientific). The cells were then visualized with DAPI (0.25 mg/mL), and the glass coverslips were put on the glass slides with antifade equipment media and then examined by a confocal fluorescence microscopy (Zeiss LSM710, Wetzlar, Germany).
siRNA Transfection
eEF2K siRNA (Supplementary Table 1) and control siRNA (si-C) were purchased from RiboBio (Guangzhou RiboBio Co., Ltd, Guangdong, China). RA FLSs were seeded in 6-well plates. When the density reached at 60%~70% confluence, a transfection mixture of 100 nM siRNA and lipofectin (10 mg/mL) in serum-free medium was added to medium-aspirated FLSs for 6h. Experiments were performed 48 hours after transfection.
Quantitative Real-Time PCR
The RA FLSs were pretreatmented with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K siRNA or si-C, then stimulated with TNF-α (10 ng/mL) for 12 h. Total RNAs was extracted by TRIzol (Sigma), then were reverse transcribed to cDNA by using miScript Reverse Transcription Kit according to the manufacturer’s protocol. The mRNA expression levels were performed on cDNA using QuantiTect SYBR Green RT-PCR Kit by the Step One Plus TM Real Time PCR System (Applied Biosystems). The primers employed for real-time PCR were listed in Supplementary Table 2. GAPDH was used as a quantitative control for RNA levels. 2-ΔΔCT method was used to quantify the relative expression of each gene. In brief, the Ct value was normalized to the endogenous reference (ΔCt = Ct target-Ct 18S rRNA) and compared with the calibrator (ΔΔCt =ΔCt sample -ΔCt calibrator). All experiments were performed in triplicate.
Western Blot Analysis
For each Western blot analysis, 2×105 cells were seeded. When subconfluence reached 80%, RA FLSs were pretreated with DMSO (5%) or NH125 for 24 h or transfected with eEF2K siRNA or si-C. Then, RA FLSs were stimulated with or without TNF-α (10 ng/mL) for 15 min. Proteins in RA FLSs were separated by SDS-PAGE (12%) and transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were incubated for 12 h at 4° C with the following primary antibodies: phosphorylated-IKK, IKK, IκBα, phosphorylated-IκBα, phosphorylated-AKT, AKT and β-actin (1:1000 dilution, all from Cell Signaling Technology, Danvers, MA, USA). Then, the PVDF membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1: 5000 dilution, Cell Signaling Technology) at room temperature for 1 h. The protein bands were visualized with chemiluminescence (Millipore, Boston, MA, USA). The membrane was scanned and densitometry quantified by ImageJ analysis system (National Institutes of Health). The intensity of each protein was First normalized to β-actin bands and represented as a ratio of controls.
Methyl-Thiazol Tetrazolium (MTT) Assays
RA FLSs were cultured with or without NH125 for 48h or transfected with eEF2K siRNA or si-C. Then, the supernatant of the cell cultures were replaced by medium containing MTT (Sigma-Aldrich) and incubated for 30 min. The resulting formazan was resuspended using acidified isopropanol and quantified at 570 nm (620 nm as reference).
ELISA Detection of Cytokines in Culture Supernatant of RA FLSs
After pretreatmented with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K siRNA or si-C, RA FLSs were stimulated with TNF-α (10 ng/mL) for 24 h or with LPS (10 ng/mL) for 24. The levels of CCL-2, IL-6, IL-8, CXCL-10, TNF-α, IL-1β and IL-17A in culture supernatant were detected ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions as we describe previously.7
Transwell Assays for Cell Migration and Invasion
The effects of eEF2K inhibition on vertical migration RA FLSs were detected using transwell assays according to the method previously described.19 RA FLSs (6.0×104 cells/mL) were suspended in serum-free DMEM in the upper well. Then RA FLSs were treated with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K siRNA or si-C. DMEM containing TNF-α as a chemoattractant was placed in the lower well. The chamber was incubated at 37°C, 5% CO2 for 8 h. After incubation, use a cotton swab to remove non-migrating cells from the upper surface of the filter. The filter was fixed in methanol for 15 min and stained with 0.1% crystal violet for 15 min. Chemotaxis was quantified by counting the stained cells that migrated to the underside of the filter using an optical microscope. Similar experiments were performed for the invasion assay using inserts coated with a Matrigel basement membrane matrix (BD Biosciences, Oxford, UK).
Wound Healing Assay for Horizontal Migration
RA FLSs were pretreated with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K siRNA or si-C, and seeded into 6-well plates to almost total confluence. An artificial homogenous wound was created onto the monolayer with a sterile 10-μL tip. After scratching, the culture dishes were washed with serum-free medium. Images of cells migrating into the wound were captured by an inverted microscope (×100), and the area of scratch was measured by ImageJ 1.47 analysis system (National Institutes of Health, Bethesda, MD, USA).
EdU Proliferation Assay
- Ethynyl-2-deoxyuridine (EdU) assay was used to explore the effects of NH125 on cell proliferation. FLS were plated into 96-well cell culturing plates at 1×104 cells/well for 24 h. RA FLSs were grown to 80% confluence and were pretreated with DMSO (5%) or NH125 for 24 h. The FLSs were incubated with 50 µM EdU for 8 h. EdU incorporation was assessed by a Cell-Light™ EdU Apollo®488 in vitro Imaging Kit (Ribobio, Guangzhou, China) following the manufacturer’s protocol. The experiment was independently repeated three times.
Measurements for Glucose Uptake and Lactate
RA FLSs pretreated with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K siRNA or si-C, then were stimulated with TNF-α (10 ng/mL) for 24 h. A glucose uptake assay kit (colorimetric, Abcam) was used to measure glucose uptake. Lactate levels in the medium were detected by a lactate oxidase-based assay at 540 nm. All of the experiments were performed in triplicate.
Fructose-2,6-Bisphosphate (F2,6BP) Assay
RA FLSs pretreated with DMSO (5%) or NH125 for 24 h, or transfected with eEF2K-siRNA or si-C, then were stimulated with TNF-α (10 ng/mL) for 24 h. Collect RA FLSs (1×106) and centrifuge at 200 g. Dissolve the precipitate in 50 mM Tris acetate (pH 8.0) and 0.1M sodium hydroxide. Intracellular levels of F2,6BP were detected by a method previously described by Van Schaftingen et al.20
Collagen-Induced Arthritis (CIA) Mice and Treatments
According to the method reported previously,21,22 a total number of 16 DBA/1 mice (male, 8–9 weeks, SLAC Laboratory Animal Company, Shanghai, China) were intradermally injected with 200 μg of bovine type II collagen diluted in acetic acid and emulsified at a 1:1 ratio (vol/vol) in Freund’s complete adjuvant at the tail base (day 0). Twenty-one days after primary immunization, the mice were intraperitoneally injected with bovine type II collagen emulsified at a 1:1 ratio (vol/vol) in incomplete Freund’s adjuvant (day 21). Then, the mice were randomly treated daily by intraperitoneal injection of DMSO (5%, n=8) or NH125 (1mg/kg/d, n=8) for 14 consecutive days, initiated on the second day of booster immunization (day 22). We also set up a normal control group including 5 mice without any treatment. The dose of NH125 was based on the previous study.23
When the symptoms of arthritis appeared, the mice were monitored daily for signs of arthritis. The arthritis severity was scored on a scale as follows: 0, normal joints; 1, swelling and redness of at least 1 joint; 2=swelling in >1 joint; 3, moderate swelling in the entire paw; and 4, joint deformity and/or ankylosis.24 The total arthritic score was the sum of 4 paws. The ankle diameter was quantified by measuring the thickness of the first hind paw with a 0.01 mm precision Vernier calliper. The serum levels of TNF-α and IL-6 in mice were measured by an ELISA kit (R&D Systems, USA), as we described previously.7 All mice were anaesthetised with 110 mg/kg ketamine and 4.8 mg/kg xylazine, hind limbs were removed and kept in 10% formalin for 48 h for fixation. After a series of dehydration, waxing and embedding, the tissues were cut into 5 micrometer (µm)-thick slices. The slices were stained with haematoxylin and eosin (H&E). Images of specimens were captured under microscope. Pathology index including inflammatory cell infiltration, hyperplasia and bone damages were scored as follows: 0=no symptom shown; 1=mild; 2=moderate; and 3=severe. All scoring was performed by two blinded pathologists. Immunohistochemistry was performed in accordance with the kit’s instructions (Zhongshan Jinqiao PV-9000 Universal Two-Step Test Kit).
Mice plasma was obtained by the centrifugation of blood in serum separator Microtainer tubes after cardiac puncture. The serum CCL-2, IL-6, IL-8, CXCL-10, TNFα, IL-1β and IL-17A was detected by ELISA assays as we described previously.25 Serum creatinine (Cr) was tested by CRE reagent kit (Enzymatic method). Serum aspartate aminotransferase (AST) was tested by AST reagent kit (Rate method), serum alanine aminotransferase (ALT) was examined by ALT reagent kit (Rate method). All the measurements were strictly performed according to the manufacturer’s instructions (NanJing JianCheng Bioengineering Institute).
Reagents and Antibodies
Recombinant human TNF-α and IL-1β were obtained from R&D Systems (Bio-Techne). The regents for cell culture including DMEM, FBS, antibiotics, trypsin EDTA, PBS, and other products were purchased from Invitrogen (Carlsbad, CA, USA). Collagenase and β-actin antibody were purchased from Sigma (St. Louis, MO, USA). NH125 was purchased from MilliporeSigma. eEK2K antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical Analysis
In the study, at least three independent experiments were conducted for each experiment, and the data of a single experiment was repeated 3 times. The continuous variables were presented as mean±Standard Error of Mean (SEM). For continuous variables with normal distribution, comparison was evaluated with 2-tailed student t test or one-way ANOVA. For continuous variables with non-normal distribution, Mann–Whitney U-test or Wilcoxon test was used to compare the difference between two-sample, and Kruskal–Wallis test was used to compare the difference between multiple groups. The categorical variables were presented as frequencies or percentages. Comparison between groups was evaluated with chi-square test or Fisher exact test for categorical variables. A P value <0.05 was considered significant. Statistical analyses of the data were performed using SPSS, version 13.0 (IBM).
Results
Demographic Characteristics of the Patients Enrolled in the Study
Twelve RA patients were recruited in this study (10 female and 2 male, aged from 42 to 63 years-old). Among them, the median of disease duration was 24 months (range 24 to 120 months), and the median of DAS28-CRP was 6.1 (range 5.2 to 7.3). There were no significant differences in sex or age between the RA patients and OA subjects.
Increased Expression of eEF2K in STs and FLSs in RA Patients
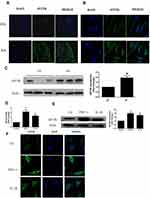
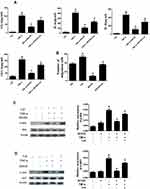
We first investigated the expression of eEF2K in STs. IF analysis showed that compared with OA STs, increased expression of eEF2K was observed in RA STs (Figure 1A). Meanwhile, IF analysis revealed that eEF2K protein was localized predominantly in the cytoplasm. We also found that RA FLSs exhibited enhanced staining for eEF2K (Figure 1B).The increased expression of eEF2K in RA FLSs was further confirmed by Western blot assays (Figure 1C). Pro-inflammatory cytokines, especially TNF-α and IL-1β, were critical in transforming RA FLSs to a more aggressive phenotype. Both of RT-qPCR and Western blot assays indicated that TNF-α (10 ng/mL) and IL-1β (10 ng/mL) significantly increased the mRNA expression and the protein level of eEF2K (Figure 1D and E). TNF-α treatment up-regulated the protein expression of eEF2K most significantly, so TNF-α was used as a stimulus in the follow-up studies. IF staining further confirmed that stimulated with TNF-α (10 ng/mL) or IL-1β (10 ng/mL) could increased the expression of eEF2K in RA FLSs (Figure 1F).
eEF2K Inhibition Suppressed Cytokines Production in RA FLSs
Interference with siRNA was used to inhibit eEF2K expression. To eliminate nonspecific interference, we designed 3 siRNA oligonucleotide sequences for eEF2K. Transfection with all 3 siRNA oligonucleotides decreased eEF2K expression (Supplementary Figure 1); however, the inhibitory effect of siRNA-3 for eEF2K was more prominent. Therefore, siRNA-3 for eEF2K (eEF2K siRNA) were used for subsequent experiments. NH125 was a potent and selective inhibitor of eEF2K. MTT assays were used to detect the toxicity of NH125 on RA FLSs. Up to 5.0 μM, NH125 did not reduce cell viability (Supplementary Figure 2A). So, we choose the concentration of NH125 (0.1, 0.5, 1.0μM) that did not affect the cell viability in the subsequent experiments. In order to rule out a non-specific effect of drugs, siRNA eEF2K was also used. The MTT assays showed that both Si-c or siRNA eEF2K did not affect the cell viability (Supplementary Figure 2B).
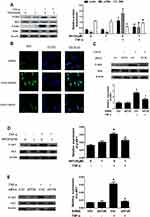
We determined that NH125 treatment or transfected with eEF2K siRNA decreased the TNF-α-induced expression of CCL-2, IL-6, IL-8, and CXCL-10 (Figure 2A and B). Consistently, NH125 treatment or transfected with eEF2K siRNA downregulated the levels of CCL-2, IL-6, IL-8, and CXCL-10 in the supernatants of RA FLSs (Figure 2C and D). Treatment with NH125 also reduced LPS-induced expression of TNF-α and IL-1β (Supplementary Figure 3A-B). However, NH125 did not affect LPS-induced expression of IL-17A in vitro (Supplementary Figure 3C). These data supported that eEF2K play an important role in regulating synovial inflammation.
eEF2K Inhibition Suppressed in vitro Migration and Invasion in RA FLSs
We next evaluated the effect of eEF2K on proliferation of RA FLSs. We demonstrated that treatment with NH125 (0.5uM, 1.0uM) inhibited proliferation of RA FLSs (Supplementary Figure 4). In addition, our data showed that NH125 and eEF2K siRNA suppressed the chemotaxis migration and horizontal migration of RA FLSs, respectively (Figure 3A and B). Then, Matrigel-coated Transwell membrane was used to explore the role of eEF2K on regulating invasive ability of RA FLSs. We found that both NH125 or eEF2K siRNA reduced the cell invasion of RA FLSs (Figure 3C). These results supported the important role for eEF2K in regulating migration and invasion of RA FLSs.
eEF2K Inhibition Suppressed TNF-α-Induced NF-κB and AKT Pathway Activation
In RA, activated NF-κB pathway was a key regulator in synovial inflammation and joint destruction. We found that NH125 decreased IKK and IkBα phosphorylation in TNF-α-stimulated RA FLSs (Figure 4A). IF staining showed that TNF-α increased p65 nuclear accumulation of RA FLSs, and treatment with NH125 significantly reduced p65 translocation into the nucleus (Figure 4B). We also observed that eEF2K knockdown with siRNA suppressed the TNF-α-induced IKK phosphorylation (Figure 4C). TNF-α has been shown to bind to two distinct receptors, TNF receptor I (TNFRI) and TNFRII. We found that the TNF-α-induced decrease in TNFRI expression was reversed by the treatment with NH125 (Supplementary Figure 5A). Treatment with NH125 also suppressed TNF-α-induced TNF-RII expression in RA FLSs (Supplementary Figure 5B).
AKT pathway played an important role in regulating inflammation and migration of RA FLSs, we next found that NH125 treatment inhibited phosphorylation of AKT in TNF-α-treated RA FLSs (Figure 4D). The similar results were also observed from RA FLSs transfected by eEF2K siRNA (Figure 4E). These data suggested that NF-κB and AKT activation might be involved in eEF2K-mediated inflammation and migration of RA FLSs.
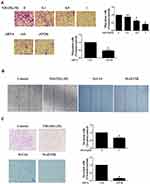
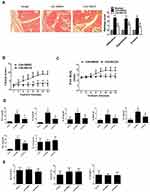
NH125 Attenuated the Severity of Arthritis in Collagen-Induced Arthritis Mice
Given the in vitro observations in RA FLSs, we next explored the in vivo effects of eEF2K inhibition by NH125 in mice with collagen-induced arthritis (CIA). The histological analysis showed that administration of NH125 attenuated the inflammatory cells infiltration, hyperplasia, and bone erosion of the ankle joints (Figure 5A). As shown in Figure 5B and C, intraperitoneal injection of NH125 reduced clinical scores and paw thickness compared with those in the DMSO-treated group. We also observed NH125 treatment reduced the serum levels of TNF-α, IL-6, IL-1β, IL-8, CCL-2, CXCL-10, but not IL-17A in CIA mice (Figure 5D). We further demonstrated that synovial expression of NF-κB and AKT was decreased in NH125-treated CIA mice compared with DMSO-treated mice (Supplementary Figure 6).
We also found no significant alterations in renal parameters (serum Cr levels), liver parameters (ALT and AST levels) in NH125-treated groups. These data demonstrate the safety of NH125 treatment in CIA mice (Figure 5E).
eEF2K Inhibition Reduced Glucose Uptake, Lactate Productions and Intracellular Levels of F2,6BP in RA FLSs
Because it has been reported that eEF2K was involved in glycolysis in multiple tumor cells,15 we investigated the role of eEF2K in glucose metabolism in RA FLSs. First, we observed treatment with TNF-α markedly increased the glucose uptake in RA FLSs, and NH125 or eEF2K siRNA decreased both basal and TNF-α-induced glucose uptake (Supplementary Figure 7A). Moreover, NH125 or eEF2K siRNA treatment also reduced intracellular levels of F2,6BP, a key regulator in the glycolysis (Supplementary Figure 7B). Finally, we found that TNF-α treatment increased the secretion of lactate, and these increases were reversed by NH125 or eEF2K siRNA treatment (Supplementary Figure 7C). Our findings suggested an important role of eEF2K in regulating glycolytic metabolism in RA FLSs.
Lactate Was Involved in eEF2K-Mediated Cytokines Production and Migration in RA FLSs
To determine whether lactate is involved in eEF2K-mediated synovial inflammation and migration, we simulated the joint micro-environment of RA as we previously described.7 The addition of lactate reversed the inhibitory effect of NH125 on cytokines productions (Figure 6A) and in vitro migration abilities (Figure 6B) in TNF-α-stimulated RA FLSs.
Lactate Was Involved in eEF2K-Mediated Activation of NF-κB and AKT Pathway
We further investigated the effects of lactate on eEF2K-mediated activation of NF-κB and AKT pathways. The results showed that the addition of lactate to NH125-pretreated RA FLS rescued the decrease of p-IKK (Figure 6C). The NH125-induced decrease in p-AKT was also reversed by the addition of lactate (Figure 6D). We further found that lactate stimulation alone had no effect on the expression of p-IKK and p-AKT (Supplementary Figure 8A-B). The data indicated that the effect of lactate on RA FLSs might through a pathway regulated by eEF2K. Collectively, our data indicated that decreased lactate in eEF2K-inhibited RA FLSs might be a major cause of NF-κB and AKT inactivation.
Discussion
In the present study, we observed the expression of eEF2K was increased in STs and FLSs from RA patients. We demonstrated that eEF2K inhibition decreased the cytokines production, migration, and invasion of RA FLSs. Furthermore, eEF2K inhibition reduced the glucose uptake, lactate secretion, and intracellular levels of F2,6BP of RA FLSs. Our studies also revealed that eEF2K regulated activation of NF-κB and AKT pathways. Interestingly, NH125 treatment attenuated the severity of arthritis in CIA mice. This study strongly suggested that increased eEF2K played an important role in regulating abnormal glycolysis and pathogenic behaviors of RA FLSs partly via NF-κB and AKT pathways.
An increased number of studies indicate that eEF2K is highly expressed in various types of solid tumors including brain,26 breast,27 and lung cancers.10 eEF2K is activated under stress conditions, which promotes cell proliferation, survival, and aggressive tumor characteristics, leading to tumor growth. For instance, over-expression of eEF2K promotes tumor progression and radioresistance in patients with esophageal squamous cell carcinoma.28 Guo et al demonstrate a small-molecule inhibitor targeting eEF2K has anti-tumor activity with apoptosis-inducing mechanisms in vitro and in vivo.29 In the present study, we demonstrated both eEF2K siRNA and NH125 decreased the cytokines production, migration, and invasion in RA FLSs. Furthermore, treatment with NH125 attenuated the the severity of arthritis in CIA mice in vivo. These data suggested that high expression of eEF2K contributed to activated RA FLSs-mediated aggressive phenotype in RA.
Energy metabolism of RA FLSs differs remarkably from that of normal cells, and glycolysis was one of the hallmarks of RA FLSs.8 It has been reported that the enhanced glycolysis of RA FLSs could promote cellular invasion/migration, secretion of pro-inflammatory mediators, abnormal angiogenesis, and pannus formation.4 We have previously reported that glycolytic inhibitor PFKFB3 significantly impaired the aggressive behaviors of RA FLSs.7 In this study, we determined that eEF2K siRANA or NH125 treatment reversed TNF-α induced glucose uptake. Similarly, Li et al found that deficiency in eEF2K significantly reduced the uptake of glucose and the productions of lactate in tumor cells.30 Indeed, consistent with our findings, a recent study demonstrated that eEF2K promoted glycolysis depended on its limitation of synthesis of the protein phosphatase 2A-A (PP2A-A), leading to slow down the ubiquitin–proteasomal degradation of c-Myc and hence pyruvate kinase M2 isoform (PKM2), a key enzyme in the glycolytic pathway.15 These data reveal a novel role of eEF-2K in promoting glycolysis of RA FLSs.
eEF2K involves in many signal pathways, such as proliferation, apoptosis, autophagy, invasion and energy metabolism. For example, eEF2K contributes to angiogenesis and tumor progression in hepatocellular carcinoma via VEGF expression and the subsequent stimulation of PI3K/AKT/STAT3 signaling.31 Targeting eEF2K suppresses the growth and peritoneal metastasis of ovarian cancer by down-regulation expression of integrin β1 and cyclin D1, as well as the activity of PI3K/AKT and NF-κB signaling pathways.32 In this study, we found that NH125 suppressed TNF-α-induced phosphorylation of IKK and IκBα, as well as the intracellular translocation of p65, suggesting that eEF2K regulated the NF-κB pathway by interfering with early cytoplasmic IKK signaling. TNFRI and TNFRII mediated different signals of RA. The cytoplasmic domain of TNFRI has been shown to mediate apoptosis and the activation of NF-κB, which then mediates inflammation of RA.33 TNFRII, in contrast, has been shown to recruit TNFR-associated factor 2 (TRAF2) through TRAF1, and it plays roles in immunoregulation, angiogenesis, and cardioprotection.34 Based the results in this study, we speculated that the eEK2K inhibition could mediate the expression of TNFR, which in turn affected the activation of downstream NF-κB and inflammatory cytokines production of RA FLSs. However, besides the TNF/TNFR pathway, the mechanisms underlying eEK2K-mediated inflammation, angiogenesis, migration, invasion of RA FLSs remain unclear and require further research.
Lactic acid is the end products of glycolysis. Excessive lactate promotes abnormal migration/invasion,35 differentiation of T cells into Th17 subtypes,36 angiogenesis,37 activates of MMPs38 and cytokines production.39 Moreover, AKT phosphorylation was found to participate in the control of glycolysis, lactate secretion, cell proliferation and protein synthesis in mitogen-stimulated thymocytes.40 Based on the above research results, we aimed to explore whether lactate linked between eEF2K and the activation of NF-κB and AKT pathways in RA FLSs. Herein, we demonstrated that TNF-α simulated higher levels of lactate. eEF2K siRNA or NH125 decreased the TNF-α-induced lactate production in RA FLSs. We also found that exogenous lactate reversed the inhibitory effect of NH125 suppression on the cytokines production and abnormal invasion. Moreover, the lactate and TNF-α-induced NF-κB and AKT activation was reversed by NH125. Collectively, our findings suggest that the synovial aggression and inflammation of RA mediated by NF-κB and AKT signalling may be partly due to the increased glycolysis and accumulation of lactate.
In summary, we uncovered eEF2K played an important role in promoting glycolysis in RA FLSs, in addition to its well-known involvement in protein synthesis. Our study also found that inhibition of eEF2K reduced the inflammation and aggressive behaviors of RA FLSs in vitro. eEF2K specific inhibitor NH125 attenuated synovial inflammation and bone erosion in vivo. Our study suggested targeting eEF2K to interfere with glucose metabolic alteration and aggressive behaviors of RA FLSs may be a novel therapeutic strategy for RA.
Acknowledgments
We thank very much for Shan Zeng, and Pro. Liuqin Liang for their contributions to the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (80603435, 81901647), Natural Science Foundation of Guangdong Province (2020A1515010201), and grant from Young Teacher Foundation of Sun Yat-sen University (19ykpy72 and 20ykpy16).
Disclosure
The authors have declared no conflicts of interest.
References
1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi:10.1016/S0140-6736(10)60826-4
2. Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316–333. doi:10.1038/s41584-020-0413-5
3. Falconer J, Murphy AN, Young SP, et al. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2018;70:984–999. doi:10.1002/art.40504
4. Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19:110. doi:10.1186/s13075-017-1303-3
5. Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Front Immunol. 2019;10:2099. doi:10.3389/fimmu.2019.02099
6. Perl A. Review: metabolic control of immune system activation in rheumatic diseases. Arthritis Rheumatol. 2017;69:2259–2270. doi:10.1002/art.40223
7. Zou Y, Zeng S, Huang M, et al. Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br J Pharmacol. 2017;174:893–908. doi:10.1111/bph.13762
8. Garcia-Carbonell R, Divakaruni AS, Lodi A, et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2016;68:1614–1626. doi:10.1002/art.39608
9. Karakas D, Ozpolat B. Eukaryotic elongation factor-2 kinase (eEF2K) signaling in tumor and microenvironment as a novel molecular target. J Mol Med. 2020;98:775–787. doi:10.1007/s00109-020-01917-8
10. Xiao M, Xie J, Wu Y, et al. The eEF2 kinase-induced STAT3 inactivation inhibits lung cancer cell proliferation by phosphorylation of PKM2. Cell Commun Signal. 2020;18:25. doi:10.1186/s12964-020-0528-y
11. Tong S, Zhou T, Meng Y, Xu D, Chen J. AMPK decreases ERK1/2 activity and cancer cell sensitivity to nutrition deprivation by mediating a positive feedback loop involving eEF2K. Oncol Lett. 2020;20:61–66. doi:10.3892/ol.2020.11554
12. Lim IK. Cunning maneuver regulating chemotherapy resistance in triple-negative breast cancer by eEF2K and autophagy. Ann Transl Med. 2020;8:845. doi:10.21037/atm.2020.04.27
13. Wang Y, Huang G, Wang Z, Qin H, Mo B, Wang C. Elongation factor-2 kinase acts downstream of p38 MAPK to regulate proliferation, apoptosis and autophagy in human lung fibroblasts. Exp Cell Res. 2018;363:291–298. doi:10.1016/j.yexcr.2018.01.019
14. Gao M, Dang F, Deng C. Beta-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur J Pharmacol. 2019;859:172528. doi:10.1016/j.ejphar.2019.172528
15. Cheng Y, Ren X, Yuan Y, et al. EEF-2 kinase is a critical regulator of Warburg effect through controlling PP2A-A synthesis. Oncogene. 2016;35:6293–6308. doi:10.1038/onc.2016.166
16. Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi:10.1016/j.cell.2016.12.012
17. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi:10.1002/art.27584
18. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi:10.1002/art.1780290816
19. Lao M, Shi M, Zou Y, et al. Protein inhibitor of activated STAT3 regulates migration, invasion, and activation of fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol. 2016;196:596–606. doi:10.4049/jimmunol.1403254
20. Van Schaftingen E, Lederer B, Bartrons R, Hers HG. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129:191–195. doi:10.1111/j.1432-1033.1982.tb07039.x
21. Li N, Xu Q, Liu Q, et al. Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Rheumatology. 2017;56:1417–1427. doi:10.1093/rheumatology/kex142
22. Liang L, Huang M, Xiao Y, et al. Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm Res. 2015;64:225–233. doi:10.1007/s00011-015-0801-5
23. Li W, Ali T, Zheng C, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021;18:38. doi:10.1186/s12974-021-02091-5
24. Shi M, Wang J, Xiao Y, et al. Glycogen metabolism and rheumatoid arthritis: the role of glycogen synthase 1 in regulation of synovial inflammation via blocking AMP-Activated protein kinase activation. Front Immunol. 2018;9:1714. doi:10.3389/fimmu.2018.01714
25. Huang M, Wu K, Zeng S, et al. Punicalagin inhibited inflammation and migration of Fibroblast-Like synoviocytes through NF-kappaB pathway in the experimental study of rheumatoid arthritis. J Inflamm Res. 2021;14:1901–1913. doi:10.2147/JIR.S302929
26. Leprivier G, Remke M, Rotblat B, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell. 2013;153:1064–1079. doi:10.1016/j.cell.2013.04.055
27. Wang RX, Xu XE, Huang L, Chen S, Shao ZM. EEF2 kinase mediated autophagy as a potential therapeutic target for paclitaxel-resistant triple-negative breast cancer. Ann Transl Med. 2019;7:783. doi:10.21037/atm.2019.11.39
28. Zhu H, Song H, Chen G, et al. EEF2K promotes progression and radioresistance of esophageal squamous cell carcinoma. Radiother Oncol. 2017;124:439–447. doi:10.1016/j.radonc.2017.04.001
29. Guo Y, Zhao Y, Wang G, et al. Design, synthesis and structure-activity relationship of a focused library of beta-phenylalanine derivatives as novel eEF2K inhibitors with apoptosis-inducing mechanisms in breast cancer. Eur J Med Chem. 2018;143:402–418. doi:10.1016/j.ejmech.2017.11.065
30. Li S, Li Y, Bai Y. What is the impact of eukaryotic elongation factor 2 kinase on cancer: a systematic review. Eur J Pharmacol. 2019;857:172470. doi:10.1016/j.ejphar.2019.172470
31. Zhou Y, Li Y, Xu S, et al. Eukaryotic elongation factor 2 kinase promotes angiogenesis in hepatocellular carcinoma via PI3K/Akt and STAT3. Int J Cancer. 2020;146:1383–1395. doi:10.1002/ijc.32560
32. Erdogan MA, Ashour A, Yuca E, Gorgulu K, Ozpolat B. Targeting eukaryotic elongation factor-2 kinase suppresses the growth and peritoneal metastasis of ovarian cancer. Cell Signal. 2021;81:109938. doi:10.1016/j.cellsig.2021.109938
33. McDermott MF. TNF and TNFR biology in health and disease. Cell Mol Biol. 2001;47:619–635.
34. Fischer R, Kontermann RE, Pfizenmaier K. Selective targeting of TNF receptors as a novel therapeutic approach. Front Cell Dev Biol. 2020;8:401. doi:10.3389/fcell.2020.00401
35. Biniecka M, Canavan M, McGarry T, et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis. 2016;75:2192–2200. doi:10.1136/annrheumdis-2015-208476
36. Wang R, Solt LA. Metabolism of murine TH 17 cells: impact on cell fate and function. Eur J Immunol. 2016;46:807–816. doi:10.1002/eji.201545788
37. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi:10.1038/s41586-019-1678-1
38. Li H, Lu S, Chen Y, et al. AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-kappaB, HIF1alpha, MMP2, and MMP9 upregulation. Cell Signal. 2019;58:99–110.
39. Abebayehu D, Spence AJ, Qayum AA, et al. Lactic acid suppresses IL-33-Mediated mast cell inflammatory responses via Hypoxia-Inducible Factor-1alpha-Dependent miR-155 suppression. J Immunol. 2016;197:2909–2917. doi:10.4049/jimmunol.1600651
40. Houddane A, Bultot L, Novellasdemunt L, et al. Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cell Signal. 2017;34:23–37. doi:10.1016/j.cellsig.2017.02.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.