Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
IncobotulinumtoxinA use in aesthetic indications in daily practice: a European multicenter, noninterventional, retrospective study
Authors Pavicic T, Prager W, Klöppel M, Ravichandran S, Galatoire O
Received 18 September 2014
Accepted for publication 3 November 2014
Published 5 March 2015 Volume 2015:8 Pages 135—142
DOI https://doi.org/10.2147/CCID.S74519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Tatjana Pavicic,1 Welf Prager,2 Markus Klöppel,3 Simon Ravichandran,4 Olivier Galatoire5
1Private Practice, Munich, Germany; 2Dr. Prager & Partner, Derma-Hamburg, Hamburg, Germany; 3Private Practice Clinic of Esthetic Surgery, MediCenter Munich-Solln, Munich, Germany; 4Clinetix Rejuvenation, Glasgow, Scotland, UK; 5Department of Ophthalmology, Adolphe Rothschild Ophthalmological Foundation, Paris, France
Purpose: To characterize utilization patterns and treatment satisfaction with incobotulinumtoxinA for aesthetic indications and assess adherence to the Summary of Product Characteristics.
Patients and methods: Data were collected retrospectively from physicians in Germany, France, and the UK regarding patients (n=638) treated with incobotulinumtoxinA for aesthetic indications. Data on indication, treatment interval, dose injected, physician and patient satisfaction, and adverse drug reactions were recorded according to routine daily practice.
Results: Most patients (76.0%) received incobotulinumtoxinA for glabellar frown lines (GFL) and were given doses of ≤20 U. The majority of treatment intervals were 5 months or longer. Overall, 64.1% of patients were treated for off-label indications, sometimes in combination with treatment for GFL. The most frequently treated off-label indications were horizontal forehead lines (38.6%) and/or crow's feet (CF; 31.7%); for CF, >95% of injected doses were ≤24 U. In Germany, a smaller proportion of patients were given incobotulinumtoxinA treatment for CF (27.6%), compared with France (40.4%) and the UK (33.2%), although country-specific differences were less prominent when treatment cycle data for CF were examined. Treatment satisfaction among physicians and patients (overall, and for GFL specifically) was very high, with excellent tolerability and only one mild adverse drug reaction reported.
Conclusion: In daily practice, incobotulinumtoxinA is mainly used for GFL; however, its use for CF and horizontal forehead lines (often in combination with GFL) is relatively common. Treatment satisfaction across aesthetic indications is high, and incobotulinumtoxinA is well tolerated, with time intervals of 5 months or longer between injections in the majority of cases. When considering factors such as dose and treatment interval, adherence to the Summary of Product Characteristics when treating GFL in daily practice is good. These results support previous reports that incobotulinumtoxinA is an effective treatment for GFL, with an excellent safety profile. Furthermore, incobotulinumtoxinA may display efficacy and tolerability in other indications.
Keywords: incobotulinumtoxinA, Xeomin®/Xeomeen®/Bocouture®/XEOMIN Cosmetic™, NT 201; free from complexing proteins, retrospective, daily practice, glabellar frown lines, crow's feet
Introduction
Botulinum neurotoxin type A (BoNT/A) preparations are approved for the aesthetic treatment of glabellar frown lines (GFL), crow’s feet (CF), mimic lines, and hyperkinetic facial lines; the latter two indications are approved in Russia and Mexico, respectively.1–4 Efficacy in these therapy areas derives from the ability of BoNT/A to block cholinergic neuromuscular transmission and, therefore, inhibit muscle activity.5,6 In facial aesthetics, botulinum toxins have been used for over 20 years,7 with several formulations commercially available, including incobotulinumtoxinA (Xeomin®/Xeomeen®/Bocouture®/XEOMIN Cosmetic™, NT 201; free from complexing proteins, Merz Pharmaceuticals GmbH, Frankfurt, Germany), onabotulinumtoxinA (Vistabel®, Vistabex®, BOTOX® Cosmetic, Allergan Inc., Irvine, CA, USA), and abobotulinumtoxinA (Dysport®, Medicis Aesthetics, Scottsdale, AZ, USA; Azzalure®, Galderma Laboratories, Lausanne, Switzerland).
IncobotulinumtoxinA differs from other marketed BoNT/A products, such as onabotulinumtoxinA, in that it is free from complexing proteins and reported to contain only active neurotoxin, thus resulting in a high specific biologic activity.1,8 In contrast, the neurotoxin present in onabotulinumtoxinA and abobotulinumtoxinA formulations constitutes part of a larger protein structure that includes complexing proteins not required for the activity of the neurotoxin.8
Currently, in aesthetics, incobotulinumtoxinA is indicated for GFL in the USA and all major European markets, hyperkinetic facial lines in Mexico, mimic lines in Russia, and (most recently) CF in the European Union;1 however, it is also an effective treatment for other aesthetic indications, including horizontal forehead lines (HFL), masseteric hypertrophy, and platysmal bands.1,9–18 OnabotulinumtoxinA is licensed to treat CF as well as GFL.4 A head-to-head study comparing onabotulinumtoxinA with incobotulinumtoxinA found that the latter was also a long-lasting, effective, and well-tolerated treatment for CF, with no significant difference between the agents when administered at the same dose.19 Similarly, a recent double-blind study with crossover evaluation found that incobotulinumtoxinA and onabotulinumtoxinA demonstrated similar clinical efficacy in the treatment of CF when administered at identical doses of 12 U per site.20 A large head-to-head comparison study has also shown that incobotulinumtoxinA was non inferior to onabotulinumtoxinA in the treatment of GFL, with both compounds displaying comparable clinical efficacy.18 Indeed, several consensus statements have recommended a 1:1 dose conversion ratio between incobotulinumtoxinA and onabotulinumtoxinA for aesthetic indications.3,21,22
The effects of incobotulinumtoxinA BoNT/A preparations for GFL occur within the first few days postinjection,1 but can potentially last for 5 months or longer.23,24 Thus, to maintain positive therapeutic effects, the Summary of Product Characteristics (SmPC) states that treatment should occur no more frequently than every 3 months at a standard dose of 20 U.1 In practice, however, doses and treatment intervals are individualized based on factors such as sex, muscle mass, and physician or patient preference.3,21,25
A retrospective analysis (n=1,256) of incobotulinumtoxinA and onabotulinumtoxinA in the treatment of upper facial lines (UFL) reported similar levels of patient and physician satisfaction, as well as similar levels of clinical efficacy at identical doses and comparable safety profiles, in daily practice.26 Retrospective monitoring of the use of incobotulinumtoxinA in daily aesthetic practice, including off-label use, can therefore provide important insights into treatment safety and efficacy beyond the standardized approach used in clinical trials.
In response to a request by the German federal body Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM), data were collected retrospectively from physicians in three European countries (Germany, France, and the UK) who used incobotulinumtoxinA for aesthetic treatment. Physicians collected anonymized data from their records regarding those patients who received incobotulinumtoxinA treatment. These data included treated indication, dose injected during each treatment cycle, length of time between treatments, and assessment of patient and physician satisfaction with incobotulinumtoxinA treatment. To assess the safety profile of incobotulinumtoxinA, the study also collected information from physicians on adverse drug reactions (ADRs) associated with its use in aesthetic indications.
Patients and methods
Study population
Physicians invited to participate (n=3,375) were selected at random from a preexisting roster of physicians, which was developed from medical registers and other sources by a contract research organization (CRO). Those who participated were asked to provide data on patients who had recently received treatment with incobotulinumtoxinA for aesthetic indications. The study aimed to collect data from at least 600 patients from across Germany, France, and the UK.
Ethical approval
Prior to the study, regulatory authorities and ethics committees in Germany, France, and the UK were consulted and study approval was received from each country.
Inclusion criteria
To participate in this study, physicians were required to be using incobotulinumtoxinA for aesthetic indications (eg, treatment of facial wrinkles or dermatologic diseases) in routine practice and to have documented sufficient patient data regarding treatment with incobotulinumtoxinA. Patient data were deemed suitable for inclusion in this retrospective study if they reported the use of incobotulinumtoxinA for ≥1 aesthetic indication at least once before the treating physician had been invited to participate in this survey.
Study design
Physicians were contacted between July and September 2012. Those who considered themselves to be eligible and who agreed to participate were asked to document patients who fulfilled the inclusion criteria, beginning with the most recently treated patient (relative to the day the physician was invited to take part in the survey) and continuing with the next most recently treated patient, and so on up to a maximum of 15 patients per physician.
Each physician was asked to document, retrospectively, all incobotulinumtoxinA treatment cycles per patient up to the day the physician was invited to participate in the study, so that all data were collected retrospectively. A treatment cycle refers to a patient visit to the physician in which ≥1 injection of incobotulinumtoxinA was administered for ≥1 indication. Cycles were documented in chronological order, with treatment cycle 1 constituting the first injection cycle with incobotulinumtoxinA and treatment cycle 2 constituting the second, and so on.
Data collection
Data were collected retrospectively by physicians and documented using an electronic Data Collection Form. Documented details included information on the physician, patients, and treatment cycles with incobotulinumtoxinA. Data on treatment cycles included the area treated, number of treatment cycles, time interval between treatment cycles, dose injected, as well as physician and patient satisfaction with treatment.
Other information collected included physician specialism, country of practice, experience with BoNTs/incobotulinumtoxinA, and whether they regarded room temperature storage as advantageous, as well as information on each patient’s relevant medical history and concomitant medications, contraindicated diseases, and general health status. Expected ADRs (as described in the SmPC) were recorded, but the study also aimed to record unexpected ADRs should any have occurred.
Statistical analysis
Statistical analysis was descriptive, and as such, no formal estimation of sample size was made. All analyses were based on the full analysis set, which comprised patients who completed at least one treatment cycle and physicians who documented at least one such patient; the full analysis set was identical to the safety analysis set. Analyses of the data were conducted using summary statistics, and statistical analyses were performed by a CRO using the SAS® software package, v9.2 (SAS Institute, Cary, NC, USA).
Results
Study population
Of the 3,375 physicians contacted from a large address pool, 123 who had used incobotulinumtoxinA expressed an interest in study participation. In total, 66 physicians fulfilled all study inclusion criteria (ie, responded, used incobotulinumtoxinA in routine practice, and had documented patient data available) and agreed to participate.
Most physicians included in this study were dermatologists within a practice and/or hospital setting (Table 1). They were all experienced in the use of BoNTs in aesthetic practice: 98.5% had been previously trained to inject any BoNTs and reported a median of 6 years’ experience in using these treatments for aesthetic indications, while 93.9% had been specifically trained to inject incobotulinumtoxinA, with a median of 3 years’ experience in using this treatment for aesthetic indications. Of this experienced population of physicians, 93.9% saw either an “important advantage” or an “advantage” in being able to store incobotulinumtoxinA at room temperature.
  | Table 1 Patient (n=638) and physician (n=66) characteristics |
A total of 638 patients were included in this study, providing data from 1,437 treatment cycles in which there were 1,972 injections of incobotulinumtoxinA. Patients were between 18 and 65 years of age (mean ± standard deviation [SD]: 46.6±10.6) and were mostly female (83.7%). The majority had a body mass index (BMI) in the normal range (mean ± SD: 23.8±4.2 kg/m2) and rated their health status as “good” or “excellent” (Table 1). All physicians followed the SmPC with respect to contraindicated conditions and concomitant medications: no patient had any contraindicated condition present or received any contraindicated medication during the study.
Treated indications
Over three quarters of patients were treated for GFL, with a similarly high proportion of treatment cycles involving GFL treatment with incobotulinumtoxinA. Around a third of patients were treated for GFL exclusively (35.9%), and 40.1% for GFL plus up to five other indications. A large proportion of patients in all countries (64.1%) were treated for indications that were off-label at the time of the study; around a third of patients were given incobotulinumtoxinA injections for CF, which, despite being on indication now, was off-label at the time of administration. This equated to a fifth of all treatment cycles. Off-label use of incobotulinumtoxinA for the treatment of HFL occurred in over a third of patients and a quarter of all treatment cycles. A small minority of patients were also treated for other off-label indications, including those related to the perioral area (ie, perioral lines, chin lines, and marionette lines; Table 2).
The proportion of patients receiving off-label incobotulinumtoxinA treatment for HFL was smaller in Germany (34.3%) than in France (36.7%) and the UK (45.8%), with a similar pattern seen for treatment cycles (Germany: 23.6%; France: 21.4%; UK: 36.7%). Additionally, the proportion of patients receiving off-label incobotulinumtoxinA treatment for CF was smaller in Germany (27.6%), compared with France (40.4%) and the UK (33.2%), although the country-specific differences were less prominent when treatment cycle data for CF were examined (Germany: 18.6%; France: 20.7%; UK: 23.2%).
Treatment intervals
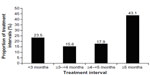
Across all indications, the majority of treatment intervals (51.6%) with incobotulinumtoxinA were 5 months or longer, with ≥6 to <9 months being the single most common treatment interval (23.3%), followed by ≥4 to <5 months (16.1%). For the treatment of GFL specifically, most treatment intervals (43.1%) were 5 months or longer (Figure 1). Overall, 61.0% of subjects treated for GFL had a treatment interval of over 4 months (≥4 to <5 month interval, 17.9%; ≥5 month interval, 43.1%). For incobotulinumtoxinA treatment of CF, 66.7% of intervals were 5 months or longer; for HFL, 64.4% were 5 months or longer; for UFL (simultaneous treatment of GFL, HFL, and CF), 67.9% were 5 months or longer.
Treatment dose
Across all indications, 75.8% of injections were ≤20 U. For incobotulinumtoxinA treatment of GFL alone, the vast majority of doses injected were less than or equal to the 20 U standard starting dose recommended in the SmPC (Figure 2A). Only 1.8% of the total number of incobotulinumtoxinA injections administered for GFL were above a dose of 30 U (dose information for one treatment cycle was missing). In Germany, doses were mostly in the range of >20U to ≤30 U (47.9%); in France, the majority of doses were exactly 20 U (75.5%); and in the UK, the majority of doses were <20 U (56.8%) or exactly 20 U (32.7%).
For the treatment of HFL, 91.0% of the total number of incobotulinumtoxinA injections were administered at a dose ≤20 U, and 22.9% were administered at a dose <10 U. For the treatment of CF, 87.3% of incobotulinumtoxinA injections were at a dose <24 U, with the rest receiving either exactly 24 U (8.2%) or >24 U (4.5%; Figure 2B). Nearly all injections of incobotulinumtoxinA for the treatment of UFL (98.7%) involved a dose ≤64 U.
Treatment satisfaction
Across all treatment indications, 95.4% of physicians regarded the success of incobotulinumtoxinA treatment as either “very good” (64.8%) or “good” (30.6%). Similarly, 97.8% of physicians rated their satisfaction with incobotulinumtoxinA treatment for GFL as “very good” (73.2%) or “good” (24.6%); this pattern was seen across individual dosing groups when satisfaction data were split according to dose injected (Figure 3). For patients, a similar level of treatment satisfaction with incobotulinumtoxinA was recorded across all indications, with 94.9% stating that they were satisfied with the result. Indeed, for GFL alone, patients were satisfied with incobotulinumtoxinA treatment in approximately 95% of cases. This pattern was seen regardless of the dose injected (Figure 4).
  | Figure 3 Physician satisfaction with incobotulinumtoxinA treatment cycles for glabellar frown lines, separated by injected dose. |
  | Figure 4 Patient satisfaction with incobotulinumtoxinA treatment for glabellar frown lines, separated by injected dose. |
Safety
After 1,972 injections in 1,437 treatment cycles for 638 patients, only one ADR was documented in one subject (0.2%). The intensity of this ADR (eyelid ptosis) was mild and it resolved during the course of the next 7 days. This subject had no violation of the SmPC with respect to age, contraindicated medication or condition, or in terms of the time interval between injections of incobotulinumtoxinA, and was treated once more a year later for GFL, HFL, CF, and platysma bands.
Discussion
This retrospective study aimed to determine common daily aesthetic practice with incobotulinumtoxinA and improve understanding of its indications, while also assessing safety and adherence to the SmPC. In addition, patient and physician satisfaction with incobotulinumtoxinA treatment were evaluated.
The study found that the majority of patients received injections of incobotulinumtoxinA for GFL, with over two-thirds of treatment cycles involving treatment of this indication. While approved aesthetic indications for incobotulinumtoxinA include GFL, hyperkinetic facial lines, mimetic lines, and (most recently) CF, research suggests that this agent may also be effective in other indications;9,11,12,19 thus, it was not surprising that approximately 30%–40% of patients in this study received incobotulinumtoxinA treatment for CF and/or HFL.
The results of this study indicate a very high degree of satisfaction with incobotulinumtoxinA treatment, with over 90% of physicians rating treatment outcome as either “good” or “very good”, and approximately 95% of patients stating that they were satisfied with the result. In patients treated for GFL, there were high levels of satisfaction similar to that seen in the overall treatment population, irrespective of the dose of incobotulinumtoxinA injected. This accords with the results of a previous retrospective analysis of incobotulinumtoxinA, which reported that 95% of patients and physicians were satisfied following treatment of GFL, CF, and HFL.26 A study of abobotulinumtoxinA reported treatment satisfaction in approximately 70%–80% of patients 3 months after treatment, and “complete satisfaction” in around 20%–40%.27 Given that most patients in this study had a GFL treatment interval of ≥4 months with incobotulinumtoxinA, and that patient satisfaction was high, these data indicate that incobotulinumtoxinA treatment was similarly long-lasting in the setting of daily clinical practice.
For incobotulinumtoxinA treatment of GFL, the SmPC recommends a standard starting dose of 20 U; however, this may be increased to 30 U based on patient needs.1 The results of this study show that the majority of injected doses for GFL were ≤20 U in the context of daily practice. While a third of doses were >20 U, global consensus papers suggest that factors such as sex and muscle mass can require such an increase in dose.3,21,25 Furthermore, research has shown that dosing for GFL based on these factors can optimize the efficacy of BoNT/A.28 Indeed, that <2% of injections for GFL were at a dose >30 U reflects the efficacy of incobotulinumtoxinA at the standard dose of 20 U in this indication.
Almost all injections of incobotulinumtoxinA for UFL were below the dose of 64 U suggested as most suitable for onabotulinumtoxinA treatment of the upper face.29 Similarly, over 95% of injected doses of incobotulinumtoxinA for CF were below the 24 U recommended starting dose for onabotulinumtoxinA,4 suggesting a good comparative potency for incobotulinumtoxinA in these indications.
For GFL, the results of this study suggest that the treatment interval between incobotulinumtoxinA injections is generally adhered to, with the majority of intervals being 5 months or longer. For treatment of CF and UFL, two-thirds of intervals between incobotulinumtoxinA injections were 5 months or longer. Shorter intervals may be due to physicians requesting a return before the initial treatment effect has waned to ensure long-term efficacy. This period may vary according to country and indication. For example, in consensus guidelines,3,21,25 a reduced dose or dose range of BoNT/A has been recommended for CF relative to GFL to avoid complete muscle relaxation so that patients are still able to close their eyes and retain some movement when smiling. Consequently, the effect that a reduced dose of BoNT/A has on CF may be more short-lived versus a higher dose of BoNT/A.
This study also demonstrates that incobotulinumtoxinA has a positive safety profile, with only 1 in 638 patients reporting an ADR (mild eyelid ptosis). This is consistent with the incobotulinumtoxinA SmPC, which estimates that <1% of patients will report eyelid ptosis after incobotulinumtoxinA injection,1 in contrast to rates of up to 10% with other BoNTs.4,30
From a daily practice perspective, physicians participating in the study were well trained and had a median of 6 years’ experience in the use of BoNT/A products in aesthetic indications. Over 90% of these physicians regarded it as advantageous that incobotulinumtoxinA could be stored at room temperature, as opposed to the refrigerated conditions required for the storage of other BoNT/A products 4,30. In other aspects, such as contraindicated conditions, concomitant medications, and patient age range, there was also evidence of excellent adherence to the SmPC.
With regard to study limitations, it is possible that the sampling method led to response bias, since response by physicians was voluntary. However, as physicians were anonymized, there seems to be no obvious reason why respondents would provide inaccurate data regarding treatment success. Consequently, this method should have produced a representative sample of physicians and an accurate set of results. Physicians were also asked to document their most recently treated patients, which was expected to result in a random sample of patients as the treatment date was unlikely to influence the study variables under consideration.
Conclusion
In daily aesthetic practice, incobotulinumtoxinA is primarily used to treat GFL, and in this study most physicians reported dosing and treatment intervals consistent with those indicated in the SmPC. Furthermore, in the majority of cases the time interval between injections (and most likely the duration of effect) was 5 months or longer, which is in line with other reports. However, a notable number of treatments were given for indications that were off-label at the time of the study, such as CF and HFL. For CF, the majority of patients received a dose below that recommended as the starting dose of onabotulinumtoxinA (24 U) and most treatment intervals were 5 months or longer. Evaluation of physician and patient satisfaction with incobotulinumtoxinA was very positive. These results support the efficacy of incobotulinumtoxinA in GFL, CF, and potentially other aesthetic indications, while also supporting its good tolerability profile.
Acknowledgments
Editorial assistance was provided by SCI Scientific Communications and Information, Oxford, UK, and funded by Merz Pharmaceuticals GmbH, Frankfurt, Germany.
Disclosure
Tatiana Pavicic has received honoraria from Merz Aesthetics and Galderma Pharma SA for speaker activities. She is a consultant for Merz Aesthetics and Galderma Pharma SA and serves on advisory boards for these companies. Welf Prager has acted as a consultant and lecturer for Allergan Inc., Merz Aesthetics GmbH, and Galderma Pharma SA. Markus Klöppel has received honoraria from Merz Aesthetics and Galderma Q-Med for speaker activities and lectures. Simon Ravichandran and Olivier Galatoire have received honoraria from Merz Aesthetics for speaker activities. The authors report no other conflicts of interest in this work.
References
Bocouture®. Summary of Product Characteristics. Germany: Merz Pharmaceuticals GmbH; 2014. | |
Wenzel R, Jones D, Borrego JA. Comparing two botulinum toxin type A formulations using manufacturers’ product summaries. J Clin Pharm Ther. 2007;32(4):387–402. | |
Carruthers J, Fournier N, Kerscher M, Ruiz-Avila J, Trindade de Almeida AR, Kaeuper G. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine – a global, evidence-based botulinum toxin consensus education initiative: part II: incorporating botulinum toxin into aesthetic clinical practice. Dermatol Surg. 2013;39(3 Pt 2):510–525. | |
Vistabel®. Summary of Product Characteristics. Irvine, CA, USA: Allergan, Inc.; 2013. | |
Alimohammadi M, Andersson M, Punga AR. Correlation of botulinum toxin dose with neurophysiological parameters of efficacy and safety in the glabellar muscles: a double-blind, placebo-controlled, randomized study. Acta Derm Venereol. 2014;94:32–37. | |
Dressler D, Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29(23):1761–1768. | |
Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18(1):17–21. | |
Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67–73. | |
Gubanova EI, Panova OS, Sanchez EA, Rodina MY, Starovatova PA. Efficacy and safety of incobotulinumtoxinA for the treatment of platysmal bands of the aging neck: an open-label, prospective pilot study. J Drugs Dermatol. 2013;12(12):1461–1466. | |
Kane MA. Nonsurgical treatment of platysma bands with injection of botulinum toxin A revisited. Plast Reconstr Surg. 2003;112(Suppl 5):125S–126S. | |
Lee JH, Park JH, Lee SK, et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseteric hypertrophy: side-by-side comparison with onabotulinum toxin A. J Dermatolog Treat. 2014;25(4):326–330. | |
Oliveira de Morais O, Matos Reis-Filho E, Vilela Pereira L, Martins Gomes C, Alves G. Comparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forehead lines in men: a pilot study. J Drugs Dermatol. 2012;11(2):216–219. | |
Prager W. Differential characteristics of incobotulinumtoxinA and its use in the management of glabellar frown lines. Clin Pharmacol. 2013;5: 39–52. | |
Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840–849. | |
Carruthers JD, Lowe NJ, Menter MA, Gibson J, Eadie N. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112(4):1089–1098. | |
Carruthers A, Carruthers J, Coleman WP 3rd, et al. Multicenter, randomized, phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(4):551–558. | |
Hanke CW, Narins RS, Brandt F, et al. A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg. 2013;39(6):891–899. | |
Sattler G, Callander M, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(Suppl 4):2146–2154. | |
Prager W, Wissmüller E, Kollhorst B, Williams S, Zschocke I. Comparison of two botulinum toxin type A preparations for treating crow’s feet: a split-face, double-blind, proof-of-concept study. Dermatol Surg. 2010;36(Suppl 4):2155–2160. | |
Muti G. Efficacy of incobotulinumtoxinA and onabotulinumtoxinA to treat crow’s feet. Summer Academy Meeting of the American Academy of Dermatology; August 6–10; 2014;Chicago, IL. | |
Lorenc ZP, Kenkel JM, Fagien S, et al. Consensus panel’s assessment and recommendations on the use of 3 botulinum toxin type A products in facial aesthetics. Aesthet Surg J. 2013;33(Suppl 1):35S–40S. | |
Poulain B, Trevidic P, Clave M, et al. Clinical equivalence of conventional onabotulinumtoxinA (900 KDa) and incobotulinumtoxinA (neurotoxin free from complexing proteins – 150 KDa):2012 multidisciplinary French consensus in aesthetics. J Drugs Dermatol. 2013;12(12):1434–1446. | |
Prager W, Bee EK, Havermann I, Zschocke I. Onset, longevity, and patient satisfaction with incobotulinumtoxinA for the treatment of glabellar frown lines: a single-arm, prospective clinical study. Clin Interv Aging. 2013;8:449–456. | |
Rappl T, Parvizi D, Friedl H, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investig Dermatol. 2013;6:211–219. | |
Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit) – part I: upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010;24(11):1278–1284. | |
Prager W, Huber-Vorlander J, Taufig AZ, et al. Botulinum toxin type A treatment to the upper face: retrospective analysis of daily practice. Clin Cosmet Investig Dermatol. 2012;5:53–58. | |
Ascher B, Zakine B, Kestemont P, Baspeyras M, Bougara A, Santini J. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223–233. | |
Kane MA, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB. Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619–1629. | |
Carruthers A, Carruthers J. A single-center, dose-comparison, pilot study of botulinum neurotoxin type A in female patients with upper facial rhytids: safety and efficacy. J Am Acad Dermatol. 2009;60(6):972–979. | |
Azzalure®. Summary of Product Characteristics Hertfordshire, UK: Galderma (UK) Ltd; 2010. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.