Back to Journals » Journal of Pain Research » Volume 13
Incidence of Opioid Overdose Among Patients Using ER/LA Opioid Analgesics Before and After Implementation of the Class-Wide Opioid Risk Evaluation and Mitigation Strategy
Authors Esposito DB, Cepeda MS , Holick CN, Knox C , Desai VCA, Liu N, Vojjala SK, Lyons JG, Wedin GP , Lanes S
Received 13 June 2019
Accepted for publication 24 December 2019
Published 16 January 2020 Volume 2020:13 Pages 157—169
DOI https://doi.org/10.2147/JPR.S219324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Daina B Esposito, 1 M Soledad Cepeda, 2 Crystal N Holick, 1 Caitlin Knox, 1 Vibha CA Desai, 1 Nianya Liu, 1 Shiva-Krishna Vojjala, 1 Jennifer G Lyons, 1 Gregory P Wedin, 3 Stephan Lanes 1 On Behalf of the REMS Program Companies Metrics Subteam
1HealthCore, Inc, Andover, MA, USA; 2Janssen Research and Development, Titusville, NJ, USA; 3Upsher-Smith Laboratories, LLC, Maple Grove, MN, USA
Correspondence: Stephan Lanes
Safety and Epidemiology, HealthCore, Inc., 300 Brickstone Square, 8th Floor, Suite 801A, Andover, MA 01810, USA
Tel +1 302-384-0673
Fax +1 978-247-6650
Email [email protected]
Introduction: The United States (US) Food and Drug Administration (FDA) required a Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting (ER/LA) opioid analgesics on 09 July 2012.
Methods: This study compared the incidence of opioid overdose before (July 2010-June 2012) and after (July 2013-September 2016) the initiation of the Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting (ER/LA) opioid analgesics. We identified patients with ≥ 1 ER/LA opioid dispensing in either time period in national data from the HealthCore Integrated Research DatabaseSM (HIRD) and in United States (US) Medicaid claims data from four states. We described each population, calculated the incidence rate (IR) of opioid overdose, and assessed crude and propensity score adjusted incidence rate ratios (IRR) comparing the overdose rate after vs before implementation of the REMS.
Results: A total of 121,229 commercially insured and 11,488 Medicaid patients were included in the analysis. Rates of overdose were substantially higher in Medicaid patients than in the commercially insured patients (IR 192.0, 95% confidence interval [CI] 162.60– 225.18 versus 102.60, 95% CI 93.0– 112.93 in the active period). The IRRs for opioid overdose were 1.01 (95% CI 0.87– 1.17) in the commercially insured population and 0.70 (95% CI 0.52– 0.93) in Medicaid.
Conclusion: This leveling off of overdose rates among commercially insured patients and decline among Medicaid patients is encouraging, but it is difficult to disentangle the specific impact of the REMS from many other ongoing initiatives with similar goals.
Keywords: opioid, overdose, REMS, Medicaid, claims
Plain Language Summary
The United States Food and Drug Administration mandated a class-wide Risk Evaluation and Mitigation Strategy (REMS) for extended-release (ER)/long-acting (LA) opioids that went into effect in July 2012. This study used administrative data from the HealthCore Integrated Research Database (HIRD) linked to the National Death Index (NDI) and Medicaid plans from four participating states to compare rates of emergency department visits or hospitalizations due to opioid overdose among those patients who were using ER/LA opioids before and after the REMS program went into effect. Overall, opioid overdose rates were stable or decreased in this population. Medicaid patients had more opioid overdoses than privately-insured patients both before and after the REMS. Opioid overdose deaths decreased slightly in privately-insured patients, but could not be assessed in Medicaid. Because many programs are concurrently working to reduce opioid harm, alternative approaches are needed to formally assess the relation between the REMS and rates of opioid overdose.
Introduction
Extended-release and long-acting (ER/LA) opioid analgesics are approved by the United States (US) Food and Drug Administration (FDA) for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.1 Although these medications are an important therapeutic option for many patients, concerns have arisen in recent years about misuse, abuse and the risk of overdose. The Centers for Disease Control and Prevention report that opioid overdose deaths more than tripled between 1999 and 2010, and abuse and misuse account for hundreds of thousands of emergency department (ED) visits each year.2 Although overall opioid overdose rates continue to climb, rates due to natural and semisynthetic opioids, which include prescription ER/LA opioid analgesics, appear to have plateaued or declined starting in 2011.2–4 Likewise, increases in the rate of overdose deaths began to slow in 2006.5 More recently, however, a renewed surge in opioid overdose and opioid overdose death has been driven by fentanyl and synthetic opioids.6
The public-health response to increasing opioid overdoses has taken a multifaceted approach that includes local and state regulations, substance abuse treatment programs, prescription drug monitoring programs, “take back programs,” and other measures. The US FDA required a Risk Evaluation and Mitigation Strategy (REMS) for ER/LA opioid analgesics on 09 July 2012 to reduce addiction, unintentional overdose, and death resulting from inappropriate prescribing, misuse, and abuse.6,7 The REMS requires educational efforts that include: (1) Medication Guides, which are provided to patients at the point of medication dispensing, address issues specific to particular drugs and drug classes, and contain FDA-approved information that can help patients avoid serious adverse events; (2) Patient Counseling Documents to facilitate education of and discussions with patients by ER/LA opioid prescribers and other health-care providers; and (3) voluntary prescriber accredited continuing education on all ER/LA opioid analgesics. Class-wide safety labeling changes were also implemented. Annual REMS assessments submitted to the FDA have shown that approximately 66,219 healthcare providers completed training as of February 2016. Patient survey data found that 96.7% of respondents had received the medication guide.8
The purpose of this study was to describe the incidence of opioid overdose among commercially insured and Medicaid patients dispensed ER/LA opioids before and after REMS implementation.
Methods
We conducted a retrospective cohort study using the HealthCore Integrated Research DatabaseSM (HIRD) as well as de-identified Medicaid data from four states. The HIRD contains longitudinal claims data for a population of over 40 million individuals with commercial insurance. With authorization from Medicaid plans in each participating state, similar claims data for individuals with Medicaid coverage administered through a private insurer were also accessed. We defined two time periods of interest: the REMS pre-implementation period (July 2010-June 2012) and the REMS active period (July 2013-September 2016). We included patients who received at least one ER/LA opioid analgesic dispensing of any duration during at least one REMS study period. Patients were required to have at least 6 months of continuous baseline health plan coverage prior to the first observed dispensing of an ER/LA opioid analgesic during a REMS study period. Subjects who were included in more than one REMS study period had different baseline periods and covariate profiles established for each applicable cohort entry date (Figure 1). Follow-up extended from the first ER/LA opioid dispensing until the end of the REMS period, the end of health plan coverage or a study outcome.
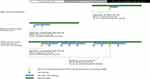 |
Figure 1 Examples of study entry and baseline covariate ascertainment windows. |
Treatment episodes were defined as beginning on the date an opioid medication was dispensed plus the number of days supplied, plus 30 days to account for intermittent use or use of medication still on hand from a prior dispensing. We assumed that concurrent or overlapping medication dispensings were used concurrently. Analyses included person-time during a treatment episode (ie, “exposed person-time”).
In the HIRD, we defined patients as either new users or non-new users upon the start of their follow-up during each REMS period. New users were individuals for whom there were no prior recorded dispensings of ER/LA opioid analgesics identified in the administrative claims data at any time prior to the start of follow-up (noting that the baseline period was a minimum of 6 months long). Non-new users were individuals for whom pharmacy dispensings for ER/LA opioid analgesics were identified within the REMS period-specific baseline period. Patients were considered as new users only in the specific REMS period during which they first started follow-up. For example, a patient who initiated treatment during the pre-implementation period and continued to obtain dispensings during the active period was considered a new user during the pre-implementation period and a non-new user during the active period. Because of the small-available sample size for Medicaid, we did not stratify this group by new versus non-new user status.
Outcomes of interest included ED visits and hospitalizations due to opioid overdose or poisoning defined using diagnosis codes in any position from facility claims (Supplemental Table 1). Validation of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 965 and E850 in Kaiser Permanente Northern California identified a positive predictive value of 83% for analgesic related overdoses or poisonings.9 For commercially insured patients, we linked claims data electronically to the National Death Index (NDI). Patient identifiers were provided to NDI, and a probabilistic matching algorithm was applied to identify matched patients.10 Because patient identifiers could not be used in this way for Medicaid, no overdose death data were available. Patients were then assigned as having a fatal overdose when any cause of death code indicated opioid overdose or poisoning.
Analyses
All analyses were performed separately for commercially insured and Medicaid patients. We first described demographics, medical history, pain conditions, psychiatric comorbidities, health-care utilization and medication use. We used these data to calculate propensity scores predicting ER/LA opioid use during the active period as compared to the pre-implementation period. We excluded patients whose propensity scores fell outside of the area of overlap between the groups by trimming the propensity score range asymmetrically using the 2.5 percentile of the propensity score distribution in the active period as a lower bound and the 97.5 percentile of the propensity score distribution in the pre-implementation period as an upper bound. The standardized difference in the distribution of each covariate included in the propensity score was reviewed within each propensity score decile to ensure balance. Any covariate that had a standardized difference of greater than 0.25 between REMS periods in any of the deciles was considered as a potential covariate along with the propensity score decile in the Poisson regression model. For the commercially insured, covariates were balanced and only indicator variables for propensity score deciles were included in the final models. For the Medicaid population, the number of prior immediate release opioid dispensings and prior use of sleep medications remained unbalanced within propensity score deciles (ie, standardized difference >0.25), and these variables were included in the regression models.
Within each REMS study period, we computed incidence rates as the number of opioid overdose events divided by the person time at risk, and for each rate, we computed an exact 95% confidence interval (CI). Rates were estimated during exposed person-time. For commercially insured patients, we also assessed rates for all users, new users and non-new users.
We presented the incidence rate ratios (IRRs) comparing the active period to the pre-implementation period, both crude and adjusted for propensity score deciles, using Poisson regression models with a generalized estimating equation correction to mitigate overdispersion (stemming from the statistical dependence introduced by having some of the same individuals appearing in both the active and the pre-implementation periods).
We performed all analyses using SAS® Enterprise Guide software version 9.4 (SAS Institute Inc., Cary, NC, USA).
This study was approved by the New England Institutional Review Board, which granted a waiver of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) authorization under 45 CFR 164.512(i). The study was carried out in accordance with the principles of the Declaration of Helsinki and the International Society for Pharmacoepidemiology Guideline for Good Pharmacoepidemiology Practices.
Results
Among the commercially insured, we identified 99,074 individuals with at least one ER/LA opioid dispensing during the pre-implementation period and 92,925 with at least one dispensing during the active period. After restricting to those with at least 6 months of health plan enrollment prior to the first ER/LA opioid dispensing that occurred during the time frame, we retained 71,800 patients in the pre-implementation period and 59,465 in the active period. Numbers in the Medicaid from three states were smaller: 4889 pre-implementation period users and 7921 active period users were included in analyses (Table 1).
 |
Table 1 Formation of the Study Cohorts* |
Comparing the active versus pre-implementation period, ER/LA users within the HIRD were similar with respect to age (median 48 years in the active period and 51 in the pre-implementation period), gender (54.6% versus 55.4% female) and residence setting (72.4% versus 72.9% urban). Medicaid ER/LA users were also similar comparing the active versus pre-implementation period, however they were younger (median age 44 years in the pre-implementation period), more often female (63% female) and less often of urban residence (68.5%) than the commercially insured. Duration of follow-up was slightly longer in the active period than the pre-implementation period for the commercially insured (13 months versus 11 months) and substantially longer for the Medicaid population (18 months versus 8 months, Table 2).
 |
Table 2 Patient Characteristics by Study Period and Insurance Type |
Across time periods, a large majority of patients were diagnosed with back pain at baseline (78.8% of the commercially insured and 87.2% of the Medicaid insured in the Active Period). Other common pain diagnoses included arthritis, abdominal pain, and chronic pain. Nearly all pain diagnoses were more often recorded during the active period than the pre-implementation period in both commercially insured and Medicaid patients (eg, fibromyalgia was recorded for 29.7% of the commercially insured in the active period and 27.1% in the pre-implementation period). Chronic pain was recorded for a higher proportion of Medicaid patients (60.8% active period, 56.4% pre-implementation period) than commercially insured patients (36.5% active period, 25.8% pre-implementation period). Conversely, malignancy was recorded for a higher proportion of commercially insured patients (31.0% active period, 28.3% pre-implementation period) than Medicaid patients (15.5% active period, 12.4% pre-implementation period). Health-care utilization was comparable when comparing the active versus pre-implementation period within each population (Table 2).
Psychiatric comorbidities were consistently more common in the active period than the pre-implementation period for both commercially insured and Medicaid insured individuals, but they were substantially more common in the Medicaid population. The most common psychiatric conditions recorded were anxiety disorders (commercially insured: 36.3% active period, 30.5% pre-implementation period, Medicaid: 51.4% active period, 46.5% pre-implementation period), depression (commercially insured: 32.4% active period, 28.4% pre-implementation period, Medicaid: 47.8% active period, 43.9% pre-implementation period), and sleep disorders (commercially insured: 34.6% active period, 30.7% pre-implementation period, Medicaid: 40.7% active period, 37.8% pre-implementation period). Opioid type dependence, other drug dependence, and history of overdose were also more prevalent at baseline in the active than pre-implementation period and were higher for Medicaid patients than for the commercially insured (eg, opioid type dependence: commercially insured: 7.4% active period, 5.6% pre-implementation period, Medicaid: 24.6% active period, 22.1% pre-implementation period, Table 2).
In terms of opioid use among the commercially insured, a large majority of ER/LA opioid users also had past or present use of immediate-release opioids (89.1% active period, 88.8% pre-implementation period). The most commonly used ER/LA opioids were oxycodone (35.1% active period, 38.6% pre-implementation period) and fentanyl (21.5% active period, 27.2% pre-implementation period), and baseline history of benzodiazepine use was common (46.2% active period, 45.7% pre-implementation period [Table 3]). In Medicaid, immediate-release opioids were nearly universal (96.1% active period, 96.6% pre-implementation period), and the most commonly used ER/LA opioids were fentanyl (48.7% active period, 32.9% pre-implementation period) and morphine (47.9% active period, 41.1% pre-implementation period). During the active period, methadone was used by 31.1% of Medicaid, but by only 7.8% of commercially insured ER/LA opioid users (data not shown). Compared to the commercially insured, baseline history of benzodiazepine use was more common in the Medicaid population (53.7% active period, 55.0% pre-implementation period [Table 3]).
 |
Table 3 Baseline Medication Use by Study Period and Insurance Type |
The commercially insured population had an IR for opioid overdose of 88.94 per 10,000 person years (95% CI 79.90–98.72) in the pre-implementation period and 102.60 (95% CI 93.00–112.93) during exposed person-time. This corresponded to an unadjusted IRR of 1.14 (95% CI 0.94–1.38) that decreased to 1.01 (95% CI 0.87–1.17) after propensity adjustment. Observed overdose rates were higher in new users than non-new users (active period incidence rate [IR] 140.03 vs 85.49). Rates appeared to decrease over time when comparing the active versus pre-implementation period for non-new users (propensity score adjusted IRR 0.87, 95% CI 0.72–1.05) and increase over time for new users (propensity score adjusted IRR 1.29, 95% CI 1.02–1.65). Opioid overdose mortality had an IR of 9.31 (95% CI 6.55–12.83) in the pre-implementation period and 7.08 (95% CI 4.74–10.17) during exposed person-time. This corresponded to an unadjusted IRR of 0.76 (95% CI 0.47–1.24) that decreased to 0.71 (95% CI 0.43–1.17) after adjusting for propensity score decile. (Table 4, Figure 2).
 |
Table 4 Opioid Overdose or Poisoning Events |
 |
Figure 2 Incidence rate ratio of opioid overdose and opioid overdose death.Abbreviation: HIRD, HealthCore Integrated Research Database. |
The rate of opioid overdose was strikingly higher in Medicaid with an IR for opioid overdose of 237.49 (95% CI 184.79–300.56) in the pre-implementation period and 192.00 (95% CI 162.60–255.18) during exposed person-time. This corresponded to an unadjusted IRR of 0.89 (95% CI 0.63–1.27) that decreased to 0.70 (95% CI 0.52–0.93) after adjusting for propensity score decile (Table 4, Figure 2).
Discussion
In this cohort study of commercially insured and Medicaid patients in a US administrative claims database, incidence rates of opioid overdose or poisoning did not meaningfully decrease after introduction of the REMS among the commercially insured patient cohort, and declined slightly in the Medicaid population. A slight numerical decrease in overdose mortality was also observed for the commercially insured.
Although substantially elevated rates of opioid overdose in Medicaid patients have been previously described,11 the comparatively higher incidence in Medicaid patients compared to commercially insured patients observed here is striking. Our inability to assess mortality data for Medicaid patients is a limitation of the study. One of the reasons for higher incidence rates among the Medicaid patients could be differences in the distributions of certain patient characteristics associated with increased opioid overdose risk that are typical of commercially insured and Medicaid insured patients, such as a higher prevalence of psychiatric comorbidities among the Medicaid insured compared to the commercially insured. It is also plausible, given substantially higher rates of diagnosed opioid type dependence and use of methadone in Medicaid patients, that a higher proportion of Medicaid patients than commercially insured patients were receiving ER/LA opioids for maintenance therapy rather than pain management. Because administrative claims do not directly capture the indication for which a medication is prescribed, it is not possible to differentiate analgesic use from use for maintenance therapy.
Although some medications used for maintenance therapy are dispensed outside of routine care and may not be captured in administrative data, rates of methadone and buprenorphine use were higher in Medicaid than commercially-insured patients. If this is the case, some of the observed ER/LA use may be comingled with illicit opioid use where individuals may relapse due to use of opioids from non-medical sources. Administrative claims cannot define whether the medication that caused an overdose was prescribed or non-prescribed; the observed rate in these patients may reflect overdoses caused by illicit use. Further, the Medicaid population included in this study derives from only four states, and we cannot exclude the possibility that they are not fully representative of other Medicaid plans.
Higher rates of opioid overdose or poisoning in new users compared to non-new users warrant further investigation. The influence of confounding by unmeasured or poorly captured characteristics such as use of medication from non-medical sources and addiction disorder may play a role. Management of patients for whom long-term pain treatment is required also may differ from new users, who may include a higher proportion of patients for whom ER/LA opioid analgesic treatment is not indicated. It is plausible, for example, that pain physicians managing long-term users are more experienced in prescribing these medications. Their patients could be more aware of the serious risks of these medications as suggested by a recent survey of commercially insured ER/LA opioid users. Further, medication tolerance, which is more likely in chronic users12 but cannot be well measured in administrative data, may have an impact on differences in findings between new and non-new users. Additionally, opioid overdose risk may not be constant over the course of treatment, with higher rates of overdose (1) early on in the course of treatment, and (2) later on for a subset of patients who develop opioid use disorders. It should be noted that the high-risk period early in the course of treatment is missed in non-new users, so this subgroup may be limited to those who did not experience adverse events early on. This may explain some differences between new and non-new users and differences in the observed overdose rates in the active and pre-implementation period for Medicaid given that follow-up was longer in the active period.
Because the REMS study periods are perfectly correlated with calendar time, the influence of the REMS on study results is difficult to disentangle from other contemporaneous factors, and results cannot be attributed solely to the REMS. Along with the implementation of the REMS, many other interventions targeting opioid analgesics have occurred. Efforts to develop abuse-deterrent formulations, enhanced requirements from both the FDA and Drug Enforcement Administration (DEA) and legislative changes at all levels of government have proceeded alongside REMS implementation. Prescription drug monitoring programs have been established in 49 states to address misuse, abuse and diversion of these controlled substances.13,14 The National Governors Association State Policy Academy on Reducing Prescription Drug Abuse is among entities promoting state-based efforts to address the “opioid epidemic.” In Oregon, for example, a task force is implementing strategies to reduce the number of pills in circulation, educate prescribers and the public on risks, improve safe disposal, and provide treatment for addicted patients.15 Health system and health plan interventions on prescribing have also been implemented.16 Given that the study REMS periods occurred during implementation of these and other measures, the extent to which any other concomitant effort has influenced rates of overdose over time is unknown. Further, whether or not each patient’s opioid prescriber(s) had completed REMS-compliant continuing education is unknown in this assessment. Given that this is one of the primary mechanisms through which the education-focused REMS may impact opioid harm, a more informative future approach may be to directly compare opioid overdose rates in patients whose health-care providers did versus did not complete continuing education activities.
Our estimates of incidence may be subject to diagnostic bias. It is plausible, for example, that increased awareness of the serious risks of ER/LA opioid analgesics among the medical community could result in more widespread attribution of overdose events to opioids and the familiarity with and use of opioid-specific ICD-9-CM or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis overdose codes. Were that the case, an opioid overdose event may be more likely to be recorded as such in the active period, which would tend to mask an effect of the REMS. Additional code availability in ICD-10 may also result in more sensitive but less specific capture of overdose in this coding system, which was implemented in 2015 in the US. The plausibility of this is increased given that the observed incidence of overdose identified by administrative claims-based sources increased sharply after the introduction of ICD-10,17 however formal validation studies have not been completed to date.
This study utilized an administrative claims database, and it is subject to the limitations inherent in the use of such data. The majority of analyses were conducted using a database that is representative of the commercially insured population in the US and is therefore not representative of individuals without medical insurance. Given differences in patient characteristics between commercially and Medicaid insured individuals and a substantially higher rate of overdose in Medicaid patients, better characterization of this at-risk population is important.
Finally, although all patients were required to have a pharmacy benefit, patients for the main analyses of ER/LA opioid analgesic users were identified on the basis of submitted pharmacy claims, excluding patients who chose not to use their pharmacy benefit from the cohort. Insurance coverage typically presents a strong financial incentive for use of the pharmacy benefit; however, it is possible that patients more likely to abuse opioids (who are therefore at higher risk for overdose, poisoning and death) could choose to pay for some or all of their prescriptions with cash.18
Conclusion
Despite these limitations, this study provides insight on the incidence of opioid overdose and poisoning in the context of the class-wide ER/LA opioid analgesic REMS in a large population. Continued efforts to reduce opioid harm are needed both overall and to reduce disparities between those with Medicaid and commercial insurance.
Disclosure
CN Holick, N Liu, SK Vojjala and S Lanes are employees of HealthCore, which received funding from the REMS Program Companies (RPC) to conduct the study. DB Esposito, C Knox, VCA Desai and J Lyons were employees of HealthCore at the time of the study. MS Cepeda is employed by Janssen Research and Development, and GP Wedin is employed by Upsher-Smith Laboratories, LLC. HealthCore, Inc. lead all aspects of the study from Protocol development through manuscript development with review, input, and contributions from the Sponsor on all documents. The authors report no other conflicts of interest in this work.
References
1. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician. 2008;11(2 Suppl):S5–S62.
2. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi:10.1056/NEJMsa1406143
3. West NA, Dart RC. Prescription opioid exposures and adverse outcomes among older adults. Pharmacoepidemiol Drug Saf. 2016;25(5):539–544. doi:10.1002/pds.v25.5
4. West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend. 2015;149:117–121. doi:10.1016/j.drugalcdep.2015.01.027
5. Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths involving opioid analgesics: united States, 1999–2011. NCHS Data Brief. 2014;166:1–8.
6. Stanos S. Evolution of opioid risk management and review of the classwide REMS for extended-release/long-acting opioids. Phys Sportsmed. 2012;40(4):12–20. doi:10.3810/psm.2012.11.1975
7. U.S. Food and Drug Administration. Risk evaluation and mitigation strategy (Rems) for extended-release and long-acting opioids; 2013. Available from: https://www.fda.gov/drugs/information-drug-class/opioid-analgesic-risk-evaluation-and-mitigation-strategy-rems. Accessed March 31, 2019.
8. Drug Safety And Risk Management Advisory Committee Anesthetic And Analgesic Drug Products Advisory Committee Briefing Book For ER/LA Opioid Analgesic REMS. Advisory Committee; 30 March 2016. Available from: https://www.fda.gov/advisory-committees/anesthetic-and-analgesic-drug-products-advisory-committee/2016-meeting-materials-anesthetic-and-analgesic-drug-products-advisory-committee. Accessed March 31, 2019.
9. Janoff S, Coplan P, Perrin N, et al. Validation of opioid overdoses and poisonings in electronic medical records with medical chart reviews. In:
10. Centers for Disease Control. National Death Index User’s Guide. Statistics NCfH, ed. Available from: https://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. Accessed March 31, 2019.
11. Sharp MJ, Melnik TA. Centers for disease C, prevention. Poisoning deaths involving opioid analgesics - New York state, 2003–2012. MMWR Morb Mortal Wkly Rep. 2015;64(14):377–380.
12. Collett BJ. Opioid tolerance: the clinical perspective. Br J Anaesth. 1998;81(1):58–68. doi:10.1093/bja/81.1.58
13. Hansen M. Using prescription drug monitoring programs to address drug abuse. NCSL Legisbrief. 2015;23(12):1–2.
14. Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33(5):319–328. doi:10.3109/01612840.2011.654046
15. McCarty D, Bovett R, Burns T, et al. Oregon’s strategy to confront prescription opioid misuse: a case study. J Subst Abuse Treat. 2015;48(1):91–95.
16. Saunders KW, Shortreed S, Thielke S, et al. Evaluation of health plan interventions to influence chronic opioid therapy prescribing. Clin J Pain. 2015;31(9):820–829. doi:10.1097/AJP.0000000000000159
17. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US Transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918–923. doi:10.1097/MLR.0000000000000805
18. Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Opioid shopping behavior: how often, how soon, which drugs, and what payment method. J Clin Pharmacol. 2013;53(1):112–117. doi:10.1177/0091270012436561
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
