Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Incidence and risk factors of poor clinical outcomes in patients with cervical kyphosis after cervical surgery for spinal cord injury
Authors Zhang Y, Li J, Li Y, Shen Y
Received 26 August 2017
Accepted for publication 14 November 2017
Published 8 December 2017 Volume 2017:13 Pages 1563—1568
DOI https://doi.org/10.2147/TCRM.S150096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yanwei Zhang,1 Jia Li,2,3,* Yongqian Li,2,3 Yong Shen2,3,*
1Department of Emergency, Xingtai People’s Hospital of Hebei Medical University, Xingtai, 2Department of Orthopedic Surgery, Third Hospital of Hebei Medical University, 3Key Laboratory of Orthopedic Biomechanics of Hebei Province, Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
*These authors contributed equally to this work
Objective: This retrospective study investigated the incidence and risk factors of poor clinical outcomes after cervical surgery for cervical spinal cord injury in a large population of patients with global or segmental cervical kyphosis.
Methods: The clinical and radiological evaluation results of 269 patients with cervical kyphosis who underwent either anterior or posterior surgery between 2008 and 2013 were collected, preoperatively and at each follow-up after surgery.
Results: All patients were followed for an average of 2.5 years. Outcomes were classified as good or poor (n=156 and 113 patients, respectively), based on the Japanese Orthopedic Association (JOA) recovery ratios. The rates of patients with good or poor outcomes were statistically comparable with regard to gender ratio, type of injury, history of diabetes or cardiovascular disease, interval between injury and surgery, and follow-up time. The multivariate logistic regression analysis indicated that the following were independent predictors of poor improvement: patient age (P=0.016, odds ratio [OR] =1.0261); preoperative JOA scores (P=0.003, OR =0.1932); and cervical instability (P=0.004, OR =2.1562).
Conclusion: This study showed that advanced age, low preoperative JOA score, and cervical instability are closely associated with a poor surgical outcome in patients with cervical kyphosis. However, these results do not suggest that the type of cervical kyphosis influences the clinical outcome of surgery.
Keywords: cervical kyphosis, segmental kyphosis, global kyphosis, spinal cord injury, spinal cord surgery, poor outcome
Introduction
Cervical kyphosis, in which the normal curve of the neck is straightened or reversed, is a complex problem to treat, whether segmental or global.1–3 Cervical kyphosis can alter the range of motion in the spinal segments and lead to dysfunctional alterations in the chin–brow vertical angle (causing a downward gaze), neck pain, and neurological deficits, the risk of which accelerates with the degree of kyphosis.4
Either segmental or global kyphosis is associated with further degeneration of the disc and cervical spine, formation of osteophytes, and hypertrophy of the ligamentum flavum.5,6 Ruangchainikom et al7 found that when cervical kyphosis was global, spinal cord compression was mostly located at the apex of the deformity. In segmental kyphosis, spinal cord compression occurred in the transition zone, irrespective of the deformity involved is a sigmoid or a reverse sigmoid curve.
In the event of low-energy trauma, a narrow cervical spinal canal increases the risk of cervical spinal cord injury (SCI).8–10 However, there is no detailed information in the literature regarding the treatment of SCI that is due to low-energy trauma in patients complicated with cervical kyphosis. It was our hypothesis that the clinical outcomes of cervical surgery vary with the type of cervical kyphosis. To determine this, the present retrospective study investigated the incidence and risk factors of poor clinical outcomes of patients with global or segmental cervical kyphosis, after cervical surgery for SCI.
Methods
The Ethics Committee of Third Hospital of Hebei Medical University approved this study. Patient consent for review of medical records was not required, as all data were de-identified. All protocols were conducted in accordance with the research principles in the Declaration of Helsinki.
This was a retrospective clinical study of 269 patients with cervical kyphosis who had sustained SCI due to low-energy trauma and underwent either anterior or posterior cervical surgery between 2008 and 2013. The low-energy trauma was due to traffic accidents, slipping, or falling down. All patients conformed to the following inclusion criteria: had SCI, suffered spinal cord contusion shown on magnetic resonance imaging (MRI), cervical stenosis >20%, and a follow-up period of ≥2 years. Patients with any of the following were excluded from this analysis: rheumatoid arthritis, cervical dislocations or fractures, infectious discitis, infectious spondylitis, or spinal tumors. Patients treated conservatively were also excluded from this study.
Among the study population of 269 patients, there were 183 men and 86 women, and the mean age was 57.6 years (range, 35–74 years). The patients underwent either anterior discectomy and interbody fusion (n=163) or posterior decompressive laminectomy and lateral mass screw fixation (n=106). The goal of the treatment was to decompress the spinal cord and nerve root, and restore the stability of the cervical spine. The decision to apply either the anterior or posterior approach mainly depended on the location of compression on the spinal cord (anterior or posterior), and the number of levels that were affected (one to three levels, anterior; more than three levels, posterior).
Associations between the presence of diabetes mellitus or cardiovascular disease and quality of outcome were also analyzed. Diabetes mellitus is a chronic systemic disease, and patients with diabetes may develop multiple neurologic sequelae, mainly associated with macrocirculatory and microcirculatory complications.11 The most frequent preoperative medical history in this study was cardiovascular disease (72 patients, 26.8%) and the second frequent was diabetes mellitus (60 patients, 22.3%).
Surgical technique
Anterior technique
Surgical procedures via the anterior approach involved a right-sided skin incision. Owing to adequate neural decompression, the posterior longitudinal ligament was excised completely. The endplates were resected with a curette or burr. A polyether ether ketone (PEEK) or mesh cage filled with bone was inserted into the disc space, and the anterior plate system was applied.
Posterior technique
For a posterior approach, a posterior midline incision was made and the paravertebral muscles were retracted laterally. After the compressed segments were identified, screws were placed bilaterally using the technique described by Pal et al.12 Rods of appropriate size were selected and bent to match the contour of the lateral masses and secured to the lateral masses by screws. An enlarged laminectomy was performed from pedicle to pedicle to ensure adequate decompression of the spinal cord.
Evaluation criteria
Clinical and radiological evaluation results were obtained baseline and at each follow-up after surgery. The last follow-up data available were used for statistical analysis. The modified Japanese Orthopedic Association (JOA) scoring system was used to determine functional status before surgery and at the final follow-up visit. The recovery rate (%) at the final follow-up visit was calculated using the Hirabayashi method: (postoperative JOA score − preoperative score)/(17 − preoperative score) ×100%. A JOA recovery rate <50% was considered a poor outcome. With these scores, for the present analysis, patients were apportioned to either a good outcome (JOA recovery rate ≥50%) or a poor outcome (JOA recovery rate <50%) group.
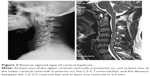
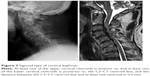
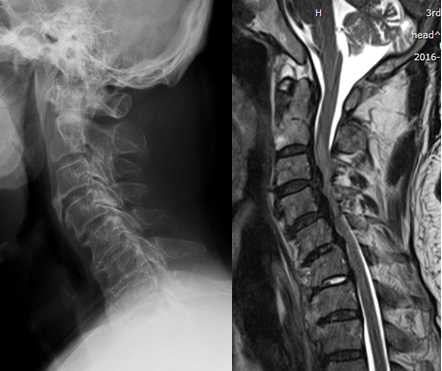
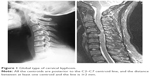
Radiographic evaluation included static and dynamic lateral images that were used to assess the severity of cervical kyphosis by two experienced radiologists without knowledge of the clinical outcomes. Sagittal alignment of the cervical spine was defined using a modified method created by Ohara et al.13 For every patient, imaging was performed to classify cervical kyphosis as global (Figure 1) or segmental. The latter was classified as either reverse sigmoid (Figure 2) or sigmoid (Figure 3). In dynamic lateral view images, cervical instability was defined as an angulation of cervical spine >11°, or >3.5 mm of translation.14
  | Figure 1 Global type of cervical kyphosis. |
We stratified the signal intensity on preoperative T2-weighted MRIs (T2WI) at the narrowest level of the spinal cord into three grades (0, 1, or 2), as follows. The signal intensity was classified as grade 0 if there was no intramedullary high-signal intensity on T2-weighted MRIs. The signal intensity was considered grade 1 if a predominantly faint and fuzzy border was noted. If a predominantly intense and well-defined border was noted, the signal intensity was defined as grade 2.15
On the T2-weighted axial image, the anteroposterior compression ratio was defined as the anteroposterior spinal canal diameter divided by the transverse diameter at the target level of the spinal cord.
Statistical analyses
Statistical analyses were performed using SPSS software (Version 22.0, Chicago, IL, USA). The descriptive analysis of the parameters is shown as mean ± SD for continuous variables, and frequencies and percentages for categorical and discrete variables. All potential risk factors were evaluated for a univariate association with poor outcomes, using independent-sample t-tests for continuous variables and chi-squared or Fisher’s exact tests for categorical or discrete variables. A multivariate logistic regression analysis was used to determine the risk factors related to poor outcome. In all the analyses, significance was defined as P<0.05.
Results
The patients were apportioned to a good postoperative outcome group (n=156; JOA recovery ≥50%) or poor postoperative group (n=113; JOA recovery <50%; Table 1). The primary cause of SCI was falling down, and next was traffic accident.
The mean postoperative JOA scores of each group were higher than the corresponding preoperative scores. The mean recovery rate of the good outcome group was significantly higher than that of the poor outcome group (P=0.000). There was no significant difference between the two groups with regard to gender ratio, cause of injury, time between injury and surgery, or follow-up time (P>0.05, all). In addition, the rates of diabetes mellitus, cardiovascular disease, type of cervical kyphosis (global or segmental), and compression ratios were comparable.
The patients in the poor outcome group were significantly older than those in the good outcome group (P=0.000; Table 1). In addition, the patients with poor outcomes had significantly lower preoperative and postoperative JOA scores (P=0.037 and P=0.000, respectively), and a higher rate of cervical instability (P=0.004). The anterior approach was used significantly more often in the good outcome group than the poor outcome group (P=0.027).
The good and poor outcome groups were identical regarding the percentages of patients with grade 1 signal intensity on preoperative T2-weighted MRIs (Table 1). However, a greater percentage of the good outcome group had a signal intensity of grade 0, while the poor group had a greater percentage with signal intensity of grade 2.
Using the clinical outcome as a dependent variable, a multivariate logistic regression model for exploring the relevant risk factors was created (Table 2). In the univariate analysis, factors were analyzed as dependent variables by a forward stepwise method, with a P-value <0.1. The multivariate logistic regression analysis showed that the following were independent predictors of poor outcome: patient age, preoperative JOA score, and cervical instability. However, no significant association was found between postoperative JOA score and type of cervical kyphosis, compression ratio, surgical approach, or grade of intramedullary signal intensity on T2WI.
  | Table 2 Multiple logistic regression analysis of risk factors for poor outcome after surgery |
Discussion
This retrospective study investigated the incidence and risk factors of poor clinical outcomes after cervical surgery for SCI in 269 patients with global or segmental cervical kyphosis. Patients were analyzed as good or poor outcome, according to the recovery rate based on the difference between preoperative and postoperative JOA scores. A poor outcome was defined as a JOA recovery rate <50%. In both the good and poor outcome groups, the mean postoperative JOA scores were higher than the preoperative scores. The following were associated with a poor outcome: patient age, cervical instability, type of surgical technique, preoperative JOA score, grade 2 of intramedullary signal intensity on T2WI, type of cervical kyphosis, compression ratio, and time from injury to surgery. The subsequent multivariate stepwise logistic regression analysis suggested that the significant predictive indicators of poor surgical outcome were advanced age, poor preoperative JOA score, and cervical instability. Of note, among these, the type of cervical kyphosis (global or segmental) was not included as a significant factor. Therefore, our hypothesis that the clinical outcome was influenced by the type of cervical kyphosis was negated.
In this study, there were only a few patients who were aware of having cervical kyphosis or stenosis before trauma. However, the combination of preexisting deformity and low-energy neck trauma can cause significant neurologic deficit.8,16–19 Previous researchers have studied the effect of cervical alignment on the kinematics of the spine and noted that both cervical kyphosis and lordosis will more likely lead to spinal cord compression in the event of low-energy trauma to the neck.20,21 In patients with cervical kyphosis, increased stress on the spinal cord with subsequent local ischemia will cause myelopathy. Continuous vascular change in the spinal cord and mechanical compression during motion of the neck can damage nerve fibers, and additional trauma may cause irreversible and severe damage to the spinal cord.22,23
C4–C5 and C5–C6 are the transition zones of both types of segmental kyphosis, while C3–C4 and C6–C7 are the apex of the sigmoid and reverse-sigmoid types, respectively.7 Overall, C3–C4 through C5–C6 are the most susceptible to spondylosis, stenosis, hyperextension injury, and SCI.24 In all subtypes of kyphotic deformity (global, and sigmoid and reverse-sigmoid segmental), spinal cord compression was observed more often in the extension position than in flexion or neutral positions on cervical kinematic MRI of patients with symptomatic neck pain.7 All subtypes showed a high percentage of spinal cord compression at C4–C5 and C5–C6, with notably more at C3–C4 in the reverse-sigmoid segmental type.
We hypothesized that patients with segmental kyphosis suffer worse damage in the event of low-energy trauma compared with patients with global kyphosis. In our experience, segmental kyphosis, rather than global, presents a greater mechanical disadvantage to the cervical spine, so that even low-energy trauma can cause excessive motion. Hyperextension injury of the cervical cord is exacerbated by cervical canal stenosis, which is often associated with kyphosis.1 At the moment of trauma, there is rapid and excessive extension of the cervical spine. During extension and flexion, the cervical cord in segmental kyphosis is more compressed than in global kyphosis.7 The neurologic deficit due to injury reflects prior spinal cord atrophy caused not only by chronic compression of the spinal cord,25 but also by the severity of compression (ie, the transverse area of the spinal cord at the level of maximum compression as observed on T1-weighted images).26 However, in the present study, according to the multivariate logistic regression analysis, segmental kyphosis was not a risk factor of poor outcome (as defined by JOA scores). This result may be due to the small number of patients.
In the present multivariate logistic regression analysis, cervical instability was a risk factor of poor clinical outcomes, in addition to advanced age and poor preoperative JOA score. Cervical instability is an important predictor of spinal cord compression and the residual space available for the spinal cord. Repeated abnormal minor motions may create irreversible damage to the spinal cord.27,28 A previous study reported that morphologic changes of the cord were related to the severity of compression on the spinal cord.29 During trauma, osteophytes or disc protrusion or extrusion from the anterior, and ligamentum flavum from the posterior caused SCI.30
Limitations
This study has several limitations. There was no control group, such as conservative treatment. Therefore, it could not be determined whether conservative treatment may be better than surgery for cervical SCI. Because this was a retrospective analysis, we could not control for many variables, such as the exact timing of trauma, and there exists the possibility of selection bias. Neither did we analyze the range and degree of signal intensity in the spinal cord nor the T1 sagittal slope, which is important to identify an association between cervical kyphosis and surgical outcome. The figures showed the condition of the cervical spine after trauma, but it is regrettable that we could not show evolution of posttraumatic kyphosis in each of its subdivisions. Prospective and large-scale studies should be performed to elucidate the association between type of cervical kyphosis and success of surgery, that is, decompression of the spinal cord and nerve root, and restoration of cervical spine stability. In a future study, we can explore a correlation between types of cervical kyphosis and cervical SCI.
Conclusion
In a relatively large population of patients with cervical kyphosis, risk factors for a poor outcome after cervical surgery for SCI were older age, low preoperative JOA score, and cervical instability. The type of cervical kyphosis, global or segmental, did not appear to influence the clinical outcome of the surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
Han K, Lu C, Li J, et al. Surgical treatment of cervical kyphosis. Eur Spine J. 2010;20(4):523–536. | ||
Ogihara S, Kunogi J. Single-stage anterior and posterior fusion surgery for correction of cervical kyphotic deformity using intervertebral cages and cervical lateral mass screws: postoperative changes in total spine sagittal alignment in three cases with a minimum follow-up of five years. Neurol Med Chir (Tokyo). 2015;55(7):599–604. | ||
Grosso MJ, Hwang R, Krishnaney AA, Mroz TE, Benzel EC, Steinmetz MP. Complications and outcomes for surgical approaches to cervical kyphosis. J Spinal Disord Tech. 2015;28(7):E385–E393. | ||
Nemani VM, Derman PB, Kim HJ. Osteotomies in the cervical spine. Asian Spine J. 2016;10(1):184–195. | ||
Harrison DD, Janik TJ, Troyanovich SJ, Holland B. Comparisons of lordotic cervical spine curvatures to a theoretical ideal model of the static sagittal cervical spine. Spine (Phila Pa 1976). 1996;21(6):667–675. | ||
Takeshita K, Murakami M, Kobayashi A, Nakamura C. Relationship between cervical curvature index (Ishihara) and cervical spine angle (C2–7). J Orthop Sci. 2001;6(3):223–226. | ||
Ruangchainikom M, Daubs MD, Suzuki A, et al. Effect of cervical kyphotic deformity type on the motion characteristics and dynamic spinal cord compression. Spine (Phila Pa 1976). 2014;39(12):932–938. | ||
Kwon SY, Shin JJ, Lee JH, Cho WH. Prognostic factors for surgical outcome in spinal cord injury associated with ossification of the posterior longitudinal ligament (OPLL). J Orthop Surg Res. 2015;10:94. | ||
Chang V, Ellingson BM, Salamon N, Holly LT. The risk of acute spinal cord injury after minor trauma in patients with preexisting cervical stenosis. Neurosurgery. 2015;77(4):561–565. | ||
Yoo DS, Lee SB, Huh PW, Kang SG, Cho KS. Spinal cord injury in cervical spinal stenosis by minor trauma. World Neurosurg. 2010;73(1):50–52. | ||
Arnold PM, Fehlings MG, Kopjar B, et al. Mild diabetes is not a contraindication for surgical decompression in cervical spondylotic myelopathy: results of the AOSpine North America multicenter prospective study (CSM). Spine J. 2014;14(1):65–72. | ||
Pal D, Bayley E, Magaji SA, Boszczyk BM. Freehand determination of the trajectory angle for cervical lateral mass screws: how accurate is it? Eur Spine J. 2011;20(6):972–976. | ||
Ohara A, Miyamoto K, Naganawa T, Matsumoto K, Shimizu K. Reliabilities of and correlations among five standard methods of assessing the sagittal alignment of the cervical spine. Spine (Phila Pa 1976). 2006;31(22):2585–2591. | ||
White AA 3rd, Johnson RM, Panjabi MM, Southwick WO. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975;109:85–96. | ||
Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J. 2015;15(5):875–884. | ||
Lee HJ, Kim HS, Nam KH, Han IH, Cho WH, Choi BK. Neurologic outcome of laminoplasty for acute traumatic spinal cord injury without instability. Korean J Spine. 2013;10(3):133–137. | ||
Dvorak MF, Fisher CG, Hoekema J, et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine (Phila Pa 1976). 2005;30(20):2303–2311. | ||
Anderson DG, Sayadipour A, Limthongkul W, Martin ND, Vaccaro A, Harrop JS. Traumatic central cord syndrome: neurologic recovery after surgical management. Am J Orthop (Belle Mead NJ). 2012;41(8):E104–E108. | ||
Boese CK, Lechler P. Prediction of prognosis in patients with cervical spinal cord injury without radiologic evidence of trauma using MRI. Orthopedics. 2014;37(5):296. | ||
Masini M, Maranhao V. Experimental determination of the effect of progressive sharp-angle spinal deformity on the spinal cord. Eur Spine J. 1997;6(2):89–92. | ||
Chavanne A, Pettigrew DB, Holtz JR, Dollin N, Kuntz C 4th. Spinal cord intramedullary pressure in cervical kyphotic deformity: a cadaveric study. Spine (Phila Pa 1976). 2011;36(20):1619–1626. | ||
Sorar M, Seçkin H, Hatipoglu C, et al. Cervical compression myelopathy: is fusion the main prognostic indicator? J Neurosurg Spine. 2007;6(6):531–539. | ||
Hasegawa K, Hirano T, Shimoda H, Homma T, Morita O. Indications for cervical pedicle screw instrumentation in nontraumatic lesions. Spine (Phila Pa 1976). 2008;33(21):2284–2289. | ||
Sun LQ, Shen Y, Li YM, Cao JM. Prediction of prognosis in patients with cervical spinal cord injury without radiologic evidence of trauma using MRI. Orthopedics. 2014;37(3):e302–e306. | ||
Koyanagi T, Hirabayashi K, Satomi K, Toyama Y, Fujimura Y. Predictability of operative results of cervical compression myelopathy based on preoperative computed tomographic myelography. Spine (Phila Pa 1976). 1993;18(14):1958–1963. | ||
Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1993;18(14):2024–2029. | ||
Luo J, Cao K, Huang S, et al. Comparison of anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy. Eur Spine J. 2015;24(8):1621–1630. | ||
Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455–461. | ||
Ohshio I, Hatayama A, Kaneda K, Takahara M, Nagashima K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976). 1993;18(9):1140–1149. | ||
Fengbin Y, Deyu C, Xinwei W, et al. Trauma-induced spinal cord injury in cervical spondylotic myelopathy with or without lower cervical instability. J Clin Neurosci. 2013;20(3):419–422. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.