Back to Journals » Drug Design, Development and Therapy » Volume 13
Increased branching and sialylation of N-linked glycans correlate with an improved pharmacokinetic profile for BAY 81–8973 compared with other full-length rFVIII products
Authors Teare JM, Kates DS , Shah A, Garger S
Received 20 September 2018
Accepted for publication 11 January 2019
Published 22 March 2019 Volume 2019:13 Pages 941—948
DOI https://doi.org/10.2147/DDDT.S188171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
John M Teare,1 David S Kates,1 Anita Shah,2 Stephen Garger1
1Biological Development, Bayer US LLC Pharmaceuticals, Berkeley, CA, USA; 2Pharmacokinetics Pharmacodynamics Hematology, Bayer US LLC Pharmaceuticals, Whippany, NJ, USA
Background: BAY 81–8973 (Kovaltry) is an unmodified full-length recombinant factor VIII (rFVIII) for treatment of hemophilia A. The BAY 81–8973 manufacturing process results in a product of enhanced purity with a consistently high degree of branching and sialylation of N-linked glycans. This study evaluated whether a relationship exists between N-linked glycosylation patterns of BAY 81–8973 and two other rFVIII (sucrose-formulated rFVIII [rFVIII-FS; Kogenate FS]) and antihemophilic factor (recombinant) plasma/albumin-free method (rAHF-PFM; Advate) and their pharmacokinetic (PK) characteristics.
Materials and methods: N-linked glycans or terminal carbohydrates were enzymatically removed from immobilized BAY 81–8973, rFVIII-FS, and rAHF-PFM proteins and analyzed using high-performance liquid chromatography to determine the percentage of individual N-linked glycan structures and degree of sialylation of each structure. PK data were available from two separate phase 1 crossover studies in which the PK profile of BAY 81–8973 was compared with that of rFVIII-FS (n=26) and rAHF-PFM (n=18) in patients with severe hemophilia A who received a single 50 IU/kg dose of each product.
Results: BAY 81–8973 and rFVIII-FS had increased N-linked glycan branching with higher levels of sialylation compared with rAHF-PFM. Levels of trisialylated glycans were 29.0% for BAY 81–8973 vs 11.5% for rFVIII-FS and 4.8%–5.5% for rAHF-PFM; tetrasialylated glycans were 12.0% vs 2.8% and 0.6%, respectively. Degree of sialylation was 96% for BAY 81–8973, 94% for rFVIII-FS, and 78%–81% for rAHF-PFM. Based on chromogenic assay results from the single-dose phase 1 PK studies, BAY 81–8973 half-life was 15% longer than that for rFVIII-FS and 16% longer than rAHF-PFM.
Conclusion: Increased N-glycan branching and sialylation were seen for BAY 81–8973 vs rFVIII-FS and rAHF-PFM. Improved PK for BAY 81–8973 relative to rFVIII-FS and rAHF-PFM as seen in single-dose crossover PK studies might be related to this greater level of branching and sialylation, which can prolong the time BAY 81–8973 remains in the circulation.
Keywords: clearance, glycan structure, glycosylation, half-life
Introduction
Hemophilia A is a congenital bleeding disorder resulting from insufficient levels of clotting factor VIII (FVIII).1 Patients with severe hemophilia A have FVIII levels <1% of normal, putting them at risk for spontaneous bleeding episodes into tissues, muscles, and joints; repeated bleeding into joints can lead to chronic hemophilic arthropathy. The current standard of care for hemophilia A is prophylactic infusions of a FVIII product. For standard-acting FVIII products, infusions are typically administered every other day or three times per week, given the ~12-hour half-life of FVIII,2,3 with frequent infusions presenting a considerable burden for patients and their caregivers.3
The circulatory half-life of recombinant FVIII (rFVIII) products is affected by glycosylation of the FVIII protein, including N-linked glycan branching and terminal sialic acid occupancy, primarily through receptor-mediated hepatic clearance (eg, asialoglycoprotein receptor [ASGPR] and lipoprotein receptor-related protein [LRP]).4,5 Through glycosylation, a post-translational modification, carbohydrate structures are covalently attached to a protein backbone;6 the most common structures are N-linked and O-linked glycans.7 The glycosylation process begins during translocation of the protein into the endoplasmic reticulum, with further modification of the carbohydrate structures on the nascent protein in the Golgi.6 These protein-bound carbohydrates are important for the structure and function of the FVIII protein, contributing to optimal protein folding and binding to ASGPR via N-linked glycans in the FVIII B domain, which plays a role in protein clearance;4,6 glycans not capped with sialic acid are rapidly cleared.6,8,9
BAY 81–8973 (Kovaltry, Bayer, Berkeley, CA, USA) is an unmodified, full-length rFVIII approved for prophylaxis and on-demand treatment of bleeding episodes in patients with hemophilia A.10 BAY 81–8973 is translated as a single-chain 330-kD precursor with a domain structure of A1-A2-B-A3-C1-C2 subunits.10 Post-translational proteolytic processing yields an A1-A2-B heavy chain and an A3-C1-C2 light chain to form a large heterodimeric structure linked by a divalent cation bridge.10 BAY 81–8973 is highly glycosylated, with multiple N-linked and O-linked glycans present on the structure, predominantly within the B domain, as well as six highly occupied tyrosine sulfation sites.10
The BAY 81–8973 manufacturing process is based on that used for sucrose-formulated rFVIII (rFVIII-FS; Kogenate FS, Bayer), with changes and enhancements made to improve production efficiency, further augment pathogen safety, and eliminate animal- and human-derived raw materials from the production processes.10 Both rFVIII-FS and BAY 81–8973 are full-length recombinant human FVIII products expressed in baby hamster kidney (BHK21) cells. The BHK21 cell line used for BAY 81–8973 was developed by introducing the gene for human heat shock protein 70 (HSP70) into the cell line used to manufacture rFVIII-FS (a full-length rFVIII expressed in BHK cells in the presence of human purified plasma); this change improved cell line robustness and productivity.10 Co-expression of HSP70 along with the FVIII gene in the production cell line is likely responsible for improving stable and reproducible FVIII expression by inhibiting apoptosis during cell culture, thus keeping FVIII producing cell viability higher. It is believed that HSP70 facilitates appropriate protein folding of the nascent intracellular rFVIII polypeptides.11,12 The chaperone function of HSP70 co-expressed with rFVIII may result in larger antennary glycan structures and a higher percentage of sialylation by reducing misfolded proteins during transport through the endoplasmic reticulum and golgi where N-linked glycosylation occurs intracellularly.13 Overall, the BAY 81–8973 manufacturing process results in a stable product with a consistently high degree of sialylation of N-linked glycans on the molecular surface, as well as enhanced purity, with low levels of high-molecular-weight impurities and protein aggregates.10
Given that rFVIII products have different patterns of glycosylation and sialylation, our objective was to evaluate the correlation between the N-linked glycosylation of BAY 81–8973, rFVIII-FS, and antihemophilic factor (recombinant) plasma/albumin-free method (rAHF-PFM; Advate, Shire, Westlake Village, CA, USA) and their pharmacokinetic (PK) profile.
Materials and methods
Glycan analysis
The N-linked glycans or terminal carbohydrates were enzymatically removed from immobilized BAY 81–8973, rFVIII-FS, and rAHF-PFM, and analyzed using high-performance liquid chromatography (HPLC) to determine the percentage of individual N-linked glycan structures (neutral, mono-, di-, tri-, and tetra-antennary glycans) and the degree of sialylation of these N-glycans. Specifically, the oligosaccharide map method was used to provide a quantitative chromatographic profile of all N-linked glycan structures. Using this method, N-linked oligosaccharides were released from the rFVIII samples and bound to nylon spin filters using PNGase F (Prozyme/Glyko, Hayward, CA, USA). The released N-glycans were then labeled with anthranilic acid (2-AA; Spectrum Chemicals, New Brunswick, NJ, USA) using reductive amination with sodium cyanoborohydride (reagent grade) as the reductant. The 2-AA labeled components of the rFVIII preparations were separated into peaks using HPLC (Agilent, Santa Clara, CA, USA) in normal phase/ion exchange mixed mode (Shodex Asahi Pak NH2P-50 2D, Showa Denko America, Inc., New York, NY, USA) and using fluorescence emission at 425 nm after excitation at 360 nm. This procedure separates the labeled components primarily according to their ionic charge, which is dependent on the number of sialic acid groups covalently bound to the oligosaccharides, and also by their size and structure.14,15
The sialylation and alpha-galactose method was used to quantify the glycosylation of each rFVIII product with respect to termination of branching of the N-linked glycan structures. This method involves binding the rFVIII samples to a nylon membrane, followed by the enzymatic release of the exposed α-linked galactose residues using α-galactosidase (Prozyme/Glyko). This step is followed by enzymatic release of the exposed β-linked galactose residues (uncapped) using β-galactosidase (Prozyme/Glyko). The “capped” β-linked galactose residues are then released using a combination of β-galactosidase and sialidase A (Prozyme/Glyko). Each digest was then analyzed using high pH anion-exchange chromatography, coupled with a pulsed amperiometric detector (HPAEC-PAD; Dionex, Sunnyvale, CA, USA), to measure the relative amount of galactose released during the respective digests. Because β-galactosidase is a glycoprotein, blank digestions (with no rFVIII) were run for both digests, the β-galactosidase and the sialidase A+β-galactosidase sample preparations, and subtracted from the peak area obtained in those digests. By calculating specific ratios for these adjusted peak areas, values for the percentage of α-galactose and the percentage of sialic acid capping were determined.
Pharmacokinetic comparison
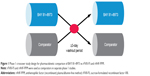
The PK of BAY 81–8973 was compared with that of rFVIII-FS and rAHF-PFM in two separate randomized phase 1 crossover studies (study design is shown in Figure 1).16,17 Each study enrolled patients aged 12–65 years (rFVIII-FS comparison) or 18–65 years (rAHF-PFM comparison) with severe hemophilia A ≥150 exposure days to any FVIII product, and no history of FVIII inhibitors.16,17 All patients received a single 50 IU/kg infusion of BAY 81–8973 or the comparator product, followed by crossover to a single infusion of the other product after a ≥3-day washout period (Figure 1).16,17 In both studies, plasma samples were collected predose and 0.25, 0.5, 1, 3, 6, 8, 24, 30, and 48 hours postdose for determination of PK parameters using one-stage and chromogenic assays.16,17
Results
Glycan analysis
N-glycan analysis of different batches of BAY 81–8973 (n=65), rFVIII-FS (n=3), and rAHF-PFM (n=6) indicated that BAY 81–8973 and rFVIII-FS had higher levels of sialylation and increased N-linked glycan branching compared with rAHF-PFM (Figures 2 and 3). Levels of trisialylated glycans were 29.0% for BAY 81–8973, 11.5% for rFVIII-FS, and 4.8%–5.5% for rAHF-PFM. For tetrasialylated glycans, levels were 12.0%, 2.8%, and 0.5%–0.6%, respectively. The degree of sialylation was higher for BAY 81–8973 (96%) compared with rFVIII-FS (94%) and rAHF-PFM (78%–81%).
  | Figure 2 Glycan structures in BAY 81–8973, rFVIII-FS, and rAHF-PFM. |
Pharmacokinetic comparison
Twenty-six patients (median age=29.5 years) completed the BAY 81–8973 vs rFVIII-FS PK study, and 18 patients (median age=36.0 years) completed the BAY 81–8973 vs rAHF-PFM PK study.16,17 In both studies, BAY 81–8973 displayed an improved PK profile vs the comparator product (Table 1).16,17
  | Table 1 Pharmacokinetic results after single 50 IU/kg dose administration of BAY 81–8973, rFVIII-FS, and rAHF-PFM using the chromogenic assay |
The half-life of BAY 81–8973 was 10% and 15% longer than that for rFVIII-FS (P=0.01 and P=0.002), based on the one-stage assay and chromogenic assay, respectively, and the area under the curve (AUC) was 19% higher for BAY 81–8973 vs rFVIII-FS, based on both assays (P<0.0001 for one-stage assay; P=0.0003 for chromogenic assay).16 Compared with rAHF-PFM, BAY 81–8973 had a half-life that was 24% longer based on the one-stage assay and 16% longer based on the chromogenic assay (P<0.0001 for both assays); AUC from time 0 to infinity was 32% and 48% higher, respectively, for BAY 81–8973 vs rAHF-PFM with both assays (P<0.0001).17 Clearance was significantly slower for BAY 81–8973 vs rFVIII-FS and rAHF-PFM with both assays (P<0.0001).16,17 The FVIII concentration-time profiles showed that, at most time points, FVIII levels were higher for BAY 81–8973 compared with rFVIII-FS and rAHF-PFM (Figure 4).17,23
  | Figure 4 FVIII concentration-time curves after single 50 IU/kg dose administration of BAY 81–8973, rFVIII-FS, and rAHF-PFM using the chromogenic assay.17,23 |
Correlation of glycan branching and sialylation with half-life
Increases in the percentage of sialylated tri-antennary and tetra-antennary N-glycans correlated well with longer half-life (adjusted R2=0.978 and 0.892 for tri-antennary and tetra-antennary N-glycans, respectively; Figure 5). Higher percentages of sialylation (ie, sialic acid capping) correlated with a longer half-life (adjusted R2=0.697), but the relationship was not as strong as that for glycan branching (Figure 5).
Discussion
Differences were seen in N-glycans among the three rFVIII products studied, with greater glycan branching and sialylation seen with BAY 81–8973 compared with rFVIII-FS and rAHF-PFM. This observation is consistent with a previously published report by Baunsgaard et al.18 In addition, in published PK studies, BAY 81–8973 showed an improved PK profile (longer half-life, higher AUC, slower clearance) vs the two comparator products.16,17
Improved PK for BAY 81–8973 vs rAHF-PFM was also seen in simulations based on population PK (popPK) models. In a popPK model developed using chromogenic assay data from the phase 1 BAY 81–8973 vs rAHF-PFM study,17 median time to reach a FVIII level of 1 IU/dL was estimated to be ~18 hours longer for BAY 81–8973 vs rAHF-PFM (80.5 vs 62.5 hours) after a 25 IU/kg infusion. In a popPK analysis based on data extracted from the Web-Accessible Population Pharmacokinetic Service–Hemophilia (WAPPS-Hemo) database, the estimated terminal half-life for BAY 81–8973 was 13.1 hours, compared with 10.5 hours for rAHF-PFM.19
The circulatory half-life of rFVIII products is affected by glycosylation of the FVIII protein, including N-linked glycan branching and terminal sialic acid occupancy, primarily through receptor-mediated hepatic clearance (eg, ASGPR and LRP).4,5 The longer circulation time and longer half-life for BAY 81–8973 compared with rFVIII-FS and rAHF-PFM might be related to the increased branching and sialylation of the N-glycans in BAY 81–8973. This increased glycan branching and sialylation may shield BAY 81–8973 from clearance by known hepatocyte receptors, such as ASGPR, which bind to exposed galactose on the glycan structure, as well as LRP. Similar observations have been made with other clotting factors such as recombinant activated factor VII and recombinant factor IX.20,21
Conclusion
The differences seen in N-glycan structure and degree of sialylation of BAY 81–8973, rFVIII-FS, and rAHF-PFM may influence their PK profile, with BAY 81–8973 displaying improved PK compared with the comparator products; increased glycan branching and sialylation correlated well with increased half-life. However, further study is needed to determine the exact mechanisms underlying the relationship between glycan branching, sialylation, and prolonged FVIII circulation.
Acknowledgments
This study was funded by Bayer. Medical writing assistance was provided by Karen L Zimmermann from Complete Healthcare Communications, LLC (North Wales, PA, USA) and was fully funded by Bayer. The abstract of this paper was presented at the American Society for Hematology Conference “ASH 2018” as a poster presentation with interim findings. The poster’s abstract was published in 2018 in the journal Blood 132:1209; doi: https://doi.org/10.1182/ blood-2018-99-113061.
Disclosure
John M Teare, David S Kates, Anita Shah, and Stephen Garger are employees of Bayer. The authors report no other conflicts of interest in this work.
References
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. | ||
Björkman S, Folkesson A, Jönsson S. Pharmacokinetics and dose requirements of factor VIII over the age range 3–74 years: a population analysis based on 50 patients with long-term prophylactic treatment for haemophilia A. Eur J Clin Pharmacol. 2009;65(10):989–998. | ||
Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemost. 2015;13(suppl 1):S176–S179. | ||
Sethuraman N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol. 2006;17(4):341–346. | ||
Lenting PJ, Neels JG, van den Berg BM, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999;274(34):23734–23739. | ||
Lenting PJ, Pegon JN, Christophe OD, Denis CV. Factor VIII and von Willebrand factor – too sweet for their own good. Haemophilia. 2010;16(suppl 5):194–199. | ||
Solá RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs. 2010;24(1):9–21. | ||
Fukuda MN, Sasaki H, Lopez L, Fukuda M. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood. 1989;73(1):84–89. | ||
Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75(3):591–609. | ||
Garger S, Severs J, Regan L, et al. Bay 81-8973, a full-length recombinant factor VIII: manufacturing processes and product characteristics. Haemophilia. 2017;23(2):e67–e78. | ||
Ishaque A, Thrift J, Murphy JE, Konstantinov K. Over-expression of Hsp70 in BHK-21 cells engineered to produce recombinant factor VIII promotes resistance to apoptosis and enhances secretion. Biotechnol Bioeng. 2007;97(1):144–155. | ||
Maas Enriquez M, Thrift J, Garger S, Katterle Y. Bay 81-8973, a full-length recombinant factor VIII: human heat shock protein 70 improves the manufacturing process without affecting clinical safety. Protein Expr Purif. 2016;127:111–115. | ||
Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(5):a013201. | ||
Anumula KR, Dhume ST. High resolution and high sensitivity methods for oligosaccharide mapping and characterization by normal phase high performance liquid chromatography following derivatization with highly fluorescent anthranilic acid. Glycobiology. 1998;8(7):685–694. | ||
Samuels N, Kates D, Liu J, Severs J. Characterizing the glycosylation state of therapeutic recombinant glycoproteins. In: Kohler J, Patrie S, editors. Mass Spectrometry of Glycoproteins. Methods in Molecular Biology (Methods and Protocols). Vol. 951. Totowa: NJ: Humana Press; 2013:323–334. | ||
Shah A, Delesen H, Garger S, Lalezari S. Pharmacokinetic properties of Bay 81-8973, a full-length recombinant factor VIII. Haemophilia. 2015;21(6):766–771. | ||
Shah A, Solms A, Garmann D, et al. Improved pharmacokinetics with Bay 81-8973 versus antihemophilic factor (recombinant) plasma/albumin-free method: a randomized pharmacokinetic study in patients with severe hemophilia A. Clin Pharmacokinet. 2017;56(9):1045–1055. | ||
Baunsgaard D, Nielsen AD, Nielsen PF, et al. A comparative analysis of heterogeneity in commercially available recombinant factor VIII products. Haemophilia. 2018;24(6):880–887. | ||
Mathew P. Increased branching and sialylation of N-linked glycans of rFVIII leads to an improved pharmacokinetic profile for bay 81–8973. East Asia Hemophilia Forum, October 6–8, 2017; Taichung, Taiwan. | ||
Seested T, Nielsen HM, Christensen EI, Appa RS. The unsialylated subpopulation of recombinant activated factor VII binds to the asialo-glycoprotein receptor (ASGPR) on primary rat hepatocytes. Thromb Haemost. 2010;104(6):1166–1173. | ||
Blasko E, Brooks AR, Ho E, Wu JM, Zhao XY, Subramanyam B. Hepatocyte clearance and pharmacokinetics of recombinant factor IX glycosylation variants. Biochem Biophys Res Commun. 2013;440(4):485–489. | ||
Cheng K, Zhou Y, Neelamegham S. DrawGlycan-SNFG: a robust tool to render glycans and glycopeptides with fragmentation information. Glycobiology. 2017;27(3):200–205. | ||
Teare, et al. Improved Pharmacokinetic Profile for BAY 81-8973. Due to Increased Branching and Sialylation of N-Linked Glycans of Recombinant Factor VIII. Poster presented at: American Society of Hematology; December 1–4; 2018; San Diago, USA. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.