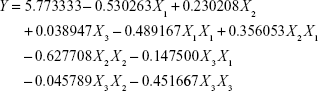Back to Journals » International Journal of Nanomedicine » Volume 11
Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: preparation optimization, in vitro dermal permeation, and in vivo bioevaluation
Authors Li Z, Liu M, Wang H, Du S
Received 25 January 2016
Accepted for publication 9 May 2016
Published 30 June 2016 Volume 2016:11 Pages 2995—3007
DOI https://doi.org/10.2147/IJN.S105035
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Lei Yang
Zehao Li,1 Meifeng Liu,1 Huijuan Wang,1 Song Du2
1School of Chemistry and Chemical Engineering, Guangdong Provincial Key Laboratory for Green Chemical Product Technology, South China University of Technology, Guangzhou, 2Guangdong Jiabao Pharmaceutical Co., Ltd., Qingyuan, People’s Republic of China
Abstract: Madecassoside (MA) is highly potent in treating skin disorders such as wounds and psoriasis. However, the topical wound healing effect of MA was hampered by its poor membrane permeability. In order to overcome this shortcoming, MA liposomes were designed and prepared by a double-emulsion method to enhance transdermal and wound healing effects. In this study, response surface methodology was adopted to yield the optimal preparation conditions of MA double-emulsion liposomes with average particle size of 151 nm and encapsulation efficiency of 70.14%. Moreover, MA double-emulsion liposomes demonstrated superior stability and homogeneous appearance in 5 months; their leakage rate was <12% even at 37°C and <5% at 4°C within 1 month. In vitro skin permeation, skin distribution, and burn wound healing of MA liposomal formulations were conducted for the first time to evaluate MA delivery efficiency and wound healing effect. The transdermal property and wound cure effect of MA double-emulsion liposomes were superior to those of MA film dispersion liposomes, and both the methods were endowed with an excellent performance by polyethylene glycol modification. In conclusion, double-emulsion liposome formulation was an applicable and promising pharmaceutical preparation for enhancing MA delivery toward wound healing effect and improving wound-healing progress.
Keywords: madecassoside liposomes, double-emulsion method, skin permeation, wound-healing effect, transdermal property
Introduction
Skin, the first barrier to protect the body, is often injured by wounds or physical trauma. The healing process of wounds is spontaneous and involves different overlapped phases, namely, inflammation (inducing hemostasis and clot formation), fibroplasia and neovascularization, generation of granulation tissue, reepithelialization, and finally the formation of new extracellular matrix and tissue remodeling.1 Minor wound damage can self-heal quickly. However, for severe damage such as large area burns, deep damage of skin, and second- and third-degree burns, the healing time will be prolonged,2 which greatly increases the risk of bacterial infection of the wound, impedes the healing process for longer time, and may even lead to life-threatening complications. The longer the spontaneous healing process extends, the more unfavorable the prognosis is. If the wound healing time is prolonged, the continuing inflammatory cascade may result in increasing tissue destruction and necrosis rather than healing.3 Scarring is the inevitable process product of human wound healing; it may cause pain, itching, and movement inflexibility.4 In addition to interfering with normal tissue function, serious postwound deformity and scarring also affect external public image.5 Because of their clearly visible and scarry appearance, wounds and scars on the body and face affect the individual not only physically but also emotionally. Therefore, the potential applications of novel formulations and new strategies are extensively desired to further enhance the healing rate and reduce scar formation.
It is well known that madecassoside (MA) not only has the effects of promoting cell growth, accelerating wound healing and scar healing6 but also has antioxidant,7 antiulcer,8 sedative, neuroprotective activities,9 germ killing around the wound, and anti-inflammatory properties.10 MA is frequently used for the treatment of surgical trauma, burns, scalds, and scars in clinical applications.11 However, many polar chemicals such as MA with large molecular weight and pharmacological activities are difficult to be absorbed through the skin by percutaneous administration because of the skin’s barrier, the stratum corneum.12 MA, a larger water-soluble drug with a glucoside chain on pentacyclic triterpene structure (Figure 1), scarcely permeates through the skin, which extremely limits its applications. Hence, it is indispensable to develop novel formula strategies to improve the dermal/transdermal penetration and enhance the bioavailability of MA applied to the skin.
  | Figure 1 The structure of madecassoside and the schematic for madecassoside-contained liposomes penetrating through the skin and fusing with the cell membrane. |
In recent years, liposomes,13 multifunctional nanofibers,14 and coaxial electrospun fibers15 have been developed and applied as drug delivery carriers. Among them, liposomes have become a promising skin drug delivery system; their use produced several-fold higher drug concentrations in the skin and lower systemic concentrations when compared with traditional dosage forms. The epidermal barrier may be weakened or eliminated by wetting the stratum corneum and strengthening hydration, which results in array configuration and structural changes among skin cells and leads to enhanced permeation of liposomes through the extracellular spaces between cells and fusion with skin cells to release the drug. The phospholipid bilayer membrane of liposomes is more flexible and inclined to fuse with the phospholipid bilayer membrane of the cell (Figure 1). Application of liposomes on the skin has many advantages: 1) liposomes are safe and biodegradable materials; the material component structures are generally similar or identical to the endogenous substances, 2) liposomes show unique compatibility and affinity with the skin, which can improve the permeability of the stratum corneum and enable the liposomes to permeate through the skin quickly, and 3) as excipients or local drug depot, liposomes can be directly applied to the skin lesion and sustain the long-term release of drugs, which would reduce the drug toxicity and side effects caused by massive release of the one time use drug in conventional drug administration.16,17
In our previous work, to improve permeation and sufficiently exert effects against skin disorders, MA film dispersion liposomes were designed for the first time and response surface methodology (RSM) was performed to optimize process conditions and yield favorable liposomal particle size to meet transdermal requirements.18 As for the hydrophilic drug-like MA, encapsulation efficiency >40% was not very low using film dispersion methods, but it still required large amount of MA so as to reach the therapeutic dosage. An average particle size of 293 nm was close to the upper limit of particle size requirement (100–300 nm) for excellent transport potency. Moreover, so far, no study has investigated the effect of liposome-based drug carrier systems on enhancing MA delivery to wound sites, despite their reliable properties of enhancing skin permeation and dermal localization of drugs. The large number of studies on liposomal process preparation, extensively transdermal investigations in vitro and in vivo are still urgently desired for evaluation of therapeutic efficacy. RSM is a powerful and efficient mathematical model with a collection of statistical techniques, wherein, interactions between multiple process variables can be identified with fewer experimental trials. On the basis of our aforementioned work and background, the double-emulsion method was adopted in RSM examination to yield MA liposomes with higher drug loading efficiency and more narrow size distribution of small liposomal particles in this present work. This work was the first one to present a novel strategy of MA liposomes with simple technology on nanocarriers to enhance MA delivery to wound sites and unique wound healing properties. Moreover, the stabilities, characteristics, and dermal localizations of MA double-emulsion liposomes were evaluated and compared with MA film dispersion liposomes, MA ethanol-injection liposomes, and conventional MA solution. To highlight the potential application of MA nanoliposome formulation, further enhanced healing rate and reduced scar formation were also assessed on experimentally induced wounds in rats.
Materials and methods
Materials and animals
Egg yolk lecithin (72%–85%) was provided by Guangzhou Hanfang Pharmaceutical Co., Ltd. (Guangzhou, People’s Republic of China). Cholesterol and sodium dihydrogen phosphate were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, People’s Republic of China). Vitamin E acetate was purchased from Tongxiang Tianhecheng Food Science and Technology (Guangzhou, People’s Republic of China). Polyethylene glycol 1500 (PEG-1500) was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, People’s Republic of China). Other chemicals and reagents used in this work were all of analytical grade. All solutions were prepared using double-distilled water.
Equal number of male and female healthy Sprague Dawley (SD) rats weighing between 200 g and 250 g were used (Laboratory Animal Center, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China). All studies involving experimental rats were conducted under the approval of the Animal Experimental Ethical Inspection of Guangzhou University of Chinese Medicine. Experimental protocols and procedures were performed strictly within national regulations and guidelines (2001/002/CME) that were approved by Animal Care and Use Committee, Guangzhou University of Chinese Medicine, People’s Republic of China. All rats were treated humanely throughout the experimental period.
Preparation of liposomes
MA double-emulsion liposome formulation was prepared by a two-step emulsification procedure.19 In the first step, phospholipid and cholesterol with the desirable ratio were dissolved in chloroform to form oil phase (O). A concentration of 5 mL of MA’s phosphate buffer solution (PBS; W1, pH=7.0) was added into the oil phase. Subsequently, high-stirring homogenization was applied for 5 minutes at the speed of 500 rpm to obtain the W1/O-type emulsions. In the second step, PBS (W2, pH=7.0) was added into the W1/O-type emulsions by stirring to form W1/O/W2 double emulsions. The volumetric ratio of W1:O:W2 was 1:2:1. Chloroform was removed by rotary vacuum evaporation at 30°C. Residual organic solvent was removed by pumping in a vacuum for 1 hour. Lipid mixture was homogenized by ultrasonic water bath for 65 seconds to form well-distributed liposomes and then filtered using a 0.45 μm membrane filter. Ultimately, the resulting suspensions of MA liposomes were stored at 4°C. The neat liposomes were prepared in the same manner as mentioned earlier, except for adding MA.
MA film dispersion liposomes were prepared according to our previous experiment. Briefly, the desirable ratio of chloroform was removed from the mixture of phospholipid and cholesterol by rotary vacuum evaporation to form lipid film; then MA film dispersion liposomes were acquired by hydrating lipid film with MA-contained PBS. MA ethanol-injection liposomes were prepared by mixing phospholipid and cholesterol in ethanol, adding to MA PBS dropwise, and then removing ethanol in water bath at 30°C under the action of stirring.20 An equal volume of PBS (pH=7.0) with 3% PEG-1500 was added into MA-wrapped liposomes and filtered by a 0.45 μm filter membrane to yield PEG modification products of MA liposomes.21,22
Response surface methodology
RSM was adopted to optimize the preparation conditions of MA double-emulsion liposomes. Based on the single-factor experiments, three different factors, namely concentration of MA (mg/mL), ratio of egg yolk lecithin to cholesterol (w/w), and stirring speed (rpm), were involved in central composite design (CCD) of RSM experiment. Among the total of 15 experimental trials in CCD, the first 12 were organized in a factorial design, and experimental trials from the 13th to the 15th were the replications of central points. Once the experiments were performed, a second-order polynomial Equation 1 was used to describe the effect of variables of linear, quadratic, and cross product.
|
|
where Y is the drug loading efficiency, μ is the regression coefficient, and λ is the number of factors studied and optimized in the experiment. Fit mass of the second-order equation was expressed by coefficient of R2, and its statistical significance was determined by F-test. The significance of each coefficient was determined using Student’s t-test. The coefficients of the equation were determined using SAS 9.0 software. Variance analysis of the predictive equation was performed using the same software package.
Physiochemical characterization of MA liposomes
Encapsulation efficiency of MA liposomes
Encapsulation efficiency of MA liposomes was analyzed by the dialysis method.23 Two microliters of liposomes were pipetted and placed in a dialysis tube (molecular weight cut-off [MWCO] 14,000) and clamped tightly. Then, the dialysis tube was immersed in 500 mL of release medium of distilled water. The release medium was stirred at 480 rpm using a magnetic stirrer and dialyzed for 12 hours. The concentration of MA was determined by high-performance liquid chromatography (HPLC, Agilent 1260; Agilent Technologies, Santa Clara, CA, USA) after being appropriately diluted by water. The total and free masses of MA were designated as W0 and Wi, respectively. Encapsulation efficiency (EE%) was calculated as follows:
|
|
Drug loading efficiency of MA liposomes
Drug loading efficiency of MA liposomes was calculated and determined in the same manner as that for encapsulation efficiency. W is the total mass of liposomal materials and MA encapsulated, and the mass of free MA is Wi. Drug loading efficiency (DL%) was calculated as follows:
|
|
Determination of particle size and zeta potential of liposomes
Particle size and zeta potential of MA liposomes were measured using Malvern zeta-size Nano ZS (Malvern Instruments, Malvern, UK). The samples with appropriate concentration were measured at 25°C in triplicate, and the mean value was calculated.
Scanning electron microscopy of MA liposomes
Scanning electron microscopy (SEM) of MA liposomes was performed using a scanning electron microscope (Carl Zeiss Meditec AG, Jena, Germany). MA liposomes prepared at the optimum conditions were diluted appropriately, placed on a glass slide, and air-dried at room temperature, and then measured by SEM to observe and analyze the morphology of liposomes.
Physical stability test of MA liposomes
Physical stability test of MA liposomes was carried out by both product appearance and fluorescent-inverted microscope scanning (Y-2 Fluorescent-Inverted Microscope; Shenzhen Aosvi Ltd., Shenzhen, People’s Republic of China). Temperature, light, and other external factors cause physical and chemical changes in the liposome membrane, which result in the inevitable changes in liposome membrane permeability and make the drug leak out of the liposomes. Except for encapsulation efficiency, leakage rate is another important factor to evaluate the performance of liposomes during their storage. The leakage rate (Lr%) was measured as follows:
|
|
where Ei is the encapsulation efficiency during storage period and E0 is the initial encapsulation efficiency.
In vitro permeation and distribution studies
In vitro permeation of MA liposomes was evaluated by the Franz diffusion cell using full-thickness dorsal skin excised from SD rats (200–250 g). The dorsal hair was shaved using a shaving machine, and residual fur was removed with 8% Na2S solution. After 24 hours, the rats were sacrificed to harvest the skin. Subcutaneous connective tissues and fat were carefully removed. Hairless rat skin was excised and mounted between donor compartment and receptor compartment of the Franz diffusion cell. A total of 0.4 mL of MA liposomes was applied on the skin and allowed to spread over the skin. The upper donor compartment was covered with parafilm, and the lower receptor compartment was filled with 6.5 mL of normal saline (NS) and maintained at 37°C in water bath. Samples (0.5 mL) were taken from the receptor compartment at time intervals of 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, 24 hours, and 48 hours, and then added into volumetric flask, diluted to 1 mL by triton solution (10%), and filtered using a 0.45 μm filter membrane for analysis. Each experiment was performed in triplicate. All samples were analyzed by HPLC. Percentage of MA permeation (P%) was calculated as follows:
|
|
where Cn is the drug concentration of the nth sampling point (mg/mL), Ci is the drug concentration of the ith sample point (mg/mL), V is the total volume (6.5 mL) of liquid in receiving pool, Vi is the volume (0.5 mL) of the ith sampling points, and M is the mass of MA.
In vitro permeation of the changing skin every 4 hours was augmented. The skin became inelastic, matt, and overly-transparent in 4 hours using the conventional method mentioned earlier, which inferred that the structure and selective permeability of the skin were destroyed. We adopted an improved method of changing skin every 4 hours for in vitro permeation to yield more accurate results. The original skin was washed three times by a small amount of normal saline. Collection of all the washed normal saline and residual of MA solution or MA liposomes was transferred to a new piece of skin. The original skin was washed three times by NS, and all the washing solution was collected and applied on the new skin surface.
In vitro skin localization of MA delivered by liposomes was studied and compared. Hairless rat skin was excised and mounted between donor compartment and receptor compartment of Franz diffusion cell, the following operations were the same as the aforementioned permeation study. The same mass of skin was cut off and collected at 0.5 hour, 1 hour, and 2 hours, and the surface was gently wiped with a cotton swab dipped with distilled water. The skin was homogenized with a biopsy punch and then placed into a centrifuge tube. Five microliters of distilled water were added into a centrifuge tube with the homogenized skin to extract MA by shaking for 30 minutes at room temperature, it was then stored at 4°C for 12 hours. Dispersions were removed and centrifuged at 10,000 rpm for 10 minutes. MA in the supernatants was determined by HPLC.
Determination of MA by HPLC
Hypersil BDS C18 (5 μm) chromatographic column (4.6×200 mm; Dalian Elite Analytical Instruments Co., Ltd., Dalian, People’s Republic of China) was used for HPLC. The mobile phase was acetonitrile–water 26:74 (v/v). The flow rate was 1.0 mL/min. The column temperature was 30°C. The detection wavelength was 205 nm, and the injection volume was 20 μL.
In vivo wound healing
SD rats were kept in individual cages with food and water and maintained at a room temperature of 20°C–25°C with alternating cycles of 12 hours light and 12 hours dark time. All the animals were anesthetized with intraperitoneal pentobarbital Na (40 mg/kg body weight) injection. The dorsal hair was shaved using a shaving machine, and the residual fur was removed with 8% Na2S solution.
Burn wound mode was induced by a cylindrical-shaped stainless steel cup with a radius of 10 mm.24 The stainless steel cup was placed in hot water (~100°C) for 1 minute and then put on the back of rats and held for 20 seconds to reach the standard of second-degree burns. Six burns were induced in each rat in the NS control group and MA solution group, while four burns were induced in the MA liposomal formulation groups. The wound sites were bound with cotton and gauze was prewet with normal saline, remarkable wounds were formed after 2 days. Experimental wounds in each group came from five different wound-induced rats (n=5). MA formulations and NS were repeatedly applied once a day to the burned sites for 12 days. The diameters of the wounds were monitored periodically by measuring the largest distance between two opposite outside edges of the wound margin.
The efficacy index (EI) of the wound contraction rate was calculated and presented by reduction percentage in the wound size. The EI% of the wound size was calculated using the following equation:
|
|
where Di refers to the diameter values (mm) measured at days 3, 6, 9, and 12, while D0 refers to the baseline value measured before treatment. The EI was evaluated on a four-rank scale specified in Table 1.
  | Table 1 Significance evaluation for EI |
Statistical analysis
Statistically significant differences were determined using two-tailed Student’s t-test. A value of P<0.05 was considered significant.
Results
RSM results
Models and data analysis
A total of 15 experiments, a 12-factorial experimental CCD together with three replications at the center points, were employed for parameter optimization. The variables were coded as follows:
|
|
where X1, X2, and X3 are the coded values for ratio of egg yolk lecithin to cholesterol, concentration of MA, and the stirring speed, respectively. A, B, and C are actual values of relevant test variable, respectively. In this experiment 6 (6:1), 20 (mg/mL), and 480 (rpm) are the actual values of A, B, and C, respectively, at the center point of the investigated field. Variable values are given in Table 2. The experimental design protocols and relevant results are presented in Table 3.
  | Table 3 Optimization on preparation conditions of MA double-emulsion liposomes using central composite design |
CCD results are presented in Table 4 and fitted with a polynomial equation (Equation 8). The fitted equation (in terms of coded values) for prediction of the maximum drug loading efficiency is as follows:
|
|
  | Table 4 Estimate of regression model |
where Y is the drug loading efficiency of MA double-emulsion liposomes. The estimated values of regression coefficients are displayed in Table 4.
Confidence level of this quadratic model was confirmed by coefficient R2 and F value that revealed that it was a highly significant model with the corresponding coefficient R2=0.9470, which demonstrated that 94.70% of the variability in the response could be explained by the model. In addition, the values of F and P in the total model were 9.93 and 0.0105, respectively, which indicated that this regression model fits the experimental results pretty well with high reliability and small experimental errors. This model could be used to analyze and forecast the drug loading efficiency of MA liposomes. Significance of each coefficient represented by P-values is shown in Table 4. The P-values implied that the main significant effects were exerted by the ratio of egg yolk lecithin to cholesterol and the concentration of MA, whereas the stirring speed had negligible effect. Linear, quadratic, three cross products, and total model adopted to test the significance of the model, and the results are shown in Table 5. The smaller the P-value, the more significant the effect on the drug loading efficiency of MA liposome. Linear and quadratic products were two highly marked factors for the drug loading efficiency of MA liposomes, and cross product was the negligible variable.
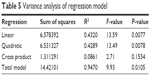  | Table 5 Variance analysis of regression model |
Response surface graphical analysis
Three-dimensional plots were employed as graphical representations of regression equation. Each surface representing two different variable combinations contributed to understanding the interactions among the three variables and locating the optimum level of each variable. The results of each experimental factor and interaction of each factor could be visually observed. The steeper the surface, the more significant the effect was. It was obviously manifested that concentration of MA and ratio of egg yolk lecithin to cholesterol were the two main factors affecting the drug loading efficiency in this experiment (Figure 2). The drug loading efficiency increased with an increase in each factor and then gradually reduced when it reached a certain height. The surface in these three dimensional plots opened downwards. The height peak value was the maximum point of the drug loading efficiency in these three-dimensional plots.
The optimum condition was obtained by SAS 9.0 software for achieving maximum drug loading efficiency, and coded variables were X1=-0.539345, X2=0.018550, and X3=0.1345. It was predicted that the maximum drug loading efficiency is 5.92%. DL% of three experiments from the 13th to the 15th trial under the optimum conditions was 5.81%, 5.84%, and 5.67%, respectively (mean value was 5.77%, and relative standard deviation was 1.56%), which confirmed the predicting accuracy of the model; these results were consistent with the predictive value (5.92%) mentioned earlier using SAS software analysis. The similar data between the predicted and experimental results verified the validity of the model.
Size distribution, zeta potential, and SEM of MA double-emulsion liposomes and neat liposomes
Liposomes with particle size of 100–300 nm had excellent potency to transport hydrophilic compounds between and into cell membranes because of their compatibility with cell lipid layer.25 Mean diameter and the polydispersity index (PDI) of MA double-emulsion liposomes prepared under the optimum conditions were 151 nm (Figure 3A [a]) and 0.243, respectively, and the zeta potential of MA liposomes was -48 mV, whereas the mean diameter, PDI, and zeta potential of neat double-emulsion liposomes were 217 nm (Figure 3A [b]), 0.278 mV, and -54 mV, respectively. Upon comparing these characteristics with those of MA film dispersion liposomes (particle size of 293 nm and PDI of 0.349) obtained in our previous work,18 it was observed that MA double-emulsion liposomes had more excellent potency to transport hydrophilic compounds.
SEM morphologies (original magnification 20,000) of MA double-emulsion liposomes and neat liposomes (Figure 3B and C) showed well-formed vesicles with spherical-shaped structure and homogeneous size distribution. Particle sizes of MA double-emulsion liposomes were <200 nm, which were close to the sizes of Malvern zeta-size Nano ZS.
Physical stability of MA liposomes
Liposome stability had a vital and direct relation with relative particle size. MA double-emulsion liposomes and film dispersion liposomes presented homogeneously intuitive appearance after 5 months (Figure 4A and B), while MA ethanol-injection liposomes exhibited aggregation and flocculation (Figure 4C). Small aggregations or flocculation of MA film dispersion liposomes could be observed using a fluorescent inverted microscope, with magnification of 400× (Figure 4B), whereas large aggregations of ethanol-injection liposomes clearly appeared with a magnification of 100× (Figure 4C). Neither aggregation nor flocculation was observed in MA double-emulsion liposomes, with magnification of 400× (Figure 4A), which manifested that MA double-emulsion liposomes had the most excellent stability.
Leakage rate of MA liposomes
According to the quantitative assay of MA by HPLC, the leakage rate at different temperatures and time was calculated using Equation 4. The leakage rate of MA double-emulsion liposomes increased slightly and slowly with an increase in temperature and time. The leakage rate of MA double-emulsion liposomes was lower than 5% at 4°C and less than 12% even at 37°C in a month (Figure 5A), which was superior to MA liposomes made by film dispersion method with the leakage rate more than 15% in 10 hours at 4°C in our previous work (Figure 5B).18 It implied that W1/O/W2 double emulsions could help liposomes resist thermodynamic motion of higher temperature. Ultimately, all resulting MA liposomes were stored at 4°C.
Results of in vitro permeation
MA film dispersion liposome was abbreviated as liposome A, and MA double-emulsion liposome was abbreviated as liposome B. The PEG-modified liposomes prepared by the film dispersion method and the double-emulsion method were named as liposome C and liposome D, respectively. NS was used as control. Two methods, namely using the same skin throughout the experiment and the changing skin every 4 hours, were adopted in this experiment. In the first method with the same skin from the beginning to the end, the permeation of MA in liposome D and liposome B increased rapidly at the initial stage before the 12th hour. Unexpected results were observed, that is, the MA transmission rate in liposome A increased rapidly after 8 hours, and was ~90% in 48 hours (Figure 6A). Because the skin rotted and became inelastic, with loosening and high transparency in 4 hours (Figure 6B), it is inferred that the texture and selective permeability of the skin were destroyed making the free drugs (MA solution) easily penetrate the skin. In contrast, encapsulation efficiency of liposome A was lowest, revealing more free MA, and hence the highest percentage of MA permeation was observed in the liposome A group in 24 hours, as shown in Figure 6A. In order to simulate the actual skin and obtain reliable results, the changing skin every 4 hours was adopted to reflect the real skin’s condition; liposome B prepared by the double-emulsion method rendered more latent capacity than liposome A prepared by the film dispersion method to deliver MA through the skin, and PEG-modified liposomes D and C generated more power and efficiency than those (liposomes B and A) without modification (Figure 6C).
MA delivered by liposomes and skin localization
In order to evaluate their storage stability, process of skin permeation, and capacity of delivering MA to skin site, MA masses delivered by liposomes and localized in skin were also compared and investigated (Figure 7). All liposomal groups demonstrated more comprehensive potency of delivering MA through skin and/or storing MA in skin than conventional MA solution. Among them, liposome B prepared by the double-emulsion method possessed better efficiency. Liposomes D, namely coating with PEG, promoted the delivery or storage capacity of MA and quickly reached the localization balance
  | Figure 7 Madecassoside delivered by liposomal formulations and skin localization. |
Results of in vivo wound healing effect
MA was helpful in reducing scars and improving the wound healing progress. However, so far, neither skin delivery nor MA liposome application has been investigated for the effect of liposome-based drug carrier systems on wound healing effect. Therefore, the present study was conducted with the aim of evaluating the cutaneous wound healing potential of MA liposomes prepared under optimal process conditions. Experiments on wound-induced rats powerfully and directly manifested the superior therapeutic wound healing effect of MA double-emulsion liposomes (Figure 8A).
Compared with NS control, MA solution and all liposomal formulation groups showed enhanced wound healing process at different degrees. No extremely significant improvement in EI was observed in any group at day 6 (Table 6). However, induced wounds treated with liposome B and liposome D exhibited more extremely significant wound closure (EI% was 98.44%±0.59% and 99.30%±0.21%, respectively, P<0.001 vs NS control) than those treated with liposome A (82.41%±2.75%, P<0.01 vs NS control) and liposome C (87.72%±6.12%, P<0.01 vs NS control) at day 12. Compared with MA solution, liposome A exhibited significant effect (P<0.05 vs MA solution), and liposomes B, C, and D demonstrated extremely significant improvement (P<0.01 vs MA solution) at day 12. Among them, liposome D possessed significantly superior therapeutic effects throughout the whole treatment process (Figure 8B).
  | Table 6 EI% values and their significance of treatment for the used formulations at days 6 and 12 (n=5) |
Effects of liposomal delivery system on wound contraction and reepithelialization were investigated (Figure 8A). Wound closure was analyzed as a percentage of reduction of wound area at days 3, 6, 9, and 12. Liposome B prepared by the double-emulsion method and liposome D prepared by double-emulsion with PEG-coating rendered outstandingly complete healing at day 12 of treatment. The results demonstrated that the healing effect of MA on scald wound had been improved significantly after being prepared and delivered in the form of liposomal formulations, especially in the double-emulsion liposomes system. Hence, double-emulsion liposomes might be a promising carrier for MA to accelerate the wound healing process, with the possibility of reducing scar formation.
Discussion
In this study, we aimed to develop a drug delivery system of MA-encapsulated liposomes with higher encapsulation efficiency and more narrow size distribution of small liposomal particles to promote cutaneous wound healing. Although the same double-emulsion method was used for preparation, the average particle size of neat liposomes (217 nm) was larger than that (151 nm) of MA double-emulsion liposomes. The same phenomenon appeared for the film dispersion method; the average particle size of neat liposomes was 612 nm and much larger than that (293 nm) of MA film dispersion liposomes. The reason why the average particle size of MA liposomes was smaller than that of neat liposomes might be due to the more ionic strength in MA liposomes, which led to the gathering trend of negative and positive charges of polarity zones dipole of lecithin, and the positive charge of choline approached the surface of liposomes, which gradually increased the adsorption of anions, until the accumulated negative charge had a balance with the cation in the suspension of liposomes. Liposome suspension gathered more easily at low ionic strength, and MA, just like cholesterol, would generate more anions to be attracted by the positive charge of phosphatidylcholine, which hampered membrane fluidity to form larger sized particles and contributed to the good stability of the phospholipid bilayer. Moreover, there seemed to be a surface adhesion effect between MA and liposomes, which weakened the aggregation and fusion effect between liposomal particles. Meanwhile, the adsorption and compatibility capacity of liposomes led toward the inclination of aggregation in neat liposomes and conversion into larger particles in a short time. Both surface and interaction effects between MA and liposomes will be investigated in further follow-up studies.
This study evaluated the improved effects of liposomes on delivering MA through the skin, storing MA into the skin, and the accelerated dermal wound repair process in different liposomal formulation groups in the wound-induced rat model. Liposomes belong to colloidal dispersion systems that are thermodynamically unstable. Liposome particles are prone to aggregate, flocculate, and fuse, so as to produce precipitates after long time of storage. The MA double-emulsion liposomes had more excellent stability and avoided aggregating in 5 months, which implied that W1/O/W2 double emulsions could resist thermodynamic motion at higher temperature. Besides remarkable stability and ease of scale-up, MA double-emulsion liposomes possess prominent capacity of delivering MA into the epidermis and dermis and superior performance in enhancing MA delivery to wound sites, healing effect, and healing progress. The fluidity of liposomal membrane plays a key role in affecting the skin penetration of liposomes; the more fluid the liposomal membrane was, the more permeable the liposomes were.26 Therefore, PEG was selected as a surfactant so as to improve the hydrophilicity and flexibility of MA liposomes. Skin penetration and absorption performances of MA liposomes, as well as in vivo wound healing, were greatly improved after PEG modification. MA liposomes ensured improving hydrophilicity, flexibility, and adhesion force by PEG modification, which led to relatively higher encapsulation efficiency, improved stability, and enhanced wound healing activity.
The permeation study was designed to mimic the absorption and diffusion of drugs through the skin. Although the magnetic stirring in the receptor compartments served the same effect as human blood flow, the isolated skin rotted and the skin texture became inelastic and loose under the conventional method of 4 hours at 37°C. Neither accurate nor reliable results could be yielded for evaluation of the long-term effect with rotted and over-transparent skin. Therefore, a method of changing skin regularly every 4 hours was established for the first time to ensure compact flexibility of the skin and evaluate the long-term effect of MA liposomes in this experiment. The transdermal property and wound cure effect of MA double-emulsion liposomes were superior to those prepared by the film dispersion method, and both the methods were endowed with more excellent performance by PEG modification.
Conclusion
In this study, according to RSM analysis, the main significant effects were the ratio of egg yolk lecithin to cholesterol and the concentration of MA, and the optimum conditions of MA double-emulsion liposomes were 20.18 mg/mL of MA, 4.9213 of ratio of egg yolk lecithin to cholesterol, 500 rpm of stirring speed with average particle size of 151 nm and encapsulation efficiency of 70.14%. Compared to the corresponding results of average particle size and encapsulation efficiency (293 nm and 40.90%) of MA liposomes obtained by the film dispersion method in our previous work,18 MA double-emulsion liposomes were more ideal and competent to meet the transdermal requirements and improve cutaneous wound healing.
Moreover, leakage rate, in vitro release, and in vivo wound-healing effect were explored for the first time on MA liposomes prepared by the double-emulsion method. The leakage rate of MA liposomes made by the film dispersion method was >15% in 10 hours at 4°C, while the leakage rate of MA double-emulsion liposomes was <5% at 4°C and <12% even at 37°C in 1 month. Microscopic appearance observation exhibited consistent results in the leakage rate; MA double-emulsion liposomes had the most excellent stability in 5 months. In vitro percutaneous absorption and diffusion experiments showed that the liposomes prepared by the double-emulsion method possessed the maximum transmittance and the largest capacity of storage of MA in skin, which made MA to be absorbed and permeated quickly by the skin. Overall, MA double-emulsion liposomes had smaller particle size, higher encapsulation efficiency, better stability, and more excellent capacities of permeation and distribution in skin so as to possess superior performance of burn wound healing effect. Double-emulsion liposome formulation might become an ideal and promising pharmaceutical preparation for MA to enhance the healing effect of a wide variety of wounds, including surgery sections, ulcers, burns, and scalds.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No 81473422) and the Fundamental Research Funds for the Central Universities (SCUT-2015zz051). The authors alone are responsible for the content and writing of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of terminalia chebula on dermal wound healing in rats. Phytother Res. 2002;16(3):227–231. | ||
Hardwicke J, Ferguson EL, Moseley R, Stephens P, Thomas DW, Duncan R. Dextrin-rhEGF conjugates as bioresponsive nanomedicines for wound repair. J Control Release. 2008;130(3):275–283. | ||
Atiyeh BS, Hayek SN. An update on management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Med Chem Rev. 2004;1(2):111–121. | ||
Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars. Am J Clin Dermatol. 2003;4(4):245–272. | ||
Stella M, Castagnoli C, Gangemi EN. Postburn scars: an update. Int J Low Extrem Wounds. 2008;7(3):176–181. | ||
Liu M, Dai Y, Yao XJ, et al. Anti-rheumatoid arthritis effect of madecassoside on type II collagen-induced arthritis in mice. Int Immunopharmacol. 2008;8(11):1561–1566. | ||
Bian DF, Liu M, Li Y, Xia Y, Gong Z, Dai Y. Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J Biochem Mol Toxicol. 2012;26(10):399–406. | ||
Cheng CL, Guo JS, Luk J, Koo MW. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci. 2004;74(18):2237–2249. | ||
Gallego A, Ramirez-Estrada K, Vidal-Limon HR, et al. Biotechnological production of centellosides in cell cultures of Centella asiatica (L) Urban. Eng Life Sci. 2014;14(6):633–642. | ||
Li H, Gong X, Zhang L, et al. Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine. 2009;16(6–7):538–546. | ||
Rougier A, Humbert P. Clinical efficacy on epidermal wound healing of topically applied madecassoside associated with copper/zinc/manganese salts. J Am Acad Dermatol. 2008;58(2):AB144. | ||
Qi R, He RR, Li BY, Liang BW. Progress of transdermal drug delivery systems. J External Ther Tradit Chin Med. 2007;16(1):3–6. | ||
Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303(9):607–621. | ||
Sharma J, Lizu M, Stewart M, et al. Multifunctional nanofibers towards active biomedical therapeutics. Polymers. 2015;7(2):186–219. | ||
Lu Y, Huang JN, Yu GQ, et al. Coaxial electronspun fibers: applications in drug delivery and tissue engineering. Wiley Interdiscip Rev Nanomed Nanobiotechnol. Epub 2016 Feb 5. | ||
Masini V, Bonte F, Meybeck A, Wepierre J. Cutaneous bioavailability in hairless rats of tretinoin acid in liposomes or gel. J Pharm Sci. 1993;82(1):17–21. | ||
Hou GR, Zeng K, Zhou ZG. The application and action mechanism of liposome in skin. J Dermatol Venereol. 2003;25(4):15. | ||
Wang H, Liu M, Du S. Optimization of madecassoside liposomes using response surface methodology and evaluation of its stability. Int J Pharm. 2014;473(1–2):280–285. | ||
Matsumoto S, Kita Y, Yonezawa D. An attempt at preparing water-in-oil-in-water multiple-phase emulsion. J Colloid Interface Sci. 1976;57(2):353–361. | ||
Miquel P, Mercè F, Joan E. Liposomes obtained by the ethanol injection method. Int J Pharm. 1993;95(93):51–56. | ||
Takeuchi H, Yamamoto H, Toyoda T, Toyobuku H, Hino T, Kawashima Y. Physical stability of size controlled small unilamellar liposomes coated with a modified polyvinyl alcohol. Int J Pharm. 1998;164(1–2):103–111. | ||
Shehata T, Ogawara K, Higaki K, Kimura T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int J Pharm. 2008;359(1–2):272–279. | ||
Fan Y, Liu J, Wang D, et al. The preparation optimization and immune effect of epimedium polysaccharide-propolis flavone liposome. Carbohydr Polym. 2013;94(1):24–30. | ||
Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of datura alba on burn wounds in albino rats. J Ethnopharmacol. 2002;83(3):193–199. | ||
Barry BW. Drug delivery routes in skin: a novel approach. Adv Drug Deliv Rev. 2002;54(suppl 1):S31–S40. | ||
Coderch L, Fonollosa J, De Pera M, Estelrich J, De La Maza A, Parra JL. Influence of cholesterol on liposome fluidity by EPR. Relationship with percutaneous absorption. J Control Release. 2000;68(1):85–95. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

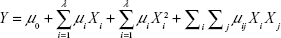
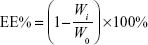
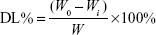
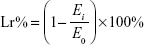
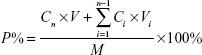
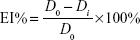
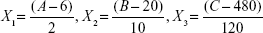

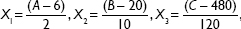 when the concentration of MA was 40 mg/mL, the coded value was 2.
when the concentration of MA was 40 mg/mL, the coded value was 2.