Back to Journals » International Journal of General Medicine » Volume 15
Incidence Trends and Survival Prediction of Malignant Skin Cancer: A SEER-Based Study
Authors Zhu S, Sun C , Zhang L, Du X, Tan X, Peng S
Received 22 November 2021
Accepted for publication 24 January 2022
Published 15 March 2022 Volume 2022:15 Pages 2945—2956
DOI https://doi.org/10.2147/IJGM.S340620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Sirong Zhu,1,* Chao Sun,1,* Longjiang Zhang,1 Xiaoan Du,1 Xiaodong Tan,1,2 Shuzhen Peng3
1School of Public Health, Wuhan University, Wuhan, Hubei, People’s Republic of China; 2School of Nursing, Wuchang University of Technology, Wuhan, Hubei, People’s Republic of China; 3The People’s Hospital of Huangpi, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Tan; Shuzhen Peng, Email [email protected]; [email protected]
Purpose: This study aimed to analyze the incidence trend and further explore the risk factors influencing the survival among patients of malignant skin cancer in America.
Methods: Age-adjusted incidence rates, annual percentage change (APC) of different sex and ethnicity in 1973– 2015 and patient records were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Univariate analysis and multivariate Cox regression were used to analyze risk factors influencing the survival in skin cancer patients. Survival curves and nomograms were constructed to evaluate the survival prediction by R.
Results: The overall age-adjusted incidence of skin cancer increased in America from 1973 to 2005 (APC = 2.8%, 95% CI: 2.6– 2.9%, P < 0.05), particularly in white patients, 66-year-old people, and males. The 3- and 5-year overall survival (OS) rates were 51.4% and 33.8%, respectively. Independent predictors for short OS include age over 65, white ethnicity, other marital status and no surgery (P < 0.05). Stage was not an independent factor of survival (P > 0.05). The nomogram with a C-index of 0.72 (95% CI: 0.71– 0.73) matched an appropriate calibration curve.
Conclusion: Incidence of skin cancer in America was on the rise during 1973– 2015 based on SEER database. Age, ethnicity, marital status and surgical history were related with survival of malignant skin cancer. Nomograms were effective tools for predicting the survival prognosis.
Keywords: malignant skin cancer, primary site surgery, SEER, incidence, overall survival, nomogram
Introduction
Skin cancer (SC) is divided into non-melanoma skin cancer (NMSC) and melanoma skin cancer (MM). Notably, melanoma is the most threatening due to its aggressiveness, caused by a malignancy of melanocytes.1–3 Incidence of melanoma is rapidly increasing worldwide, which results in public health problems. Primary melanomas can be ocular, gastrointestinal, mucosal, leptomeningeal, genitourinary, and lymphatic.4 SCC and BCC are often referred to as non-melanoma skin cancer (NMSC). Although NMSCs are relatively non-lethal and curable by surgery, its incidence rate (case number) is about 10 times of that for melanoma.5 These three types of cancer share many clinical characteristics and trends.
The incidence of SC varies widely around the world, high in white populations, the highest in southern Australia.6–8 The disparities of different sex and ethnicity have also been reported, generally men worse than women, white populations or Caucasians worse than others.9–12 Studies have shown that factors influencing the survival period were age, sex, ethnicity, socioeconomic status, etc.13–16 The classification of tumor stage made varying degrees of influence to the incidence and prognosis before and after the screening program.17,18 Owing to the poor treatment effects, high risk of recurrences and metastasis,19 especially melanoma, exploring the long-term incidence trend and prognostic factors of malignant skin cancer efficiently was of vital importance.
In this study, we analyzed long-term incidence trend and the association between survival status of malignant skin cancer patients and different demographics and clinical characteristics. Nomograms are computational diagrams that expresses the functional relations between several variables with lines or curves, widely used in risk prediction of diseases.20–23 Although some descriptive studies of skin cancer and the development mechanism have been analyzed, few of them analyzed the long-term trend and survival prediction with nomograms in America. We drew nomograms with R to analyze the incidence trend during 1973–2015 and prognostic factors, using the data from the Surveillance, Epidemiology, and End Results (SEER) database, which can provide guidance for the prevention and clinical guidance of malignant skin cancer.
Materials and Methods
Case Selection and Definition of Variables
Data extracted from the SEER database in 1973–2015 were analyzed. Patients of SC diagnosed by the AJCC-6th in 2004–2015 were selected together with the following corresponding variables: age (0–49, 50–65, 66-), sex (female, male), ethnicity (white, black, others (American Indian/AK Native, Asian/Pacific Islander)), marital status (married, others), year of diagnosis (2004–2007, 2008–2011, 2012–2015), region (Alaska, East, Northern Plains, Pacific Coast, Southwest), site (melanoma of the skin, other non-Epithelial Skin), stage (I, II, III, IV), tumor size (unclear, clear), surgery (no surgery, primary site surgery), survival months, outcome (alive, dead). The inclusions are as follows: (1) Pathologically confirmed skin cancer. (2) The age, ethnicity, gender, survival status, survival time and other data of the patients were not missing. The exclusion criteria are: (1) Patients with non-malignant skin cancer; (2) Missing data on corresponding variables. The specific flow chart is shown in Figure 1.
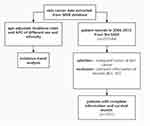 |
Figure 1 Flowchart of inclusion and exclusion. |
Statistical Analysis
The age-standardized incidence and annual percentage change (APC) of malignant skin cancer were explicitly depicted in the SEER database. The incidence trend we analyzed was classified by overall, age, and ethnicity. R studio 4.0.2 was used to presenting morbidity trends. Overall survival (OS) refers to the period from being diagnosed to death. The data were divided into case and death groups using Excel 2016 and were analyzed separately for demographic characteristics. OS and survival curves were estimated by applying with the Kaplan–Meier method and the log rank test. Univariate and multivariate Cox regression were utilized to analyze relationships between cases demographics, clinical characteristics and survival months. Variables of which P values were less than 0.05 in univariate analysis were then brought into the multivariate cox analysis.
Nomograms are visualization of results of a regression equation, and often used to present the results of logistic regression or cox regression. Nomogram results were evaluated by the concordance index (C-index), which was associated with the accuracy of the prognostic prediction. According to the predicted survival along with observed survival, calibration curve of the nomogram was performed. All P values were two-sided and P < 0.05 was considered statistically significant eventually.
Results
Case Demographics
The total cases we finally selected from SEER were 3551 and 1080 of them ended to death outcome. Specific information about demographics is shown in Table 1.
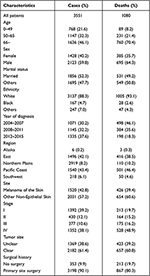 |
Table 1 Patients’ Demographics and Clinical Characteristics of Malignant Skin Cancer |
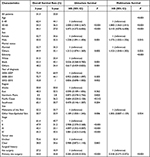 |
Table 2 Survival, Univariate and Multivariate Analysis in Patients with Skin Cancer |
Incidence Trends
The overall age-adjusted incidence of SC increased from 7.4/100,000 to 27.9/100,000 during 1973–2015, with an APC of 2.8% (95% CI = 2.6–2.9, P<0.05) (Figure 2A). A significant increase was shown in both male and female population with an APC of 3.1% (95% CI = 2.9–3.3, P<0.05) and 2.4% (95% CI = 2.2–2.5, P<0.05) (Figure 2B). Incidence among white population increased remarkably from 8.1 per 100,000 to 34.7 per 100,000 during this period (APC = 3.1, 95% CI = 2.9–3.2, P<0.05) whereas black population (APC = 0.3, 95% CI: −0.2–0.7, P>0.05) and other ethnicities (APC = 1.4, 95% CI = 1.0–1.8, P<0.05) change slightly (Figure 2C). Evidently, the incidence of males was far greater than females, white greater than other ethnicities.
 |
Figure 2 Age-adjusted incidence rate of skin cancer in 2004–2015. Notes: Stratified by total (A), sex (B), ethnicity (C). |
 |
Figure 3 Continue. |
Survival
Overall survival rate of patients with 3-year and 5-year was 51.4% and 33.8%, respectively. Overall, 1080 (30.4%) patients died during follow-up. The related risk factors of short OS encompassed white ethnicity, over 66 years old, unmarried, regions of Northern plains, male, stage III, other non-epithelial skin and no surgery (P < 0.05). Year of diagnosis (P = 0.089) and tumor size (P = 0.45) did not seem to impose an impact on OS rate (Table 2, Figure 3). Specifically, age 50–65 (vs age 0–49: hazard ratio (HR) = 2.000, 95% CI = 1.558–2.567, P<0.001) and 66- (vs age 0–49: HR = 5.475, 95% CI = 4.372–6.856, P<0.001), male (vs female: HR = 1.238, 95% CI = 1.091–1.404, P<0.001), other marital status (vs married; HR = 1.213, 95% CI = 1.074–1.369, P=0.002), black ethnicity (vs white: HR = 0.536, 95% CI = 0.368–0.780, P<0.001), other ethnicity (vs white: HR = 0.617, 95% CI = 0.459–0.830, P<0.001), other non-epithelial skin (vs melanoma of skin; HR = 1.189, 95% CI = 1.050–1.345, P=0.006), stage II (vs stage I: HR = 2.908, 95% CI = 2.370–3.568, P<0.001), stage III (vs stage I: HR = 4.097, 95% CI = 3.352–5.007, P<0.001), stage IV (vs stage I: HR = 3.081, 95% CI = 2.623–3.618, P<0.001), primary site surgery (vs no surgery: HR = 0.285, 95% CI = 0.244–0.333, P<0.001) were correlated with the overall survival rate. Year of diagnosis, region, tumor size were not significantly statistical (P > 0.1).
According to the results of univariate Cox regression analysis and the log rank test, the covariates with P-values greater than 0.05 and not satisfying the Cox proportion hazard regression model (the curves have clear crossovers) were eliminated. The final variables incorporated are age, sex, surgical history, site, marital status. Age 50–64 (vs age 0–49: HR = 1.880, 95% CI = 1.463–2.415, P<0.001) and age 65- (vs age 0–49: HR = 5.110, 95% CI = 4.076–6.408, P<0.001), male (vs female: HR = 1.175, 95% CI = 1.032–1.336, P=0.015), other marital status (vs married: HR = 1.153, 95% CI = 1.018–1.305, P=0.025), primary site surgery (vs no surgery: HR = 0.318, 95% CI = 0.271–0.372, P<0.001) remain associated with the overall rate. Site (P=0.934) was not the significant factor.
Nomogram
As shown in Figure 4, We drew nomograms after multivariate Cox regression analysis, which comprised all significant prognostic factors. In Figure 4A, variables in the prediction model are marked with a scale on a line segment, which represents the range of available values of variable, and the length reflects the contribution of factors to the outcome. Points at the top of the graph indicate the scores corresponding to each variable at different values, and the sum of all variable scores is Total Points. The risk at the bottom of the graph represents the probability of cancer occurrence. It depicted that age was the most significant predictor of overall survival, followed with surgical history, ethnicities and marital status. Figure 4B is a case study of Figure 4A, which shows that a patient who were 66-years old, white ethnicity, married and a history of primary site surgery scored 0.336. It showed a cumulative probability of death of 0.325 and 0.424 at 3-years and 5-years separately. The C-index 0.71 (95% CI: 0.70–0.72) displayed well to predict overall survival. The model calibration diagram indicated that the model exhibits high accuracy (Figure 5) since the black line roughly coincided with the reference line (the dashed line in the diagram). High prediction consistency was also found through validation group data after verifying the model calibration diagram of the modeling group.
 |
Figure 5 Calibration curves for overall survival of malignant skin cancer patients. Notes: (A) 3-year; (B) 5-year. |
Discussion
From the SEER-based study, we analyzed the incidence trend of malignant skin cancer patients stratified by total, sex and ethnicities in 1973–2015. Furthermore, 3- and 5-year overall survival predictions based on univariate and multivariate analysis were evaluated through nomograms with a C-index of 0.76, of which calibration was reliable. Although incidence trends of skin cancer were previously studied, few of them analyzed the long-term trend over the past 30–40 years in America. With regard to the survival prediction, prognostic factors were assessed insufficiently.
In our study, the result of an overall increasing trend of skin cancer patients was in accordance with descriptions in most regions, such as Puerto Rico.12 Singapore,24 and a SEER-based study in America.25 It might be the result of increasing awareness of physical examination, access to healthcare and screening methods, cumulative intense and complex sunlight exposure.26–28 In terms of sex differences, men were usually more likely to be exposed in sunlight than women due to the different social conditions. In addition, women pay more attention to skin maintenance and protection against sunburn. Concerning the ethnicity, it was universally accepted that white populations were more sensitive to the ultraviolet radiation due to lack of pigment cells of skin. European ancestry and fair skin phenotype are known factors of SC, reported by Dimitriou et al and Schmerling et al.29,30 In view of the increasing incidence of malignant skin cancer and significant difference between sex and ethnicity, main focus should be on men and white people. Daily skincare protection and early clinical screening will be recommended to prevent malignant skin cancer. In this long-term trend of incidence, we did not consider the specific age, histology and predilection site of SC. The next step to explore the age-specific, site-specific incidence and how BCC, SCC or other types of skin cancer were distributed were worth discussing.
Our study has shown that OS rate of malignant SC with 3- and 5-year was 51.4% and 33.8%, respectively, much lower than the 5-year survival rate (nearly 90%) for melanoma of skin cancer in the United States from 2010 to 2014.31 This may be because we incorporated a malignant stage of skin cancer with lymph node metastases and distant metastases. Another study indicated that the survival rate for advanced melanoma metastasis is only 15–20%, with a 5-year survival of 6% in metastatic melanoma patients.32
In the present study, age, marital status, ethnicity and surgical history were recognized as independent prognostic factors. Especially, surgical history and age were top predictors of OS. Compared to those age under 44, the mortality was 5.11 times higher in the 66-year-old group. This could indicate that aging gradually becomes an important cause of SC patients’ death. In addition to that, cancer survival rate was also affected by marital status.33 Previous researches pointed out that the married group had longer OS than the unmarried, which is in accordance with our study.34,35 It could be speculated that married patients may be more accessible to companionship and moral support. It was reported that white ethnicity was a risk factor of survival, which reflects that the melanoma occurred more frequently in people with paler skin.36 This may be caused by fair-skinned individuals having the highest risk of sunburn and skin cancer. In the present analysis, other ethnicities had the longest OS, whereas the white group had the shortest OS. The hazard ratio of people who had surgery was 0.318 times lower than those not having a surgery, indicating that surgery was a protective factor. These data proved that an increasing number of people were able to acquire a cure by surgical resection at present. Given that surgical management was improved, the 5-year OS rate for skin cancer people raised to more than 30%. What differed from the previous studies was that stage did not seem to be an independent factor for SC survival in our study.37,38 This might be related to the fact that the variables were not incorporated comprehensively due to database privilege limitations.
We constructed a nomogram that incorporated both demographic and clinical characteristics, with univariate and multivariate analysis in advance. Supported by the bootstrap-corrected C-index and calibration curve, the nomogram performed well indeed. Nomograms are graphical calculation instruments based on statistical predictive models that provide predictions on likely treatment outcomes. Nomograms provide individualized risk estimates by incorporating and illustrating important prognostic factors, and are superior to other available decision aids in more accurately predicting outcomes in patients with cancer. There are some limitations in our study as well. Firstly, variables about cancer metastasis and chemotherapy regimen information were not included due to some objective factors. Second, the sample size we brought eventually was still relatively not big enough after integrating corresponding variables. Thirdly, malignant skin cancer was not specifically classified limited by sample size and it is worth exploring further. Finally, for some reasons, we did not do a validation queue, even though a validation queue is not a required procedure for nomograms. However, the high-quality database SEER can be well trusted because of the high-volume facilities to case finding, which ensured the data accuracy, completeness, and reporting timelines.39 In the future, large databases such as SEER can provide a more integrative and interconnected strategy combined with big data for skin cancer prevention and management, which is a valuable and necessary tool to collect multinational data.40
Conclusions
Our study demonstrated incidence trends of malignant skin cancer and constructed a nomogram to predict their long-term survival based on SEER database. Overall, the incidence of SC was increasing, higher in male and white population. The nomogram which displayed great accuracy and consistency selected out several variables associated with the survival of skin cancer: age, ethnicity, married status, and surgical history. It showed that the nomogram was an effective tool for predicting the prognosis.
Abbreviations
APC, annual percentage change; SEER, the Surveillance, Epidemiology, and End Results; OS, overall survival; SC, skin cancer; SCC, squamous cell carcinoma; BCC, basal carcinoma; NMSC, non-melanoma skin cancer; APC, annual percentage change; C-index, concordance index; HR, hazard ratio.
Data Sharing Statement
The datasets analyzed during the current study are available in the SEER repository, https://seer.cancer.gov/.
Ethics Approval and Informed Consent
Consent requirement was waived for this study from The Ethics Committee of Wuhan University School of Medicine as it was based on a publicly available database with anonymized and confidential patient information. The waiver number is 2021YF0066.
Consent for Publication
All authors consent for publication.
Acknowledgments
Sirong Zhu and Chao Sun are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Ingraffea A. Melanoma. Facial Plast Surg Cl. 2013;21(1):33. doi:10.1016/j.fsc.2012.11.007
2. Lin Z, Du J, Lu C, Wang J. Risk factors of new symptomatic vertebral compression fractures after percutaneous vertebroplasty. Int J Clin Exp Med. 2019;12(1):949–954.
3. Tuong W, Cheng LS, Armstrong AW. Melanoma: epidemiology, diagnosis, treatment, and outcomes. Dermatol Clin. 2012;30(1):113. doi:10.1016/j.det.2011.08.006
4. Ahmed B, Qadir MI, Ghafoorb S. Malignant melanoma: skin cancer-diagnosis, prevention, and treatment. Crit Rev Eukar Gene. 2020;30(4):291–297. doi:10.1615/CritRevEukaryotGeneExpr.2020028454
5. Liu-Smith F, Jia J, Zheng Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Adv Exp Med Biol. 2017;996:27–40.
6. Ploydaeng M, Rajatanavin N, Pinyowiwat P. 15106 Prevalence of skin cancer, photoprotection behaviors, and photoaging in a Southeast Asian population. J AM ACAD Dermatol. 2020;83(6S). doi:10.1016/j.jaad.2020.06.651
7. Han WH, Yong SS, Tan LL, et al. Characteristics of skin cancers among adult patients in an urban Malaysian population. Australas J Dermatol. 2019;60(4):E327–E329. doi:10.1111/ajd.13106
8. Kim HS, Ahn HS. Response to “the incidence and survival of melanoma and nonmelanoma skin cancer in patients with vitiligo: a nationwide population-based matched cohort study in Korea” - reply from the authors. Brit J Dermatol. 2020;183(6):1150–1151. doi:10.1111/bjd.19465
9. Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. Ca Cancer J Clin. 2012;62(2):118–128. doi:10.3322/caac.20141
10. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44(12):1016–1021. doi:10.1111/j.1365-4632.2004.02322.x
11. Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of non melanoma skin cancer. Facial Plast Surg Cl. 2012;20(4):419. doi:10.1016/j.fsc.2012.07.004
12. De La Torre-lugo EM, Figueroa LD, Sanchez JL, Morales-Burgos A, Conde D. Skin cancer in Puerto Rico: a multiannual incidence comparative study. P R Health Sci J. 2010;29(3SI):312–316.
13. Fillon M. Darker-skinned patients have lower cancer survival rates. J Natl Cancer Inst. 2016;108(11):11. doi:10.1093/jnci/djw285
14. Charles AJ, Otley CC, Pond GR. Prognostic factors for life expectancy in nonagenarians with nonmelanoma skin cancer: implications for selecting surgical candidates. J Am Acad Dermatol. 2002;47(3):419–422. doi:10.1067/mjd.2002.122740
15. de Vries E, Steliarova-Foucher E, Spatz A, Ardanaz E, Eggermont AMM, Coebergh JWW. Skin cancer incidence and survival in European children and adolescents (1978–1997). report from the automated childhood cancer information system project. Eur J Cancer. 2006;42(13):2170–2182. doi:10.1016/j.ejca.2006.07.001
16. Dunn EC, Moore KJ, Miao F, Kirsner RS, Koru-Sengul T. Survival of children and young adults with skin cancer: analysis of a population-based Florida cancer registry: 1981–2013. Pediatr Dermatol. 2018;35(5):597–601. doi:10.1111/pde.13588
17. Eisemann N, Waldmann A, Katalinic A. Incidence of melanoma and changes in stage-specific incidence after implementation of skin cancer screening in Schleswig-Holstein. Bundesgesundheitsbla. 2014;57(1):77–83. doi:10.1007/s00103-013-1876-1
18. Kai Y, Ishikawa K, Goto M, et al. Results of second-stage screening for skin cancers in Oita Prefecture, Japan. J Dermatol. 2015;42(12):1160–1164. doi:10.1111/1346-8138.13016
19. Bander TS, Nehal KS, Lee EH. Cutaneous squamous cell carcinoma updates in staging and management. Dermatol Clin. 2019;37(3):241. doi:10.1016/j.det.2019.03.009
20. Graefen M, Augustin H, Karakiewicz PI, et al. Can nomograms derived in the US applied to German patients? A study about the validation of preoperative nomograms predicting the risk of recurrence after radical prostatectomy. Urologe A. 2003;42(5):685. doi:10.1007/s00120-002-0251-x
21. Dotan ZA, Ramon J. Nomograms as a tool in predicting prostate cancer prognosis. Eur Urol Suppl. 2009;8(9):721–724. doi:10.1016/j.eursup.2009.06.013
22. Borque Fernando A, Esteban Escano LM, Sanz Saiz G, Rioja Sanz LA. Predictive models for biochemical recurrence of prostate cancer after local treatment nomograms. Arch Esp Urol. 2012;65(1):39–50.
23. Hinev AI, Anakievski D, Kolev NH, Hadjiev VI. Validation of nomograms predicting lymph node involvement in patients with prostate cancer undergoing extended pelvic lymph node dissection. Urol Int. 2014;92(3):300–305. doi:10.1159/000354323
24. Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore cancer registry data 1968–97. Brit J DermatoL. 2003;148(6):1161–1166. doi:10.1046/j.1365-2133.2003.05223.x
25. Stern RS. Prevalence of a history of skin cancer in 2007 results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282. doi:10.1001/archdermatol.2010.4
26. Rundle CW, Militello M, Barber C, Presley CL, Rietcheck HR, Dellavalle RP. Epidemiologic burden of skin cancer in the US and worldwide. Curr Dermatol Rep. 2020;9(4):309–322. doi:10.1007/s13671-020-00311-4
27. Brunssen A, Waldmann A, Eisemann N, Katalinic A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: a systematic review. J Am Acad Dermatol. 2017;76(1):129. doi:10.1016/j.jaad.2016.07.045
28. Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134(1):43–50. doi:10.1038/jid.2013.304
29. Schmerling RA, Loria D, Cinat G, et al. Cutaneous melanoma in Latin America: the need for more data. Rev Panam Salud Publ. 2011;30(5):431–438. doi:10.1590/S1020-49892011001100005
30. Dimitriou F, Krattinger R, Ramelyte E, et al. The world of melanoma: epidemiologic, genetic, and anatomic differences of melanoma across the globe. Curr Oncol Rep. 2018;20(11):11. doi:10.1007/s11912-018-0732-8
31. Claudia A, Tomohiro M, Veronica DC, et al. Global surveillance of trends in cancer survival 2000–14 (concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
32. Zhang Y, Song Y, Gao Q. Increased survival time of a patient with metastatic malignant melanoma following immunotherapy: a case report and literature review. Oncol Lett. 2015;10(2):883–886.
33. Abegaz TM, Ayele AA, Gebresillassie BM. Health related quality of life of cancer patients in Ethiopia. J Oncol. 2018;2018:1467595. doi:10.1155/2018/1467595
34. Luo P, Zhou J, Jin S, Qing M, Ma H. Influence of marital status on overall survival in patients with ovarian serous carcinoma: finding from the surveillance epidemiology and end results (SEER) database. J Ovarian Res. 2019;12(1). doi:10.1186/s13048-019-0600-7
35. Sartorius B, Veerman LJ, Manyema M, Chola L, Hofman K. Determinants of obesity and associated population attributability, South Africa: empirical evidence from a national panel survey, 2008–2012. PLoS One. 2015;10:6. doi:10.1371/journal.pone.0130218
36. O’Shea SJ, Rogers Z, Warburton F, Ramirez AJ, Newton-Bishop JA, Forbes LJL. Which symptoms are linked to a delayed presentation among melanoma patients? A retrospective study. BMC Cancer. 2017;17(1). doi:10.1186/s12885-016-2978-6
37. Metri R, Mohan A, Nsengimana J, et al. Identification of a gene signature for discriminating metastatic from primary melanoma using a molecular interaction network approach. Sci Rep Uk. 2017;7(1):1–4.
38. Herbreteau G, Vallee A, Knol A, et al. Circulating tumour DNA Is an independent prognostic biomarker for survival in MetastaticBRAForNRAS-mutated melanoma patients. Cancers. 2020;12(7). doi:10.3390/cancers12071871
39. Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun. 2019;39(1):1–9.
40. Lancellotta V, Luis Guinot J, Fionda B, et al. SKIN-COBRA (consortium for brachytherapy data analysis) ontology: the first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer. J Contemp Brachyther. 2020;12(2):105–110. doi:10.5114/jcb.2020.94579
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


