Back to Journals » Infection and Drug Resistance » Volume 13
Incidence of Mortality and Its Predictors Among Adult Visceral Leishmaniasis Patients at the University of Gondar Hospital: A Retrospective Cohort Study
Authors Yeshaw Y , Tsegaye AT , Nigatu SG
Received 14 January 2020
Accepted for publication 6 March 2020
Published 24 March 2020 Volume 2020:13 Pages 881—891
DOI https://doi.org/10.2147/IDR.S245991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Yigizie Yeshaw,1 Adino Tesfahun Tsegaye,2 Solomon Gedlu Nigatu2
1Department of Medical Physiology, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 2Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Yigizie Yeshaw Email [email protected]
Background: Visceral leishmaniasis (VL) is a neglected tropical disease, affecting the poor and productive age group of a country, resulting in a huge impact on its economic development. Even though anti-leishmanial drugs reduce the incidence of mortality among VL patients, there is still death of these patients while on treatment. In this aspect, there are limited studies in Ethiopia; therefore, this study aimed to determine the incidence of mortality and its predictors among adult VL patients at the University of Gondar Hospital.
Methods: Institution-based retrospective cohort study was conducted among 586 adult visceral leishmaniasis patients who were admitted to the University of Gondar Hospital from 2013 to 2018. Data were collected from the patients’ charts and registration books, and analyzed using Stata 14 software. Kaplan–Meier failure curve and Log rank test was used to compare the survival probability of patients with independent variables. A multivariable stratified Cox regression model was used to identify predictors of mortality among VL patients. P≤ 0.05 was employed to declare statistically significant factors. Adjusted hazard ratio (AHR) and 95% confidence interval (95% CI) were estimated for potential risk factors included in the multivariable model.
Results: A total of 586 VL patients were included in the study. The age of patients ranged from 18 to 55 years with a median age of 27 years. The incidence of mortality was 6.6 (95% CI: 5.2– 8.4) per 1000 person-days of observation. Independent predictors of mortality were presence of comorbidity (AHR=2.29 (95% CI: 1.27– 4.11)), relapse VL (AHR=3.03 (95% CI: 1.25– 7.35)), treatment toxicity (AHR=5.87 (95% CI: 3.30– 10.44)), nasal bleeding (AHR=2.58 (95% CI: 1.48– 4.51)), jaundice (AHR=2.84 (95% CI: 1.57– 5.16)) and being bedridden at admission (AHR=3.26 (95% CI: 1.86– 5.73)).
Conclusion: The incidence of mortality among VL patients was high. Mortality was higher among VL patients with concomitant disease, relapse VL, treatment toxicity, nasal bleeding, jaundice, and those who were bedridden at admission, which implies that great care should be taken for these risky groups through strict follow-up and treatments.
Keywords: mortality, visceral leishmaniasis, Ethiopia
Background
Visceral leishmaniasis (kal-azar) is a neglected tropical disease caused by a protozoa parasite called Leishmania donovani complex (L. donovani and L. infantum), transmitted by a female phlebotomine sand fly. It is characterized by prolonged fever, weight loss, decreased appetite, anemia, and hepatosplenomegaly.1–3
Globally about 500,000 new cases of visceral leishmaniasis (VL) occur every year. Of these, over 90% of the global burden of VL occurs in poor, rural and suburban areas in seven countries including Ethiopia.2,4 Among Eastern Africa countries, Ethiopia is the second affected country following Sudan.5 The mortality rate of VL is 3.7% in Eastern Uganda6 and 4.8% in Ethiopia.7 The finding of another two pocket studies conducted in Tigray, Ethiopia, reported 12.4%8 and 18.5%9 proportion of death among VL patients.
Visceral leishmaniasis is associated with 2,357,000 disability-adjusted life years (DALYs).3,10 If not appropriately treated; over 95% of VL cases will eventually die. This rate is surpassed among parasitic diseases only by malaria.11
The emergence of VL in Ethiopia places a huge burden on society in terms of mortality, and impact on country’s economy. This is because the disease is more prevalent in Kola to Weina Dega agro-ecological zones of Ethiopia, areas where major agricultural projects exist.12
Predictors of mortality among VL patients include presence of drug toxicity,13 malnutrition,3,14,15 VL-HIV co-infection,5,8,15–22 thrombocytopenia,5,10,16–19,23 leukopenia,5,16–19,24 jaundice,5,16–19,24 relapsing course of the disease,10,20,23 high parasite load,25–27 renal failure (creatinine >1.5 mg/dl),18,24 diarrhea,9,10,23 nasal bleeding,5,9,16–19 anemia,10,15,23 inability to walk at admission,8 longer duration of illness,15 concomitant disease,5,16–19 late diagnosis6,8,28 and edema.5,16–19
Even though the introduction of more effective anti-leishmanial drugs has reduced the case fatality rate of VL nowadays,4 it is still one of the leading health problems in Ethiopia and causing a reduction in productivity by affecting a significant portion of the poor, rural, and productive age group of the country.29
However, there is a scarcity of data about the incidence of mortality and its predictors among adult VL patients. Hence, considering VL severity, lethality rate as well as its impact on a country, early identification of factors associated with mortality among VL patients is relevant to the establishment of appropriate measures. Therefore, the objective of this study is to determine the incidence of mortality and its predictors among adult VL patients at the University of Gondar Hospital.
Methods
Study Area, Period and Population
An institution-based retrospective cohort study was employed at the University of Gondar Hospital from January 1, 2013 to December 30, 2018. The University of Gondar Hospital is a tertiary health care center located 727 km far away from the capital city, Addis Ababa, in the northwest direction. The hospital serves for a population of around five million across the region and has a Leishmaniasis Research and Treatment Center (LRTC), which was established in 2004 in collaboration with Drugs for Neglected Diseases initiatives (DNDi). Visceral leishmaniasis-suspected patients are referred to the hospital from its different units and other health facilities in the country for further investigation and treatment. Patients admitted for VL treatment at LRTC are routinely evaluated, and the findings are documented in their own chart and registration books. The LRTC currently serves for more than 300 VL patients per year.7 Adult VL patients who received treatment for anti-leishmaniasis drug in the study period were the study population. Those patients with unknown treatment outcomes, no recorded date of treatment initiation and treatment outcome were excluded.
Diagnostic Procedures and Protocol of Treatment
A VL case was suspected based on the World Health Organization’s clinical case definition: A person who presents with fever for more than two weeks and an enlarged spleen (splenomegaly) and/or enlarged lymph nodes (lymphadenopathy), or either loss of weight, anemia or leucopenia while living in a known VL endemic area or having traveled to an endemic area. Patients who met this case definition were eligible for confirmatory VL tests and VL diagnostic tests were done using microscopic detection of the parasite in tissue aspirates (spleen, bone marrow, lymph node) and serology. Individuals who had confirmed VL were treated with any of these three groups of treatment: Sodium stibogluconate only (for 28 days), a combined therapy of Sodium stibogluconate and Paromomycin (for 17 days) and ambisome (for 6 days). Therefore, these patients’ were followed retrospectively from the time of treatment initiation until treatment ends. Hence, the follow-up time ranges from 6 to 28 days depending on the type of treatment they took. At the end of the follow-up time, patients were said to be censored if they did not die or event if they died.
Software and Sample Size
The sample size for this study was calculated through Stata 14 software using 12.4% probability of an event (death) in another similar setting,8 80% power, hazard ratio of two, 5% significance level, and 10% for incomplete data. Accordingly, the final sample size was 586. A computer-generated simple random sampling technique was employed to select those sampled patients’ charts from a total of 1899 patients that had been on VL treatment from 2013 to 2018.
Study Variables
The dependent variable was time until the death of the patient. Independent variables include socio-demographic variables (age, sex, residence, migration status), clinical and laboratory-related variables such as visceral leishmaniasis parasite load, leukopenia, thrombocytopenia, hemoglobin level, treatment type, toxicity during treatment, late diagnosis, VL episode, concomitant disease, condition of patient at admission, creatinine level, diarrhea, jaundice, body mass index (BMI), nasal bleeding and edema.
Operational Definitions
Any documented death of VL patients while taking the treatment during the follow-up period was considered as event and patients who were transferred out or loss to follow-up or treatment failure or became initial cured were censored.
Initial cure: declared when a patient shows an improvement of signs and symptoms at the end of treatment (depending on the category of treatment). Treatment failure: defined as a positive test of cure (parasitological failure) and/or persisting clinical signs/symptoms at the end of treatment or failure to continue first-line treatment for safety reasons.
Loss to follow-up: a patient who started VL treatment but interrupted treatment due to the patient leaving the hospital during the study period.8,13,30
Primary VL case: a patient who is diagnosed with visceral leishmaniasis for the first time in which diagnosis relies on a positive serological test for VL (rK39-based rapid test and/or DAT direct agglutination test) and/or a positive parasitological test (microscopic detection of Leishmania parasites in spleen, lymph node, and bone marrow aspirates).
Relapse VL: a patient with a history of previous VL and discharged improved or with a negative test of cure (TOC) after treatment and who then presents with symptoms of VL after four weeks of initial VL treatment and is parasitologically confirmed and documented as relapse VL.31
High parasite load: a parasite load grade of more than 4+ (1–10 parasites per field). If a parasite load grade is less than or equal to 3(1–10 parasites per 10–1000 fields) it is called low parasite load.
Concomitant disease: the presence of one or more of a documented case of diseases such as tuberculosis, pneumonia, malaria, and HIV.
Toxicity during treatment: the presence of one or more of documented toxicity such as cardiac arrest, pancreatitis, jaundice (liver disease) and kidney failure.18
Data Collection Procedure and Tools
Data were collected from VL patient registration books and charts. Pretested, structured data extraction checklist was used to collect the data.
Four BSc nurse data collectors were recruited and trained about ways of extracting data. Clinical and laboratory parameters such as parasite load, leukopenia, thrombocytopenia, hemoglobin level, treatment type, late diagnosis, VL episode, concomitant disease, general condition of the patient at admission, diarrhea, jaundice, BMI, nasal bleeding, edema, and creatinine level were extracted. The presence of toxicity during treatment was also assessed. The presence or absence of those abnormalities was decided based on the documentation made by the physicians. Laboratory results were also collected and their values were compared with their reference values to decide on the presence of derangement on these parameters.
Data Quality Management
To assure the data quality, high emphasis was given in designing the data collection instrument. Training was given for data collectors to create a common understanding of the data extraction checklist and chart reviewing skills. The data extraction checklist was pre-tested. Throughout the data collection period, data collectors were supervised by the principal investigator.
Data Analysis Procedure
Data were checked for completeness, clarity, accuracy on daily bases and entered into Epi-data version 3.1. Then, it was exported to Stata 14 software for analysis.
Person-days of observation (PDO) were calculated by subtracting the date of starting anti-leishmanial treatment from the date of death or censored. The failure probability of patients during VL treatment to different independent variables was described with the Kaplan–Meier (KM) curve. A Log rank test was also used to test the failure differences among the categories of each independent variable.
Schoenfeld residuals test (both global and scaled) and graphical methods were used to check the Cox proportional hazard assumption. Model adequacy was also checked using the Cox Snell residuals.
A multivariable stratified Cox model was used to identify predictors of mortality among VL patients. All variables with a p-value of <0.2 at bi-variable analysis were entered into the final model. P ≤ 0.05 was employed to declare the statistically significant variables. Adjusted Hazard Ratio (AHR) and its corresponding 95% confidence interval (95% CI) were estimated for potential risk factors included in the multivariable stratified Cox model.
Results
Sociodemographic and Baseline Clinical Characteristics of VL Patients
A total of 586 visceral leishmaniasis patients were included in the study. Almost all 584 (99.7%)) of them were males. The age of patients ranged from 18 to 55 years with a median age of 27 years. Most of the patients (470 (80.2%)) were migrant workers.
The majority of VL patients, 561 (95.7%), had primary visceral leishmaniasis. From a total of 586 patients, 169 (28.8%) of them had concomitant disease at admission. Of these, about half of them had pneumonia (49.7%). Sixty-seven (11.4%) of the study participants had a high parasite load. Forty-one (7.0%) of study participants had toxicity during treatment. Of these, 15 (36.6%) of them had cardiac arrest followed by pancreatitis, 10 (24.4%). Regarding the duration of illness, 258 (44%) of them had more than 30 days of illness duration at admission (Table 1).
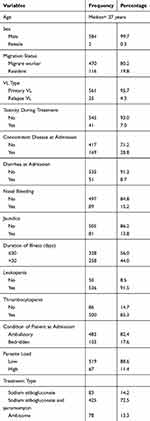 |
Table 1 Sociodemographic and Baseline Clinical Characteristics of VL Patients at the University of Gondar Hospital, 2019 (n=586) |
Comparison of Failure Functions
Kaplan–Meier’s failure curve was used to compare death probability among categories of each independent variable visually. A Log rank test was also used to objectively judge the presence or absence of a difference in death probabilities among different categories of each independent variable. Accordingly, the Kaplan–Meier failure curve was done for all possible predictors. For instance, relapse VL patients had shorter survival experience than primary VL cases. This visually observed difference was also statistically significant (Log-rank, p<0.001). Visceral leishmaniasis patients with comorbidity at admission had shorter survival experience than those VL patients without comorbidity (Log-rank, p<0.001). Visceral leishmaniasis patients with jaundice at admission had shorter survival experience than those VL patients without jaundice (Log-rank, p<0.001) (Figure 1).
 |
Figure 1 Kaplan–Meier failure curves for some of the variables among the cohort of VL patients at the University of Gondar Hospital, 2019. |
Assessing Proportional Hazard Assumption
The proportional hazard assumption was checked both graphically and Schoenfeld residuals test (global and scaled) for all possible predictors of VL mortality. Just to show for some of the variables –Ln (-Ln (survival probability) to Ln (analysis time) for jaundice, comorbidity, and residence was demonstrated graphically. Accordingly, the hazards do not cross between categories of jaundice and comorbidity, which means that the proportional hazard assumption was satisfied for these variables. However, it crosses between categories of residence, which means that the proportional hazard assumption was not satisfied for residence (Figure 2).
 |
Figure 2 Proportional hazard plot for some of the variables among the cohort of VL patients at the University of Gondar Hospital, 2019. |
Moreover, to test the proportional hazard assumption objectively, the Schoenfeld residuals test (global and scaled) was done. Accordingly, all variables except residence satisfy the proportional hazard assumption (p>0.05) (Table 2).
 |
Table 2 Proportional Hazard Assumption Test for the Study on Incidence of Mortality and Its Predictors Among VL Patients at the University of Gondar Hospital, 2019 |
Incidence and Predictors of Mortality Among VL Patients
From a total of 586 VL patients who started anti-leishmanial treatment during the study period, 65 (11.09%) of them died, 483 (82.4%) cured, 26 (4.4%) lost to follow-up, 9(1.5%) treatment failure, and the rest, 3 (0.5%) were transferred out. The total cohort contributed 9830 person-days, resulting in the overall mortality rate of 6.6 deaths (95% CI: 5.2–8.4) per 1000 person-days of observation. Of the 65 deaths, 39 (60%) of them occurred within the first 10 days of treatment initiation.
Variables with p<0.2 on bivariable analysis were entered into the multivariable stratified Cox model and six variables were found to be independent predictors of mortality among VL patients while on treatment (p≤ 0.05). These were concomitant disease, episode of visceral leishmaniasis, toxicity during treatment, nasal bleeding, jaundice, and condition of a patient at admission.
The hazard of death among patients with relapse VL was 3 (AHR=3.03 (95% CI: 1.25–7.35)) times higher than patients with primary VL. The hazard of death was 5.9 (AHR=5.87 (95% CI: 3.30–10.44)) times higher among patients who had toxicity during treatment as compared to those patients who did not have toxicity. The hazard of death among VL patients with comorbidity was 2.3 (AHR=2.29 (95% CI: 1.27–4.11)) times higher than those who did not have. The hazard of death among VL patients who had nasal bleeding was 2.6 (AHR=2.58 (95% CI: 1.48–4.51)) times higher than those patients who did not have nasal bleeding. Visceral leishmaniasis patients who had jaundice at admission were 2.8 (AHR=2.84 (95% CI: 1.57–5.16)) times more at risk of death than their counterparts. Those patients who were bedridden had 3.3 (AHR=3.26 (95% CI: 1.86–5.73)) times increased risk of death compared to ambulatory patients (Table 3).
 |
Table 3 Multivariable Stratified Cox Regression Analysis for Incidence of Mortality Among VL Patients at the University of Gondar Hospital, 2019 |
Discussion
This study aimed to identify the incidence of mortality and its predictors among adult VL patients on treatment. It shows that concomitant disease, episode of visceral leishmaniasis, toxicity during treatment, nasal bleeding, jaundice, and condition of the patient at admission were the predictors of mortality among VL patients. This finding is important to reconsider the frequency of follow-up time and quality of care among VL patients.
The overall incidence rate of mortality among VL patients was 6.6 (95% CI 5.2–8.4) per 1000 person-days of observation, with most of the deaths (60%) occurred within the first 10 days of follow-up period, implying that great attention should be there especially in the early phase of treatment initiation.
In this study, the proportion of death among VL patients was 11.09% (95% CI: 10.85% −13.6%), which is in line with a study conducted in Kahsay Abera Hospital (12.4%).8 This consistency might be due to the similarity in the quality of care given for VL patients in these Hospitals. Moreover, most of the patients in the current and earlier study were rural migrant workers, which share a similar economic level to have comparable risk of death.
However, the current finding is less than the finding in Tigray (18.5%).9 The possible explanation for this difference might be differences in type of antileishmanial drugs used, in which patients in the earlier study used only sodium stibogluconate, which is often poorly tolerated, and toxic drug, to result in a significant incidence of serious adverse events such as toxicity of pancreas, liver, kidney, and heart than other anti-leishmanial drugs.32
On the contrary, the current finding is higher than the finding of Eastern Uganda (3.7%),6 and Northwest Ethiopia (4.8%).7 The reason for this discrepancy in the case of Eastern Uganda might be that only primary VL cases were included in the study, which may underestimate the death rate, as death is more common among relapse cases than primary VL patients.20,23 In the case of an earlier study of Ethiopia, VL patients who were taking Amphotericin B only were included, a drug with less toxicity and more tolerability than that taken by participants of this study such as Sodium stibogluconate.11
In our study, the hazard of death among relapse VL patients was 3 times higher than that of primary VL patients. This implies that a significant proportion of patients with relapse VL are dying compared to patients with primary VL. This finding is similar to the finding of two studies in Brazil.20,23 This might be due to the fact that the majority of the relapse cases in this study were HIV positive (64%). As a result, both VL and HIV attack the immune system of the body to cause a profound immune deficiency state. The presence of HIV complicates the management of VL as well. Visceral leishmaniasis lowers the total lymphocyte count (TLC) and Cluster of Differentiation four (CD4) count to a great extent by depressing the bone marrow and the splenic activities.33 All these mechanisms lead to a higher incidence of death among patients with relapse VL compared to primary once.
VL patients who had toxicity during treatment were at increased risk of mortality than those patients who had no toxicity. This finding in line with the finding of a study in Uganda,6 and national guideline reports of Somali11 and Ethiopia.10 Since most anti-leishmanial drugs are toxic, the development of drug toxicity such as arrhythmia, pancreatitis, and others are common, leading to poor compliance and further deterioration of the patient to cause death.13
The hazard of death among VL patients with comorbidity was higher than those without comorbidity. This finding is in agreement with the finding of studies in Eastern Uganda, Brazil, India, and Ethiopia.6,20–22,28 Probably ascribed to the double burden associated with the comorbidity. Moreover, patients with concomitant disease/comorbidity had to take more drugs so they might have more risk of toxicity and drug–drug interaction, which causes a severe form of the disease to end up with the death of the patient.
The hazard of death among VL patients who had nasal bleeding was higher than those patients who did not have nasal bleeding. This finding is consistent with the results of studies conducted in Northern Ethiopia (Tigray), America and Sudan.5,9,19 Nasal bleeding among VL patients occurs probably due to a combination of deficient clotting factors and platelet count, which increases the risk of death among VL patients.34
In this study, VL patients who had jaundice at admission were at increased risk of death than their counterparts. This finding is also similar with those of studies conducted in Gedaref state of Sudan, America and Brazil.5,16–19,24 This could be possibly due to the presence of liver dysfunction among VL patients with jaundice (jaundice is usually the sign of liver dysfunction); therefore, VL patients with jaundice might have decreased plasma protein synthesis, inability to detoxify drugs and impairment of other liver functions compared to VL patients without jaundice.
Those bedridden VL patients had an increased risk of death compared to ambulatory VL patients. The current finding is in agreement with the finding of a study in Kahsay Abera Hospital.8 This might be linked to the majority of bedridden patients (64%), in this study, had concomitant diseases such as HIV/AIDS, tuberculosis and pneumonia, which ultimately increases the risk of death compared to ambulatory patients. This explanation is supported by studies conducted in Brazil, which states that most severely ill patients have an increased risk of concomitant diseases that can increase their risk of death.16,35 Furthermore, severely ill patients usually do not respond to their medication easily and do not take adequate food as well.
We authors strongly believe that the present study is very important in providing evidences about the incidence of mortality and its predictors among VL patients. However, due to its retrospective nature, there are unmeasured confounders such as blood glucose level, serum albumin level and income of the patient. So we cannot assess the effect of these variables on the incidence of mortality among VL patients.
Conclusion
The incidence of mortality among VL patients on treatment was high. The risk of death was higher among VL patients with concomitant disease, relapse VL, treatment toxicity, nasal bleeding, jaundice and those who were bedridden at admission. Therefore, it is better to strictly follow and treat VL patients especially for those who had toxicity during treatment, nasal bleeding, jaundice, relapse VL and bedridden once. High emphasis should also be given for these VL patients with other comorbidities such as pneumonia, HIV/AIDS, and tuberculosis.
Abbreviations
AHR, Adjusted Hazard Ratio; BMI, Body Mass Index; LRTC, Leishmaniasis Research and Treatment Center; TOC, Test of Cure; VL, Visceral Leishmaniasis.
Data Sharing Statement
All data relevant to the study are included in the article. Datasets used for the analysis of the study can be provided with a reasonable request of the corresponding author.
Ethics and Consent Statement
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar (Reference number: IPH/180/06/2011). Written permission letter for extracting data from the patients’ chart was also obtained from the University of Gondar Hospital. Privacy and confidentiality of information were kept properly and names of patients, as well as other personal identifiers, were not recorded.
Acknowledgments
We would like to thank the University of Gondar for giving this chance to conduct the study. Our honest gratitude also goes to LRTC of the University of Gondar Hospital, staff working at the center especially Helina Fikre, data collectors and chart room workers.
Author Contributions
YY conceived and designed the study, collect the data, conducted data analysis, interpret the data and drafted the manuscript for publication. SGN and ATT conceived and designed the study, supervised the data collection process, analyze and interpret the data, and drafting the article. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Funding
No specific funding was obtained to conduct the study.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Biologics I for international cooperation in animal. Leishmaniasis Leishmaniasis (cutaneous and visceral). Cent Food Secur Public Health. 2017;1–18.
2. WHO FR. Book review: working to overcome the global impact of neglected tropical diseases. Perspect Public Health. 2012;132(4):192. doi:10.1177/1757913912449575
3. Marinkelle CJ. The control of leishmaniases. Bull World Health Organ. 2010;58(6):807–818. doi:10.1088/1751-8113/44/8/085201
4. Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):1–12. doi:10.1371/journal.pone.0035671
5. Mueller YK, Nackers F, Ahmed KA, et al. Burden of visceral leishmaniasis in villages of Eastern Gedaref State, Sudan: an exhaustive cross-sectional survey. PLoS Negl Trop Dis. 2012;6(11):1–6. doi:10.1371/journal.pntd.0001872
6. Mueller Y, Mbulamberi DB, Odermatt P, Hoffmann A, Loutan L, Chappuis F. Risk factors for in-hospital mortality of visceral leishmaniasis patients in eastern Uganda. Trop Med Int Heal. 2009;14(8):910–917. doi:10.1111/j.1365-3156.2009.02305.x
7. Tamiru A, Tigabu B, Yifru S, Diro E, Hailu A. Safety and efficacy of liposomal amphotericin B for treatment of complicated visceral leishmaniasis in patients without HIV, North-West Ethiopia. BMC Infect Dis. 2016;16(1):1–7. doi:10.1186/s12879-016-1746-1
8. Welay GM, Alene KA, Dachew BA. Visceral leishmaniasis treatment outcome and its determinants in northwest Ethiopia. Epidemiol Health. 2016;39:1–6. doi:10.4178/epih.e2017001
9. Lyons S, Veeken H, Long J. Visceral leishmaniasis and HIV in Tigray, Ethiopia. Trop Med Int Heal. 2003;8(8):733–739. doi:10.1046/j.1365-3156.2003.01088.x
10. Director H promotion and D prevention DG. Guideline for diagnosis, treatment and prevention of leishmaniasis in Ethiopia. 2017;91:1–88.
11. Government SF. Guidelines for diagnosis, treatment and prevention of visceral leishmaniasis in Somalia. 2012:1–82.
12. Gadisa E, Tsegaw T, Abera A, et al. Eco-epidemiology of visceral leishmaniasis in Ethiopia. BMC. 2015;8(1):1–10. doi:10.1186/s13071-015-0987-y
13. Singh P, Kumar M. Current treatment of visceral leishmaniasis (Kala-azar): an overview. Int J Res Med Sci. 2014;2(3):810. doi:10.5455/2320-6012.ijrms20140808
14. Okwor I, Uzonna J. Review article social and economic burden of human leishmaniasis. Am Soc Trop Med Hyg. 2016;94(3):489–493. doi:10.4269/ajtmh.15-0408
15. Collin S, Davidson R, Ritmeijer K, et al. Conflict and Kala‐Azar: determinants of adverse outcomes of Kala‐Azar among patients in Southern Sudan. Clin Infect Dis. 2004;38(5):612–619. doi:10.1086/381203
16. Queiroz A, Cavalcanti NV. Risk factors for death in children with visceral leishmaniasis. PLoS Negl Trop Dis. 2010;4(11):1–5. doi:10.1371/journal.pntd.0000877
17. Gerais M, Sérgio A, De AC, Juniorii CT, Rabelloiii A. Factors of poor prognosis of visceral leishmaniasis among children under 12 years of age. A Retrospective Monocentric Study in Belo. Rev Soc Bras Med Tro. 2013;46(1):1–7. doi:10.1063/1.3482623
18. Costa DL, Rocha RL, de Brito Ferreira Chaves E, et al. Predicting death from kala-azar: construction, development, and validation of a score set and accompanying software. Rev Soc Bras Med Trop. 2016;49(6):728–740. doi:10.1590/0037-8682-0258-2016
19. Belo VS, Struchiner CJ, Barbosa DS, et al. Risk factors for adverse prognosis and death in American visceral leishmaniasis: a meta-analysis. PLoS Negl Trop Dis. 2014;8(7):1–9. doi:10.1371/journal.pntd.0002982
20. Druzian AF, Souza AS, Campos DN, et al. Risk factors for death from visceral leishmaniasis in an urban area of Brazil. PLoS Negl Trop Dis. 2015;9(8):1–11. doi:10.1371/journal.pntd.0003982
21. Alemayehu M, Wubshet M. Magnitude of visceral leishmaniasis and poor treatment outcome among HIV patients: meta-analysis and systematic review. HIV/AIDS - Res Palliat Care. 2016;8:75–81. doi:10.2147/HIV.S96883
22. Gebreyohannes EA, Bhagvathula AS, Abegaz TM, Seid MA. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: A systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):1–9. doi:10.1186/s40249-018-0491-7
23. Assumpção Mourão MV, Toledo A, Gomes LI, Freire VV, Rabello A. Parasite load and risk factors for poor outcome among children with visceral leishmaniasis. A cohort study in Belo Horizonte, Brazil, 2010-2011. Mem Inst Oswaldo Cruz. 2014;109(2):147–153. doi:10.1590/0074-0276140257
24. de Araújo VEM, Morais MHF, Reis IA, Rabello A, Carneiro M. Early clinical manifestations associated with death from visceral leishmaniasis. PLoS Negl Trop Dis. 2012;6(2):1–10. doi:10.1371/journal.pntd.0001511
25. Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014;8(5):14–17. doi:10.1016/j.arth.2017.02.037
26. Verma S, Kumar R, Katara GK, et al. Quantification of parasite load in clinical samples of leishmaniasis patients: Il-10 level correlates with parasite load in visceral leishmaniasis. PLoS One. 2010;5(4). doi:10.1371/journal.pone.0010107
27. Soares MRA, Ishikawa EAY, Silva JM, Zacarias DA, Costa DL, Costa CHN. Bone marrow parasite burden among patients with new world Kala-Azar is associated with disease severity. Am J Trop Med Hyg. 2014;90(4):621–626. doi:10.4269/ajtmh.13-0376
28. Das A, Karthick M, Dwivedi S, Banerjee I, Mahapatra T. Epidemiologic correlates of mortality among symptomatic visceral leishmaniasis cases: findings from situation assessment in high endemic foci in India. PLoS Negl Trop Dis. 2016;1–12. doi:10.1371/journal.pntd.0005150
29. Desta A, Shiferaw S, Kassa A, Shimelis T, Dires S. Leishmaniasis. 2005;1–99.
30. Aderie EM, Diro E, Zachariah R, et al. Does timing of antiretroviral treatment influence treatment outcomes of visceral leishmaniasis in Northwest Ethiopia? Trans R Soc Trop Med Hyg. 2017;111(3):107–116. doi:10.1093/trstmh/trx023
31. Abongomera C, Diro E, Vogt F, et al. The risk and predictors of visceral leishmaniasis relapse in human immunodeficiency virus-coinfected patients in Ethiopia: a retrospective cohort study. Clin Infect Dis. 2017;65(10):1703–1710. doi:10.1093/cid/cix607
32. Croft SL, Olliaro P. Leishmaniasis chemotherapy — challenges and opportunities. Clin Microbiol Infect. 2011;17:1478–1483. doi:10.1111/j.1469-0691.2011.03630.x
33. Ministry of Health. Diagnosis and treatment of visceral leishmaniasis (Kal-azar) in Kenya. 2017:2–77.
34. Sigdel B, Bhandary S, Rijal S. Epistaxis in visceral leishmaniasis with hematological correlation. Int J Otolaryngol. 2012;2012:10–13. doi:10.1155/2012/809056
35. Werneck GL, Batista MSA, Gomes JRB, Costa DL, Costa CHN. Prognostic factors for death from visceral leishmaniasis in Teresina, Brazil. Infection. 2003;31(3):174–175. doi:10.1007/s15010-003-3139-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
