Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Incidence of febrile neutropenia during chemotherapy among patients with nonmyeloid cancer receiving filgrastim vs a filgrastim biosimilar
Authors Schwartzberg LS , Lal LS, Balu S, Campbell K, Brekke L , Elliott C, Korrer S
Received 15 March 2018
Accepted for publication 8 June 2018
Published 3 September 2018 Volume 2018:10 Pages 493—500
DOI https://doi.org/10.2147/CEOR.S168298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Lee S Schwartzberg,1,2 Lincy S Lal,3 Sanjeev Balu,4 Kim Campbell,4 Lee Brekke,3 Caitlin Elliott,3 Stephanie Korrer3
1West Cancer Center, Memphis, TN, USA; 2Division of Hematology and Oncology, University of Tennessee, Memphis, TN, USA; 3Health Economics and Outcomes Research, Optum, Eden Prairie, MN, USA; 4US Clinical Development and Medical Affairs, Sandoz Inc., Princeton, NJ, USA
Background: Filgrastim and other granulocyte colony-stimulating factors are recommended to decrease febrile neutropenia (FN) incidence among patients with nonmyeloid cancers undergoing chemotherapy. Data comparing biosimilar filgrastim-sndz with reference filgrastim (filgrastim-ref) are limited outside of clinical trials in the US.
Objective: To compare the incidence of FN across chemotherapy cycles 1–6 between patients treated with filgrastim-sndz vs filgrastim-ref.
Materials and methods: This was a retrospective claims analysis of patients with nonmyeloid cancer enrolled in commercial or Medicare Advantage plans from March 2015 to June 2016 and receiving filgrastim-sndz or filgrastim-ref during ≥1 completed chemotherapy cycle. Patients undergoing hematopoietic stem cell transplantation, pregnant patients, and those with missing data were excluded. FN was identified using the diagnosis codes for neutropenia + fever, neutropenia + bacterial/fungal infection, and neutropenia + infection + fever. Equivalence testing for FN incidence at the cycle level across chemotherapy cycles 1–6 was conducted for filgrastim-sndz vs filgrastim-ref after adjusting for baseline characteristics using inverse probability of treatment weighting. Results were considered equivalent if the 90% CIs for between-cohort differences were within ±6.0%.
Results: The analysis included 3,459 patients (162 filgrastim-sndz and 3,297 filgrastim-ref). Before weighting, the filgrastim-sndz cohort was younger than filgrastim-ref and had a higher proportion of men, a higher proportion with commercial insurance, and lower proportions with granulocyte colony-stimulating factor prophylaxis or metastatic cancer. After weighting, baseline characteristics were similar between cohorts. Adjusted FN incidence was equivalent for filgrastim-sndz vs filgrastim-ref, respectively: neutropenia + fever, 0.81% vs 0.61% (difference [90% CI]=0.20 [−0.57 to 1.56]); neutropenia + infection, 1.21% vs 1.33% (difference [90% CI]=−0.12 [−1.17 to 2.28]); neutropenia + infection + fever, 0.0% vs 0.14% (difference=–0.14; CI not calculated because filgrastim-sndz had 0 events).
Conclusion: Filgrastim-sndz and filgrastim-ref are statistically equivalent for preventing FN across chemotherapy cycles 1–6 among patients with nonmyeloid cancer.
Keywords: biosimilar pharmaceuticals, febrile neutropenia, filgrastim, granulocyte colony-stimulating factor, retrospective studies
Introduction
Patients with cancer who receive myelosuppressive chemotherapy are susceptible to febrile neutropenia (FN),1 a potentially life-threatening side effect that increases infection risk and frequently requires hospitalization and treatment with broad-spectrum antibiotics.2–4 Individuals who develop FN during chemotherapy treatment not only have increased rates of morbidity and mortality4,5 and higher health care costs,5–7 but are also more likely to experience treatment delay, reduction in chemotherapy dose intensity, or even treatment discontinuation,4,8,9 all of which can lead to reduced treatment response and lower survival.10,11
Prophylaxis with granulocyte colony-stimulating factors (G-CSFs) such as filgrastim (Neupogen®, Amgen Inc., Thousand Oaks, CA, USA), which was approved in the US in 1991, has been shown to reduce the risk of these negative outcomes and improve chemotherapy-related quality of life among patients with solid tumors or nonmyeloid malignancies.12,13 Accordingly, current practice guidelines in the US and Europe recommend G-CSF prophylaxis for patients whose chemotherapy regimens are associated with high (≥20%) risk of FN,14–16 as well as for some patients on chemotherapy regimens with intermediate FN risk (10%–20%), depending on patient characteristics such as age, comorbidities, disease stage, and previous history of FN.16 However, only about 17% of patients who meet the high-risk criteria actually receive prophylaxis,17 and there is evidence that the high cost of G-CSFs – which, like many biologics, are expensive drugs – may constitute a treatment barrier for some patients.17,18
Approval of the filgrastim biosimilar filgrastim-sndz (US trade name Zarxio®, Sandoz Inc., Princeton, NJ, USA), which in 2015 became the first biosimilar available in the US via the abbreviated pathway established by the Biologics Price Competition and Innovation Act,19 was an important step in potentially expanding access to G-CSF treatment. Filgrastim-sndz was approved on the basis of a substantial body of analytical, preclinical, and clinical data demonstrating that there were no clinically meaningful differences between the biosimilar and the reference drug (filgrastim-ref) for preventing FN among patients receiving myelosuppressive chemotherapy.19 A subsequent multicenter prospective observational study (MONITOR-GCSF) demonstrated that safety and efficacy outcomes among patients treated with biosimilar filgrastim (EU trade name Zarzio®, Sandoz Inc.) were similar to those reported historically for filgrastim-ref,20 and studies conducted in the US and Europe have since indicated that biosimilar filgrastim is the most cost-efficient approach for preventing FN among patients undergoing myelosuppressive chemotherapy in routine clinical practice.21,22
Until recently, real-world comparative data regarding clinical outcomes among patients treated with biosimilar filgrastim had been lacking. We conducted one of the first retrospective observational studies to compare outcomes between filgrastim-sndz–treated vs filgrastim-ref–treated patients undergoing chemotherapy treatment for nonmyeloid malignancies in US clinical practice, demonstrating that the incidence of FN was statistically equivalent between these groups during the first chemotherapy cycle.23 A contemporaneous analysis comparing FN incidence between filgrastim-sndz–treated vs filgrastim-ref–treated patients in the first 14 days after G-CSF use had similar results.24 While neutropenic complications are most common in the first cycle of chemotherapy,4,25 examination of subsequent cycles is warranted to confirm equivalence over a longer period. We conducted the present study to expand on earlier findings by extending the equivalency analysis up through the first six cycles of chemotherapy.
Materials and methods
Study design and data source
This was a retrospective claims analysis conducted from 01 September 2014 through 31 July 2016 (study period) using data from the Optum Research Database (ORD). The ORD contains fully de-identified medical and pharmacy claims and enrollment information for commercial and Medicare Advantage health plan members, is geographically diverse across the US, and, for individuals 18 years and older, is linked to dates of death (month and year) from the Social Security Administration death master file. The information included in medical claims and outpatient pharmacy claims within the ORD was described in detail in our previous report.23 Because no identifiable protected health information was extracted or accessed for the purpose of this study, institutional review board approval or waiver of authorization was not required.
Study sample
The study population comprised adult patients (aged 18 years or older) undergoing chemotherapy for nonmyeloid cancer who were treated with filgrastim-sndz or filgrastim-ref in at least one completed cycle of a chemotherapy treatment regimen from 01 March 2015 through 30 June 2016 (patient identification period). Patient selection and cohort assignment (filgrastim-sndz or filgrastim-ref) are illustrated in Figure 1 and described in detail in our previous report.23 The end of the follow-up period was defined as the end of continuous health plan enrollment, death, or 31 July 2016 (whichever came earliest).
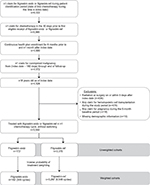  | Figure 1 Sample selection and attrition flow diagram. |
Chemotherapy lines of therapy and cycle definitions
This analysis used up through the first six observed chemotherapy cycles of the first observed course of adjuvant, neoadjuvant, or metastatic chemotherapy (line of therapy [LOT]) for each patient, which by definition began on the index date. The methodology for determining LOT end dates was described previously.23 The start of the first observed chemotherapy cycle began on the first day of the first observed LOT and included all chemotherapy regimens received during the first 6 days of the cycle. The end of the first observed chemotherapy cycle was defined as the earlier of the next fill/infusion of cancer therapy occurring on or after day 8 of the LOT, or the end of the LOT. Cancer therapy fills/infusions that occurred on or after day 8 of the LOT were defined as subsequent chemotherapy cycles. Chemotherapy cycles that ended due to censoring (ie, disenrollment or study end) were considered to be incomplete and excluded from the analysis.
Study measures
Baseline characteristics
Patient demographic and clinical characteristics were assessed during the 6 months prior to the index date (baseline period). Comorbidity burden was evaluated using the Quan–Charlson comorbidity index.26 Because FN rarely occurs within the first 5 days of chemotherapy, use of filgrastim-sndz or filgrastim-ref that was initiated on or before day 5 of a chemotherapy cycle was categorized as prophylactic.27
Incidence of FN
FN was defined on the basis of claims with a combination of diagnosis codes from the International Classification of Diseases (ICD), ninth or tenth edition, Clinical Modification (ICD-9-CM or ICD-10-CM) for neutropenia (ICD-9-CM 288.0 or ICD-10-CM D70*), fever (ICD-9-CM 780.61 or ICD-10-CM R50.81), or bacterial or fungal infection (codes available from the authors upon request) in the first or second position, starting after the last dose of filgrastim-sndz or filgrastim-ref of a chemotherapy cycle or the fifth day after the index date, whichever occurred earlier in the cycle. Outcomes were assessed using three different definitions of FN: neutropenia + fever, neutropenia + infection (bacterial or fungal), and neutropenia + infection (bacterial or fungal) + fever. These definitions have previously been shown to effectively identify patients with FN from administrative claims data.28
Statistical analysis
As described in our previous report,23 weighted cohorts were created using inverse probability of treatment weighting (IPTW)29 to control for possible confounding of the relationship between the outcome (development of FN) and the independent variable of interest (G-CSF agent received). In the IPTW method, weights based on propensity scores are used to create a synthetic sample in which the distribution of baseline covariates is independent of treatment assignment. The covariates used in propensity scoring were number of completed cycles, age, sex, geographic region, insurance type, Quan–Charlson index score, number of CSF doses, length of chemotherapy cycle 1, use of G-CSF prophylaxis, cancer type, metastatic cancer in general and specific to bone, infection, radiation, surgery, renal or hepatic dysfunction, and prior use of CSF agents. As in our previous analysis of cycle 1, only complete cycles were used and patients whose first cycle was censored were dropped from the study.23 For the present multiple-cycle analysis, the loss of some cycles 2–6 due to censoring was adjusted for by applying additional inverse probability of censoring weights to the non-censored (ie, complete) cycles. These additional weights depended only on the cycle number and the treatment cohort based on cycle 1 for the patient. The probability of censoring used in the weights was calculated using separate Kaplan–Meier analyses for each cycle number 2 through 6, stratified by treatment cohort.30 Baseline characteristics were analyzed descriptively on the cycle level and presented by unweighted and weighted treatment cohorts; numbers and percentages were provided for categorical variables, and means were provided for continuous variables. Standardized differences were calculated to compare characteristics between filgrastim-sndz vs filgrastim-ref cohorts (unweighted and weighted); values <10% are by convention considered to indicate high similarity.29 All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Statistical equivalence testing
FN incidence was calculated among the weighted cohorts for all three definitions of FN. Bootstrap methods were used to calculate 90% CIs of the difference in FN incidence between groups.31 CIs within ±6% were considered to denote statistical equivalence between cohorts on the basis of the 0%–11% range of neutropenic fever incidence reported in the PIONEER trial.32
Results
Study sample
A total of 3,542 patients (172 filgrastim-sndz and 3,370 filgrastim-ref) met the study criteria and were included in the unweighted treatment cohorts (Figure 1). There were 162 patients in the weighted filgrastim-sndz cohort and 3,297 in the weighted filgrastim-ref cohort after IPTW, including a total of 349 chemotherapy cycles for filgrastim-sndz–treated patients and 6,546 chemotherapy cycles for filgrastim-ref–treated patients (excluding censored cycles and patients; Figure 1). Before weighting, the filgrastim-sndz cohort was younger than the filgrastim-ref cohort (mean age 60.9 vs 64.8 years) and had a higher proportion of men (42.1% vs 35.6%), a higher proportion of patients with commercial insurance (61.0% vs 44.1%), a lower proportion of patients with G-CSF prophylaxis (45.6% vs 51.6%), and a lower proportion of patients with metastatic cancer (39.0% vs 46.6%), as shown in Table 1. The unweighted mean numbers of G-CSF doses received for filgrastim-sndz and filgrastim-ref were 2.4 and 2.2, respectively, with prophylaxis occurring during 45.6% of chemotherapy cycles among filgrastim-sndz patients and 51.6% of chemotherapy cycles among filgrastim-ref patients. After weighting, baseline characteristics were similar between cohorts and standardized differences were acceptably low (Table 1).
Incidence of FN
FN incidence in chemotherapy cycles 1–6 was statistically equivalent between the weighted treatment cohorts (90% CI of difference within ±6%; Table 2). Adjusted FN incidence in the filgrastim-sndz vs filgrastim-ref cohorts, respectively, was 0.81% vs 0.61% for neutropenia + fever (difference [90% CI]=0.20 [−0.57 to 1.56]), 1.21% vs 1.33% for neutropenia + infection (difference [90% CI]=−0.12 [−1.17 to 2.28]), and 0.0% vs 0.14% for neutropenia + infection + fever (difference=–0.14; CI was not calculated for this definition because there were no FN events in the filgrastim-sndz cohort).
Discussion
In this real-world analysis, the incidence of FN among patients undergoing chemotherapy for nonmyeloid cancers was statistically equivalent between those treated with filgrastim-sndz vs filgrastim-ref up through six chemotherapy cycles. These findings build upon those of our previous first-cycle analysis by demonstrating equivalence over a longer period among the same study group,23 and bolster existing evidence from randomized controlled trials regarding the noninferiority of biosimilar filgrastim compared with the reference drug.32
In the present study, the incidence of FN ranged from 0.0% to 1.2% of cycles in the filgrastim-sndz cohort and from 0.14% to 1.33% of cycles in the filgrastim-ref cohort (depending on the FN definition used), similar to the patient-level FN incidence ranges found in chemotherapy cycle 1 in our previous study (filgrastim-sndz, 0.0%–2.3%; filgrastim-ref, 0.3%–1.7%).23 Our findings are also comparable to those of the only other real-world, head-to-head comparison of these two drugs to date, a claims analysis conducted by Douglas et al in a similar patient group.24 In the Douglas et al study, the incidence of FN within the first 14 days after G-CSF administration was 2.0% for filgrastim-sndz and 1.1% for filgrastim-ref, when FN was defined as hospitalization plus diagnoses for both neutropenia and infection.24 Taken together, these contemporary results suggest a lower FN rate than the 1.3%–3.5% reported in previous claims analyses,1,27,33–35 which may be partly attributable to the fact that earlier studies defined FN as hospitalization with a diagnosis code for neutropenia. This broader definition has higher sensitivity, but a lower positive predictive value for FN than definitions that also require diagnoses of infection and/or fever,28 such as the ones used in the present study, as well as in clinical trials.32 In addition, earlier analyses were conducted prior to a 2006 modification of the American Society of Clinical Oncology guidelines that lowered the FN risk cutoff for G-CSF prophylaxis from 40% to the current 20%.36
It is well established that prophylactic administration of G-CSF among patients receiving myelosuppressive chemotherapy reduces the risk of FN, FN-related hospitalization, and death, while allowing higher chemotherapy regimen intensity.12 Filgrastim prophylaxis is recommended to be administered daily until neutrophil counts return to normal;37 in clinical trials, this has been shown to require ~9–14 injections.38 However, while the proportion of chemotherapy cycles with G-CSF prophylaxis in our study sample ranged from 45.6% (filgrastim-sndz) to 51.6% (filgrastim-ref), the mean numbers of G-CSF injections administered per cycle for filgrastim-sndz and filgrastim-ref were only 2.4 and 2.2, respectively, similar to the patient-level mean found in our previous study.23 These findings suggest that many patients receive G-CSF dosing that is inconsistent with existing guidelines, a phenomenon that has been observed in a number of other real-world studies.1,17,20,39,40 Unfortunately, such practices may be putting patients at risk, as shorter courses of G-CSF have been associated with increased rates of FN, neutropenia-related hospitalization, and chemotherapy regimen disruptions.1,39–41 Potential reasons for G-CSF underutilization cannot be discerned from the present analysis, but may include factors such as physicians’ lack of familiarity with neutropenia guidelines, patients’ aversion to additional injections, or decreased level of monitoring after chemotherapy administration. In addition, there is evidence that the high cost of filgrastim precludes treatment for some patients.17,18 Given the lower cost of filgrastim-sndz compared with filgrastim-ref,22,42 increased use of the biosimilar has the potential to improve drug access and guideline compliance. In Europe, for example, not only have significant cost savings been achieved since the 2008 approval of the filgrastim biosimilar Zarzio, but also the use of G-CSF prophylaxis has become substantially more widespread.43 Recent US analyses have demonstrated that prophylaxis with filgrastim-sndz in routine clinical practice is cost-efficient compared with filgrastim-ref and pegfilgrastim;22 moreover, use of filgrastim-sndz in place of filgrastim-ref could potentially increase access to curative cancer care, as the resulting cost savings could be applied to provide expensive curative treatments to more patients on a budget-neutral basis.44
Limitations
This study is subject to several limitations, which were described in detail in our previous report23 and are outlined briefly below. Our findings may not be generalizable to populations that were excluded from the study sample (eg, uninsured patients and those on fee-for-service health care plans). The analysis did not determine causality or severity of the study outcomes, as this information is not available in claims data. In addition, because it cannot be definitively determined from claims data whether G-CSF was given as treatment or prophylaxis, we did not attempt to exclude patients with prophylaxis from the study; however, prophylaxis was included as a propensity scoring covariate to avoid confounding the equivalence analysis. The number of patients using filgrastim-sndz was low because the drug was relatively new to the US market during our patient identification period; however, the effect of small sample size on study outcome was mitigated by adjustment with IPTW. Finally, possible misidentification of patients with the outcome of interest due to reliance on diagnosis codes from medical claims is an inherent limitation of claims analyses, and exists for this study also.
Conclusion
The findings of this study not only demonstrate statistical equivalence between filgrastim-sndz vs filgrastim-ref for preventing FN among patients with nonmyeloid cancer undergoing chemotherapy, but also suggest that G-CSF may be underutilized in this population. With burgeoning evidence for its safety and efficacy in clinical practice, biosimilar filgrastim has the potential to improve patient access to G-CSF treatment and increase physician compliance with existing prophylaxis guidelines.
Acknowledgments
Medical writing services were provided by Yvette Edmonds, PhD, an employee of Optum. This work was funded by Sandoz Inc., which participated in the design of the study, interpretation of the data, writing and revision of the manuscript, and the decision to submit the manuscript for publication.
Disclosure
SB and KC are employees of Sandoz Inc., which is the manufacturer of the filgrastim biosimilars Zarzio® and Zarxio®. LSL, LB, CE, and SK are employees of Optum, which was contracted by Sandoz Inc. to conduct this study. LSS is a consultant for Sandoz Inc. The authors report no other conflicts of interest in this work.
References
Weycker D, Barron R, Edelsberg J, Kartashov A, Legg J, Glass AG. Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis. BMC Health Serv Res. 2014;14:189. | ||
Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368(12):1131–1139. | ||
National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN guidelines). Prevention and treatment of cancer-related infections. 2017. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed June 19, 2017. | ||
Culakova E, Thota R, Poniewierski MS, et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 2014;3(2):434–444. | ||
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. | ||
Dulisse B, Li X, Gayle JA, et al. A retrospective study of the clinical and economic burden during hospitalizations among cancer patients with febrile neutropenia. J Med Econ. 2013;16(6):720–735. | ||
Michels SL, Barron RL, Reynolds MW, Smoyer Tomic K, Yu J, Lyman GH. Costs associated with febrile neutropenia in the US. Pharmacoeconomics. 2012;30(9):809–823. | ||
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–4531. | ||
Shayne M, Crawford J, Dale DC, Culakova E, Lyman GH. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100(3):255–262. | ||
Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24(10):2475–2484. | ||
Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77(3):221–240. | ||
Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167. | ||
Vanderpuye-Orgle J, Sexton Ward A, Huber C, Kamson C, Jena AB. Estimating the social value of G-CSF therapies in the United States. Am J Manag Care. 2016;22(10):e343–e349. | ||
National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN guidelines). Myeloid growth factors. 2017. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed June 19, 2017. | ||
Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2015;33(28):3199–3212. | ||
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 20112011;47(1):8–32. | ||
Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103(12):979–982. | ||
Mulcahy A.W, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. 2014. Available from: https://www.rand.org/content/dam/rand/pubs/perspectives/PE100/PE127/RAND_PE127.pdf. Accessed July 12, 2017. | ||
Raedler LA. Zarxio (filgrastim-sndz): first biosimilar approved in the United States. Am Health Drug Benefits. 2016;9(Spec Feature):150–154. | ||
Gascón P, Aapro M, Ludwig H, et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer. 2016;24(2):911–925. | ||
Aapro M, Cornes P, Abraham I. Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract. 2012;18(2):171–179. | ||
Mcbride A, Campbell K, Bikkina M, Macdonald K, Abraham I, Balu S. Cost-efficiency analyses for the US of biosimilar filgrastim-sndz, reference filgrastim, pegfilgrastim, and pegfilgrastim with on-body injector in the prophylaxis of chemotherapy-induced (febrile) neutropenia. J Med Econ. 2017;20(10):1083–1093. | ||
Schwartzberg LS, Lal LS, Balu S, et al. Clinical outcomes of treatment with filgrastim versus a filgrastim biosimilar and febrile neutropenia-associated costs among patients with nonmyeloid cancer undergoing chemotherapy. J Manag Care Spec Pharm. 2018:1–9. | ||
Douglas AG, Schwab P, Lane D, Kennedy K, Slabaugh SL, Bowe A. A comparison of brand and biosimilar granulocyte-colony stimulating factors for prophylaxis of chemotherapy-induced febrile neutropenia. J Manag Care Spec Pharm. 2017;23(12):1221–1226. | ||
Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–118. | ||
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. | ||
Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13:11. | ||
Weycker D, Sofrygin O, Seefeld K, Deeter RG, Legg J, Edelsberg J. Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res. 2013;13:60. | ||
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. | ||
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. | ||
Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192–196. | ||
Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948–1953. | ||
Weycker D, Malin J, Kim J, et al. Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther. 2009;31(5):1069–1081. | ||
Weycker D, Malin J, Barron R, Edelsberg J, Kartashov A, Oster G. Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol. 2012;35(3):267–274. | ||
Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin. 2011;27(1):79–86. | ||
Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. | ||
Zarxio (filgrastim-sndz) injection [prescribing information]. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fe707775-a0ae-41b5-a744-28c41889fce8. Accessed June 19, 2017. | ||
Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer. 2010;18(5):529–541. | ||
Weycker D, Li X, Tzivelekis S, et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer. 2017;25(2):439–447. | ||
Bokemeyer C, Gascón P, Aapro M, et al. Over- and under-prophylaxis for chemotherapy-induced (febrile) neutropenia relative to evidence-based guidelines is associated with differences in outcomes: findings from the MONITOR-GCSF study. Support Care Cancer. 2017;25(6):1819–1828. | ||
Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402–407. | ||
Stanton D. Biosimilars land in US and Sandoz launches Zarxio. 2015. Available from: http://www.biopharma-reporter.com/Markets-Regulations/Biosimilars-land-in-the-US-as-Sandoz-launches-Zarxio. Accessed July 12, 2017. | ||
Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925–2932. | ||
Mcbride A, Balu S, Campbell K, Bikkina M, Macdonald K, Abraham I. Expanded access to cancer treatments from conversion to neutropenia prophylaxis with biosimilar filgrastim-sndz. Future Oncol. 2017;13(25):2285–2295. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


