Back to Journals » Clinical Epidemiology » Volume 13
Incidence of Atrial Fibrillation in Persons with Very High Serum Levels of N-Terminal Pro-B-Type Natriuretic Peptide: The Multi-Ethnic Study of Atherosclerosis
Authors Weiss NS , Perez Trejo E, Kronmal R, Lima J, Heckbert SR
Received 24 January 2021
Accepted for publication 26 March 2021
Published 7 April 2021 Volume 2021:13 Pages 265—272
DOI https://doi.org/10.2147/CLEP.S303560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Irene Petersen
Noel S Weiss,1 Esther Perez Trejo,2 Richard Kronmal,3 Joao Lima,4 Susan R Heckbert1
1Department of Epidemiology, University of Washington, Seattle, WA, USA; 2School of Public Health, University of Montreal, Montreal, QC, Canada; 3Department of Biostatistics, University of Washington, Seattle, WA, USA; 4Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
Correspondence: Noel S Weiss
Department of Epidemiology, University of Washington, 18372 Ridgefield Road NW, Shoreline, WA, 98177, USA
Email [email protected]
Objective: While persons in the upper fourth or fifth of the distribution of serum levels of N-terminal pro-B type natriuretic peptide (NT-proBNP) are at a sharply increased risk of developing atrial fibrillation, their absolute risk of this condition (about 20 per 1000 per year) is not clearly high enough to justify prevention or early detection measures. We sought to determine whether the incidence of atrial fibrillation among persons with VERY high levels of NT-proBNP might be sufficiently high to warrant further action.
Design and Setting: Among persons enrolled in the Multi-Ethnic Study of Atherosclerosis, we documented rates of new onset atrial fibrillation in those with increasingly high serum levels of NT-proBNP.
Results: There was a monotonic increase in the incidence of atrial fibrillation with increasing serum level of NT-proBNP, reaching rates of about 50– 70 cases per 1000 person-years among those in the upper 3.1% of the distribution (above 422 pg/mL). In this group the incidence tended to be somewhat higher still among persons who were at increased risk of atrial fibrillation for other reasons (eg older age), but in no subgroup did the incidence reach 100 per 1000 person-years.
Conclusion: Serum levels of NT-proBNP have a considerable ability to predict the development of atrial fibrillation. However, the value of screening middle aged and older adults for these levels hinges largely on the ability of interventions in screen-positive people to lead to a reduced incidence of atrial fibrillation and its complications.
Keywords: atrial fibrillation, NT-proBNP
Introduction
Atrial fibrillation (AF) is a commonly occurring condition. While the presence of this arrhythmia produces diminished cardiac output and can give rise to a variety of symptoms, many people with AF are unaware of its presence. Whether symptomatic or not, the impaired contraction of the atria that is a feature of AF is associated with an increased risk of cardiac thrombosis and arterial embolism.1 When a person’s AF is recognized, he/she can be treated in an attempt to restore sinus rhythm, and/or with anticoagulant medication.
Screening for AF takes place by providers of healthcare when they check a patient’s pulse for rate and regularity. However, many persons seek health care only occasionally, and even those who have regular checkups can go up to one year or more without their pulse being examined. If persons at unusually high risk of developing AF could be identified, more frequent pulse measurements (by a healthcare provider or the patient him/herself) would have the potential to lead to earlier treatment of AF, and therefore a reduction in the risk of untoward consequences of cardiac emboli, especially embolic stroke.
The prevalence of AF is strongly related to age, and also is modestly increased among men, whites, and persons with elevated blood pressure.2 AF prevalence also is increased among persons with relatively high serum levels of natriuretic peptides, substances produced in the heart and released into the circulation in response to volume overload. An increased level of one of these peptides, N-terminal pro-B-type natriuretic peptide (NT-proBNP), has been associated with a monotonic increase in AF risk, even after adjusting for demographic, anthropometric and clinical characteristics.2–8
Despite its strong association with the occurrence of AF, the predictive value of an elevated serum level of NT-proBNP is not high: typically, the incidence of AF among participants in the highest fourth or fifth of the distribution has been only about 20 per 1000 person-years.2,3 However, it is possible that AF incidence is much higher in persons with a VERY high level of NT-proBNP (eg persons in the highest tenth of the distribution). Also, AF rates could be particularly high in demographic, anthropometric, or clinically-defined subgroups of persons with a very high serum level of NT-proBNP.
Using data gathered in the Multi-Ethnic Study of Atherosclerosis (MESA), we sought to determine if there are some persons with an elevated serum level of NT-proBNP in whom there is a high enough risk of developing AF so that cardiac rhythm screening and augmented patient education might be justified – and possibly in whom measures to prevent the development of AF could be undertaken.
Methods
The MESA takes place in six locations of the United States: Baltimore city and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. From July, 2000 to August, 2002, the participating institutions recruited residents of these communities ages 45–84 years who were white, African-American, Hispanic, or Chinese. Persons eligible to take part were free of clinically apparent cardiovascular disease. All participants gave informed consent. The study was approved by the committees on human subjects at the investigators’ institutions. Details regarding the means of recruitment have previously been published.9
At the time of enrollment in the study, a baseline exam was done, which included the assessment of anthropometric characteristics and other exposures and characteristics potentially relevant to the occurrence of cardiovascular disease (eg smoking). NT-proBNP levels were measured in serum samples that had been obtained from participants at baseline and again at about 3 and about 10 years into the follow-up period (and then stored at −70 degrees C). Measurements (described in greater detail elsewhere2) were performed at the University of California, San Diego (at baseline and 3-year follow-up), the University of Maryland (3-year follow-up), and the Laboratory for Clinical Biochemistry Research, University of Vermont (at 10-year follow-up) using the Roche ElecSys immunoassay (Roche Diagnostics, Indianapolis, IN).
Information on demographics, smoking, and medications were collected via questionnaire at the initial examination. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Resting blood pressure was measured three times in the seated position, and the average of the last two measurements was used in analysis. Hypertension was defined as diastolic blood pressure ≥90mmHg, systolic blood pressure ≥140mmHg, or use of antihypertensive medications. Persons were categorized as having diabetes if their fasting blood glucose concentration exceeded 125 mg/dl or if they were taking a medication used to treat diabetes. Current smoking was defined as answering “yes” to the question, “Have you smoked cigarettes during the last 30 days?” For those answering “no”, former smoking was defined as answering “yes” to the question, “Have you smoked at least 100 cigarettes in your lifetime?” Physical activity was assessed using a detailed, semi-quantitative instrument.9 At the baseline exam, MESA participants also underwent 12-lead electrocardiography, carotid ultrasonography, and cardiac magnetic resonance imaging,9,10 as well as a measurement of coronary artery calcium by CT.11
In this cohort study, incident AF during follow‐up was identified (a) through MESA event surveillance, (b) for participants age 65 years or older and enrolled in fee‐for‐service Medicare, from inpatient and outpatient Medicare claims data, and (c) from a 12-lead electrocardiogram (ECG) completed during the 2010–2012 study exam. As part of MESA event surveillance, a telephone interviewer contacted each participant or a proxy every 9–12 months to ask about hospital admissions that had taken place since the prior phone call. MESA investigators requested copies of medical records, from which discharge diagnosis and procedure codes were abstracted. (The MESA surveillance phone calls also identified deaths that occurred among cohort participants. Mortality ascertainment was augmented by annual searches of the National Death Index.) AF was considered to be present in the hospitalization data or Medicare claims data if an International Classification of Diseases, Ninth Revision diagnosis code 427.31 or 427.32 was present. AF diagnoses associated with open cardiac surgery were excluded. If the first AF claim occurred before the baseline MESA examination, the participant was considered to have prevalent AF and thus was excluded from the analysis. Participants contributed to the person-time denominator on which incidence rates were based until they were diagnosed with AF, or until they died. The incidence of AF was ascertained through the end of 2014, resulting in a median duration of follow-up of 12.2 years.
A prior study of the incidence of AF in MESA participants,2 with a median of 7.6 years of follow-up, observed a particularly high rate among those in the upper fourth of the serum NT-proBNP distribution. Therefore, we focused our analysis on this group, first splitting them further into approximate additional fourths based on level, and then splitting the highest of these (the upper 6.3%) into yet four more groups. Based on an examination of these incidence rates, we chose to assess the additional contribution of other risk factors for AF on the incidence of AF among persons in the upper 3.1% (>422 pg/mL) of the serum NT-proBNP distribution. The “other” factors included in our analysis were those which among MESA cohort members bore the strongest independent relation to AF incidence.
The rates we calculated are based on the participant’s most recently measured level of serum NT-proBNP. As a result, during the course of follow-up a given person could contribute to the numerator and denominator of the incidence rate of different serum NT-proBNP categories. Similarly, if at the beginning of a given follow-up interval a person’s level of NT-proBNP was below the threshold required to enter a particular analysis (or was not measured), during that interval the person’s experience was not considered.
Results
Of the 6814 participants enrolled in MESA, 94 did not have their serum level of NT-proBNP measured, and an additional 24 were not followed for cardiac events after their baseline visit. Among the remainder of the participants, 2458 (top 25%) had at least one value of serum NT-proBNP of 133 pg/mL or greater. The baseline characteristics of the study population, according to highest level of NT-proBNP at any time point prior to the diagnosis of AF, are presented in Table 1. Persons with high levels of NT-proBNP tended to be older than persons with lower levels, and a higher proportion of them had hypertension and calcium in their coronary arteries.
 |
Table 1 Baseline Characteristics of MESA Participants According to Highest NT-proBNP Level |
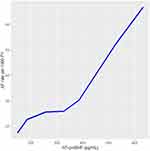
There was a monotonic increase in AF incidence with increasing serum level of NT-proBNP (Table 2, Figure 1), the rate rising from 17.3 per 1000 person-years for participants whose level was 133–162.7 pg/mL to 45.1 per 1000 person-years in those whose level exceeded 303.7 pg/mL. When the latter group was further divided into approximate fourths based on level of NT-proBNP there was a further monotonic rise, the incidence being 52.3 and 66.7 per 1000 person-years associated with levels of 422.1–631.4 and >631.4 pg/mL, respectively. Similar results were obtained when analyses were based only on the participants’ levels of NT-proBNP obtained at the baseline exam.
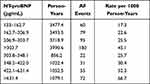 |
Table 2 Incidence of AF in MESA Participants Whose Most Recently Measured Level of NT-proBNP Exceeded 133 pg/ml |
In Table 3, we present the incidence of AF in persons whose serum level of NT-proBNP exceeded 422.0 pg/mL according to characteristics and exposures that themselves have a bearing on the incidence of AF. In these persons with very high serum levels of NT-proBNP there was some variation in AF incidence, with higher rates in older persons, men, Whites, and those with a relatively large waist circumference, evidence of carotid artery pathology, and renal dysfunction. Nonetheless, the magnitude of this additional variation was not large, and in none of the subgroups evaluated was the incidence of AF as high as 100 per 1000 per year.
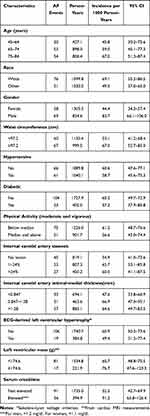 |
Table 3 AF Incidence (per 1000 Person-Years) in Persons Whose Serum Level of NT-proBNP Exceeded 422 pg/ml at Some Point During Follow-Up, by Level of Other Characteristics That Influence AF Incidence |
Discussion
One limitation of our analysis stems from the fact that AF can be asymptomatic and intermittent. As a result, AF that developed in some MESA participants may not have been identified, and so the incidence rates presented here could be modest underestimates of the true rates.
To our knowledge, this is the first report of the incidence of AF among persons with the very highest serum levels of NT-proBNP. The steady rise in incidence with increasing levels that we observed warrants replication in the experience of members of other cohorts in whom serum levels of NT-proBNP have been measured. Our suspicion is that this pattern of results WILL be replicated, at least in broad outline, given the steady (and dramatic) rise in AF incidence with increasing NT-proBNP levels among MESA participants.
In the present study, the overall incidence of AF was 11.7 per 1000 person-years, whereas that in persons in the upper 3% of the serum NT-proBNP distribution was some five times higher. Nonetheless, even in this group at relatively high AF risk, the absolute incidence (and that in subgroups defined by the presence of additional predictors of AF occurrence) did not exceed 100 per 1000 per year.
Is such an incidence high enough to justify screening middle aged and older persons for serum levels of NT-proBNP? The answer to that question requires consideration, first, of the cost of such screening. In the US at present, this is between $40 and $200. Second, it is necessary to gauge the sensitivity and cost of the means of assessing the development of AF in patients with high levels of NT-proBNP. Periodic pulse palpation by health care providers (accompanied by an ECG for those persons in whom AF is suspected) ought to be relatively inexpensive, and has been observed12 to have over 90% of the sensitivity of an ECG performed in all patients. Also, it has been observed that among the patients of physicians to whom the identification of AF has been emphasized and to whom reminders to conduct pulse palpation have been issued, the incidence of previously-occult AF was identical to that of patients who underwent systematic ECG evaluations.13
Third, we would need information regarding the efficacy of treatment of persons with high serum levels of NT-proBNP in preventing the development of AF. To the best of our knowledge, this has not yet been evaluated for any potential intervention. Finally, a decision to screen would require a judgment regarding the efficacy of treatment of persons with AF regarding the occurrence of stroke and other potential cardiovascular complications. In randomized trials of patients with atrial fibrillation, administration of oral anticoagulant drugs (whether a vitamin K antagonist or another type of anticoagulant) led to a large reduction in the rate of stroke and all-cause mortality.14,15 A cohort study of patients with incidentally-detected AF obtained similar findings.16
A randomized trial of the efficacy of screening for atrial fibrillation against stroke incidence has been initiated, the intensity of screening dictated by serum levels of NT-proBNP.17 However, at present the European Society of Cardiology (ESC) recommends opportunistic evaluation of the presence of AF in ALL patients 65 years and greater.18 If the ESC approach proves to be cost-effective, then screening for serum levels of NT-proBNP would be superseded, though still might be considered in somewhat younger persons. If the ESC recommendation for patients 65 years and above is not deemed to be cost-effective, then NT-proBNP screening might itself prove to be cost-effective in the elderly. However, as suggested above, an informed cost-effectiveness analysis would have to await the development of additional information, most notably the efficacy of the management of persons with very high levels of serum NT-proBNP in the prevention of AF.
MESA IRB Names and Numbers
Wake Forest University IRB number IRB00008492 under Federal-wide Assurance – FWA00001435
Columbia University IRB number IRB00002973 under Federal-wide Assurance – FWA00002636
Johns Hopkins University IRB number 00001656 under Federal-wide Assurance – FWA00005752
University of Minnesota IRB number IRB00000438 under Federal-wide Assurance – FWA00000312
Northwestern University IRB number IRB00005003 under Federal-wide Assurance – FWA00001549
University of California Los Angeles IRB number 00000172 under Federal-wide Assurance – FWA00004642
University of Washington IRB number IRB00005647 under Federal-wide Assurance – FWA00006878.
Ethics Statement
The MESA study was approved by the Institutional Review Boards at the participating institutions, and written informed consent was given by all participants. The study was conducted in accordance with the Declaration of Helsinki.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grant R01-HL127659 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclosure
Dr Susan R Heckbert reports grants from NIH, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Chugh SS, Blacksheer J, Shen WK, et al. Epidemiology and natural history of atrial fibrillation. J Am Coll Cardiol. 2001;37:371–378. doi:10.1016/S0735-1097(00)01107-4
2. Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incidence atrial fibrillation: the effects of age, sex, and ethnicity. Heart. 2013;99:1832–1836. doi:10.1136/heartjnl-2013-304724
3. Svennberg E, Lindahl B, Berglund L, et al. NT-proBNP is a powerful predictor for incident atrial fibrillation – validation of a multimarker approach. Int J Cardiol. 2016;223:74–81. doi:10.1016/j.ijcard.2016.08.001
4. Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. JACC. 2010;56:1712–1719. doi:10.1016/j.jacc.2010.05.049
5. Schnabel RB, Wild PS, Wilde S, et al. Multiple biomarkers and atrial fibrillation in the general population. PLoS One. 2014;9:e112486. doi:10.1371/journal.pone.0112486
6. Sinner MF, Stepas KA, Moser CB, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF consortium of community-based cohort studies. Eurospace. 2014;16:1426–1433. doi:10.1093/europace/euu175
7. Kaffer K, Geisel MH, Mohlenkamp S, et al. B-type natriuretic peptide for incident atrial fibrillation – the Heinz Nixdorf Recall Study. J Cardiol. 2015;65:453–458. doi:10.1016/j.jjcc.2014.08.003
8. Kumarathurai P, Mouridsen MR, Mattson N, et al. Atrial ectopy and N-terminal pro-B-type natriuretic peptide as predictors of atrial fibrillation: a population-based cohort study. Eurospace. 2017;19:364–370.
9. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi:10.1093/aje/kwf113
10. Chrispin J, Jain A, Soliman EZ, et al. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA. JACC. 2014;63:2007–2013. doi:10.1016/j.jacc.2014.01.066
11. Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–1320. doi:10.1161/01.CIR.0000157730.94423.4B
12. Cooke G, Doust J, Sanders S. Is pulse palpation helpful in detecting atrial fibrillation? A systematic review. J Fam Pract. 2006;55:130–134.
13. Fitzmaurice DA, Hobbs FDR, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 years or over: cluster randomized controlled trial. BMJ. 2007;335(7616):383. doi:10.1136/bmj.39280.660567.55
14. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have non-valvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi:10.7326/0003-4819-146-12-200706190-00007
15. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of the new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet. 2014;383:955–962. doi:10.1016/S0140-6736(13)62343-0
16. Freedman B, Martinez C, Katholing C, et al. Residual risk of stroke and death in anticoagulant-treated patients with atrial fibrillation. JAMA Cardiol. 2016;1:366–368. doi:10.1001/jamacardio.2016.0393
17. Gudmundsdottir KK, Fredriksson T, Svennberg E, et al. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the Strokestop II study. Europace. 2020;22:24–32. doi:10.1093/europace/euz255
18. Kirchhof P, Benussi S, Kotechka D, et al. Guidelines for the management of atrial fibrillation. Eur Heart J. 2016;37:2893–2962.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.