Back to Journals » International Journal of General Medicine » Volume 14
Incidence and Risk Factors for Adjacent Segment Disease After Transforaminal Lumbar Interbody Fusion in Patients with Lumbar Degenerative Diseases
Authors Ye J, Yang S, Wei Z , Cai C, Zhang Y, Qiu H, Chu T
Received 8 September 2021
Accepted for publication 21 October 2021
Published 15 November 2021 Volume 2021:14 Pages 8185—8192
DOI https://doi.org/10.2147/IJGM.S337298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jiawen Ye, Sizhen Yang, Zihan Wei, Chenhui Cai, Ying Zhang, Hao Qiu, Tongwei Chu
Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, People’s Republic of China
Correspondence: Tongwei Chu
Department of Orthopedics, Xinqiao Hospital, Army Medical University, Shapingba District, Chongqing, People’s Republic of China
Tel +86 13708388336
Email [email protected]
Purpose: To explore the incidence and risk factors for adjacent segment disease (ASD) in patients with lumbar degenerative diseases after transforaminal lumbar interbody fusion (TLIF).
Patients and Methods: The clinical data of 1258 patients who underwent transforaminal lumbar interbody fusion (TLIF) for lumbar degenerative diseases in our hospital from January 2011 to December 2017 were retrospectively analyzed. Patients were divided into the ASD group and non-ASD (N-ASD) group, and the incidence of ASD was calculated. We compared age, BMI, comorbidities, surgery-related parameters, and imaging parameters before surgery between the two groups and used univariate analysis and logistic regression analysis to explore the risk factors for ASD.
Results: Among the 1258 patients who underwent TLIF due to lumbar degenerative diseases, 65 patients developed ASD and received surgical treatment for it, for an incidence of 5.2%. The average onset time of ASD was 68.3± 25.1 (20– 123) months. Univariate analysis showed that BMI, hypertension, preoperative adjacent segment disc degeneration and preoperative adjacent intervertebral disc height were significantly different between the ASD and N-ASD groups (P< 0.05). Incorporating the above indicators into the logistic regression model, the results showed that BMI and preoperative adjacent intervertebral disc degeneration were risk factors for ASD after TLIF.
Conclusion: The incidence of ASD after TLIF in patients with lumbar degenerative disease is approximately 5.2%. High BMI and preoperative adjacent segment disc degeneration are risk factors for ASD after TLIF.
Keywords: lumbar degenerative disease, transforaminal lumbar interbody fusion, adjacent segment disease, risk factors
Introduction
With the acceleration of demographic aging, the incidence of lumbar degenerative diseases is approximately 3.36%1 worldwide and is on the rise. Posterior lumbar interbody fusion (PLIF) is one of the classic surgical procedures for lumbar degenerative diseases.2 It can achieve stable intervertebral fusion and sufficient nerve structure decompression,3 thus significantly relieving the symptoms. Transforaminal lumbar interbody fusion (TLIF) can preserve more posterior structures while achieving the above-mentioned surgical effects and maintaining the biomechanical stability of the spine,4,5 so TLIF is increasingly used to treat lumbar degenerative diseases. Although most patients have alleviated symptoms and improved quality of life after lumbar fusion, the biomechanical changes in the spine caused by fusion may accelerate the degeneration of adjacent segments. Adjacent segment disease (ASD) is a potential long-term complication after lumbar fusion.6 In different studies, due to the different definitions of ASD, the incidence of ASD after lumbar fusion has ranged from 5.0% to 49%.7–11 The risk factors for ASD after lumbar fusion are still controversial, and there have been few articles on ASD after TLIF using the same technique with a large number of case follow-ups. To the best of our knowledge, there have been only 2 articles with more than 1000 cases.10,12 This study aims to explore the incidence of ASD and its risk factors in patients with lumbar degenerative diseases after TLIF with the hope of reducing the incidence of ASD.
Patients and Methods
Subjects
This study complied with the Declaration of Helsinki and was approved by the ethics committee of Xinqiao Hospital of Army Medical University. All patients signed an informed consent form. A total of 1258 patients with lumbar degenerative disease treated by TLIF performed by the same surgeon in our department from January 2011 to December 2017 were included. They were 566 males and 692 females with an average age of 56.4 ±12.4 years. Inclusion criteria: clinical diagnosis of lumbar degenerative disease, including lumbar disc herniation (LDH), degenerative spondylolisthesis (DS), isthmic spondylolisthesis (SO), and lumbar spinal stenosis (LSS); TLIF treatment; complete preoperative and postoperative imaging data; follow-up time ≥24 months; and ≤2 fusion segments. Exclusion criteria: age <18 years; complications with spinal fractures, tumors, infections, ankylosing spondylitis or other spinal diseases; previous history of lumbar fusion surgery; and scoliosis with a Cobb angle ≥ 20°.
In this study, ASD was defined as a symptomatic degenerative disease in an adjacent segment after lumbar fusion, for which a second operation was performed on the adjacent fusion segment.9
Radiological and Clinical Evaluation
The patient’s age, body mass index (BMI), comorbidities (hypertension; diabetes mellitus, DM), smoking history, bone mineral density (BMD), operation time, blood loss, number of fixed segments, number of fusion segments, etc. According to whether ASD had occurred by the last follow-up, patients were divided into the ASD group and the non-ASD (N-ASD) group. To evaluate the preoperative lumbar spine imaging data, we used the Pfirrmann classification (I–V) to evaluate the degeneration of the intervertebral disc at adjacent segments13 and used intervertebral height to judge the collapse of the intervertebral space.
Surgical Procedures
After general anesthesia, the patient was placed in the prone position, and the subperiosteal dissection fully revealed the facet joints of both sides of the vertebral body. Positioning under the guidance of the 3D digital C-arm, one pedicle screw was implanted on each side of the surgical vertebral body. We used an osteotome to remove the inner part of the facet joint on the side of the vertebral body of the diseased segment, remove the ligamentum flavum with a Kerrison rongeur, cut the posterior longitudinal ligament, remove the intervertebral disc, and completely remove the nucleus pulposus. This was followed by curettage of the annulus fibrosus and cartilage endplates of the intervertebral space. After biting the excised lamina into pieces, a part of it was densely implanted in the intervertebral space, and a suitably sized cage filled with autologous bone was implanted in the intervertebral space. The dura mater and nerve roots were fully loosened, and the bilateral internal fixation was firm. We placed 2 fully flush negative-pressure drainage tubes and sutured the incision. The drainage tube was removed 48–72 hours after the operation, at which time the patient first got out of bed. The patient wore a hard waistband until 3 months after the operation.
Statistical Analysis
Data were analyzed using Statistical Product and Service Solutions software (version 23.0; SPSS). Continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as frequencies or percentages. The independent t-test, χ2 test and rank-sum test were used to perform single-factor correlation analysis on various variables to find potential risk factors for ASD. The potential risk factors were further analyzed by logistic regression to find the independent risk factors for ASD after TLIF. Odds ratios (ORs) and their 95% confidence intervals were calculated.
Results
This study included 1258 patients who underwent TLIF due to lumbar degenerative disease, including 566 males and 692 females with an average age of 56.4±12.4 years and an average follow-up time of 35.0±17.8 months. Among them were 988 patients with single-segment fusion and 270 patients with two-segment fusion. The average number of fusion segments was 1.2±0.4. The patient’s diagnoses were as follows: LDH, N=560; DS/SO, N=438; and LSS, N=260 (Table 1). No significant difference was found in the ASD incidence or ASD period of these three diseases (p>0.05).
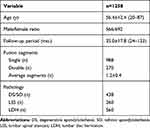 |
Table 1 Characteristics of the Patients |
Among the 1258 patients, 65 developed ASD and underwent reoperation. The total incidence was 5.2%, and the average onset time of ASD was 68.3±25.1 (20–123) months after TLIF. Two of these patients had ASD again after the second operation and received a third operation. The segment of the previous operation in patients with ASD was as follows: L2–L4, N=2; L3–L4, N=2; L3–L5, N=6; L4–L5, N=37; L4–S1, N=4; L5–S1, N=14. The ASD segment was as follows: L2/3, N=2; L2/3 and L3/4, N=1; L2/3 and L5/S1, N=2; L3/4, N=30; L3/4 and L5/S1, N=1; L4/5, N=17; L5/S1, N=12. ASD occurred in 46 cases at the cranial fusion segment, 16 cases at the caudal segment, and 3 cases on both sides (Table 2).
 |
Table 2 Characteristics of the Patients with ASD |
The 65 ASD reoperation procedures were TLIF (N=43), oblique lumbar interbody fusion (OLIF, N=8), percutaneous endoscopic lumbar discectomy (PELD, N=12) and microendoscopic discectomy (MED, N=2). The symptoms of all ASD patients were relieved, and the function was improved after reoperation (Table 2).
Univariate analysis showed that there were no significant differences between the two groups in terms of age, sex, DM, smoking history, BMD, operative time, blood loss, number of fixed segments, or number of fusion segments (P>0.05) (Table 3). BMI (t=11.945, P=0.000), history of hypertension (χ2=6.675, P=0.010), preoperative adjacent intervertebral disc degeneration (χ2=96.923, P=0.000) and preoperative intervertebral height (t=−8.172, P=0.000) were significantly different between the ASD and N-ASD groups (Tables 3 and 4).
 |
Table 3 Comparison of Demographic and Surgical Parameters Between the ASD Group and N-ASD Group |
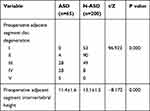 |
Table 4 Comparison of Radiological Variables Between the ASD Group and N-ASD Group |
BMI, history of hypertension, preoperative adjacent intervertebral disc degeneration, preoperative adjacent spinal canal stenosis, and height of the intervertebral disc, meeting the cutoff in the univariate analysis, were incorporated into a logistic regression model. Logistic regression analysis showed that BMI (P=0.000, OR=2.073, 95% CI=1.558–2.759) and preoperative adjacent intervertebral disc degeneration (P=0.000, OR=6.093, 95% CI=3.127–11.871) were risk factors for ASD after TLIF. Preoperative intervertebral height (P=0.035, OR=0.713, 95% CI=0.521–0.976) may be a protective factor against ASD after TLIF (Table 5).
 |
Table 5 Logistic Regression Analysis of Risk Factors in ASD Patients |
Discussion
TLIF is a classic surgical method for the treatment of lumbar degenerative diseases. It relieves the symptoms and improves the quality of life of the patient.14 However, the biomechanical changes to the spine caused by lumbar fusion may accelerate the regression of adjacent segments, which could also affect the occurrence of ASD. In the present study, 5.2% of patients experienced ASD, the cranial segment being more often involved than the caudal segment, which is consistent with the previously reported 5–24% reoperation rate of adjacent segments after lumbar fusion surgery.7,8,10 Different researchers may define ASD differently. Some researchers10 divide ASD into radiological ASD (R-ASD), symptomatic ASD (S-ASD) and operative ASD (O-ASD). Our study used the definition of O-ASD, and all patients with O-ASD underwent reoperation. During the follow-up period, we excluded patients with incomplete imaging data, less than 2 years of follow-up or loss to follow-up. Therefore, the actual incidence of ASD after TLIF may be higher.
We found that the type of preoperative lumbar degenerative disease had no significant effect on the incidence or onset time of ASD (Figure 1). This may be due to the small difference in the incidence of various lumbar degenerative diseases and the large sample, so the likelihood of selection deviation was low.
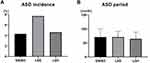 |
Figure 1 The incidence (A) and onset delay (B) of ASD between the three types of lumbar degenerative diseases. No difference was seen in the ASD incidence or ASD onset time. |
The exact cause of ASD is not yet known, but the spinal biomechanical changes caused by fusion, including increased load on the facet joints, increased pressure in the intervertebral disc, and excessive movement of adjacent segments, are believed to play key roles.7 The occurrence of ASD after fusion may be affected by many factors, including age, BMI, smoking history, hypertension, BMD, osteoporosis, preoperative adjacent intervertebral disc degeneration, preoperative or intraoperative superior facet joint violation, long-segment fusion, sagittal vertical axis (SVA), postoperative lumbar lordosis (LL), and preoperative pelvic incidence (PI).6–8 There is still much controversy regarding the risk factors for ASD after fusion. This study tried to explore the risk factors for ASD through the follow-up of more than 1000 patients to provide a reference for the prevention of ASD and the formulation of surgical plans. After single-factor analysis and logistic regression analysis, we found that BMI and preoperative adjacent intervertebral disc degeneration were risk factors for ASD. In the logistic regression, the OR of preoperative adjacent intervertebral height was less than 1, so we speculate that it may be a protective factor against the occurrence of ASD.
BMI is an objective and simple indicator that is generally accepted. The World Health Organization defines overweight and obesity as a BMI greater than 25 and 30 kg/m2, respectively.15 In our experience, it seems that overweight people are more likely to suffer from lumbar disc herniation, but whether BMI is associated with the occurrence of ASD after fusion is still controversial. Our univariate analysis of the BMI of the ASD and N-ASD groups showed that it was significantly different between the two groups (t=11.945, P=0.000). Further logistic regression analysis showed that P=0.000, OR=2.263, 95% CI=1.622–3.157, indicating that BMI is an independent risk factor for ASD after TLIF; the higher the BMI, the greater the risk of ASD. Wang et al16 showed that patients with a high preoperative BMI had a significantly increased risk of ASD after undergoing lumbar fusion surgery. Zheng et al17 and Ushio et al18 also showed that patients with higher BMI are more likely to develop ASD. All of these are consistent with our findings. We think this may be related to the increase in the motion of the segments adjacent to the fusion and the mechanical stress between the vertebrae after the patient undergoes lumbar fusion surgery.19,20 Compared with patients with normal BMI, patients with high BMI will have a greater increase in the degree of motion of adjacent vertebrae and in the mechanical stress of the intervertebral vertebrae, which will further increase the workload of adjacent intervertebral discs and accelerate the degeneration of the adjacent intervertebral discs, leading to ASD. Although the effect of BMI on ASD is still unclear, it is necessary to recommend appropriate weight loss for patients with higher BMIs after surgery.
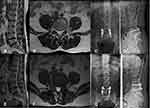
Preoperative degeneration of the intervertebral disc in the adjacent segment may be a risk factor for ASD. In this study, through univariate analysis and logistic regression analysis, we found that preoperative adjacent intervertebral disc degeneration was a risk factor for ASD (P=0.000, OR=19.550, 95% CI=6.951–54.983). Wang et al6 reported that a preoperative Pfirrmann classification of ≥3 was significantly associated with a higher incidence of ASD. Anandjiwala et al21 prospectively studied 74 consecutive patients undergoing lumbar/lumbosacral fusion who were followed up for at least 5 years, and the results showed that patients with preoperative adjacent segment disc degeneration were at higher risk of ASD. This is consistent with our results. Fixation and fusion lead to reduced flexibility and increased stiffness of the lumbar spine, which lead to biomechanical changes in adjacent motion segments, including the concentration of stress in adjacent segments, overmobility and increased pressure in the intervertebral disc.22 For healthy, freely moving segments adjacent to a lumbar fusion, these biomechanical changes and an older age will lead to accelerated disc degeneration after surgery.23 In a previously degenerated lumbar intervertebral disc, the inherent function is reduced, and biomechanical changes make it easier for the degeneration to accelerate after fusion surgery, which will promote ASD. However, there is still much controversy about whether fusing severely degenerated segments causes symptoms. On the one hand, if the adjacent degenerative segment is not within the fusion range, it may aggravate the degeneration of the adjacent intervertebral disc due to its vulnerability. On the other hand, if the adjacent degenerative segment is included in the fusion range, it will lead to prolongation of the fusion, thereby increasing the likelihood of new ASD.24 Therefore, regardless of the surgical method selected, the surgeon should fully inform the patient of the risk of ASD in the adjacent degenerative segment before surgery (Figure 2).
The height of the intervertebral space can also reflect the degeneration of the adjacent intervertebral disc. Collapse of the intervertebral space is often accompanied by severe degeneration of the intervertebral disc and the appearance of neurological symptoms. When we analyzed the preoperative adjacent segment intervertebral height, we found that there were significant differences between the two groups with ASD and no ASD (t=−8.172, P=0.000). Further logistic regression analysis showed that preoperative adjacent segment intervertebral height may be a protective factor against ASD (P=0.000, OR=0.713, 95% CI=0.521–0.976). There have been few reports about the correlation between preoperative adjacent segment intervertebral height and ASD after lumbar fusion. Our findings need to be confirmed by further studies. As far as we know, within the normal range, the higher the intervertebral space height, the better the function of the intervertebral disc. After lumbar fusion surgery, a greater height makes the disc better able to resist the biomechanical changes and the movement increase brought by fusion.
There are some limitations to this study. First, it was a single-center retrospective study. A prospective randomized controlled multicenter study is necessary to further study the risk factors for ASD after TLIF. Second, this study did not include spine-pelvic parameters, including lumbar lordosis, sacral slope, pelvic tilt, pelvic incidence, pelvic incidence minus lumbar lordosis, sagittal vertical axis, etc.
Conclusions
A retrospective study of more than 1000 cases found that the incidence of adjacent segment disease (ASD) after transforaminal lumbar interbody fusion (TLIF) in patients with lumbar degenerative disease was 5.2%. High body mass index (BMI) and preoperative adjacent intervertebral disc degeneration were risk factors for ASD after TLIF. Encouraging patients to lose weight and trying to avoid lumbar fusion surgery at the vertebrae adjacent to a degenerated intervertebral disc may reduce the occurrence of ASD.
Funding
Chongqing Technology Innovation and Application Development Special Project, China (cstc2019jscx-gksbX0014).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Global Spine J. 2018;8(8):784–794. doi:10.1177/2192568218770769
2. Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ ATP,LLIF and ALIF. J Spine Surg. 2015;1(1):2–18. doi:10.3978/j.issn.2414-469X.2015.10.05
3. Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int. 1994;3:577–590.
4. Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118–126. doi:10.1007/s12178-009-9053-8
5. Audat Z, Moutasem O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J. 2012;53(3):183–187.
6. Wang T, Ding W. Risk factors for adjacent segment degeneration after posterior lumbar fusion surgery in treatment for degenerative lumbar disorders: a meta-analysis. J Orthop Surg Res. 2020;15(1):582. doi:10.1186/s13018-020-02032-7
7. Bagheri SR, Alimohammadi E, Zamani Froushani A, et al. Adjacent segment disease after posterior lumbar instrumentation surgery for degenerative disease: incidence and risk factors. J Orthop Surg. 2019;27(2):2309499019842378. doi:10.1177/2309499019842378
8. Zhong ZM, Deviren V, Tay B, et al. Adjacent segment disease after instrumented fusion for adult lumbar spondylolisthesis: incidence and risk factors. Clin Neurol Neurosurg. 2017;156:29–34. doi:10.1016/j.clineuro.2017.02.020
9. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi:10.1016/j.spinee.2004.07.007
10. Okuda S, Nagamoto Y, Matsumoto T, et al. Adjacent segment disease after single segment posterior lumbar interbody fusion for degenerative spondylolisthesis: minimum 10 years follow-up. Spine. 2018;43(23):E1384–E1388. doi:10.1097/BRS.0000000000002710
11. Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29(17):1938–1944. doi:10.1097/01.brs.0000137069.88904.03
12. Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18(11):1637–1643. doi:10.1007/s00586-009-1060-3
13. Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. PMID: 11568697. doi:10.1097/00007632-200109010-00011
14. Lan T, Hu SY, Zhang YT, et al. Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. World Neurosurg. 2018;112:86–93. doi:10.1016/j.wneu.2018.01.021
15. World Health Organization. World Health Organization obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 1997.
16. Wang H, Ma L, Yang D, et al. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine. 2017;96(5):e6032. doi:10.1097/MD.0000000000006032
17. Zheng G, Wang C, Wang T, et al. Relationship between postoperative lordosis distribution index and adjacent segment disease following L4–S1 posterior lumbar interbody fusion. J Orthop Surg Res. 2020;15(1):129. doi:10.1186/s13018-020-01630-9
18. Ushio S, Hirai T, Yoshii T, et al. Preoperative risk factors for adjacent segment degeneration after two-level floating posterior fusion at L3-L5. Spine Surg Relat Res. 2019;4(1):43–49. doi:10.22603/ssrr.2019-0003
19. Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25(13):1617–1624. doi:10.1097/00007632-200007010-00004
20. Weinhoffer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20(5):526–531. doi:10.1097/00007632-199503010-00004
21. Anandjiwala J, Seo JY, Ha KY, et al. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: a prospective cohort study with a minimum five-year follow-up. Eur Spine J. 2011;20(11):1951–1960. doi:10.1007/s00586-011-1917-0
22. Ruberté LM, Natarajan RN, Andersson GB. Influence of single-level lumbar degenerative disc disease on the behavior of the adjacent segments–a finite element model study. J Biomech. 2009;42(3):341–348. doi:10.1016/j.jbiomech.2008.11.024
23. Liang J, Dong Y, Zhao H. Risk factors for predicting symptomatic adjacent segment degeneration requiring surgery in patients after posterior lumbar fusion. J Orthop Surg Res. 2014;9:97. doi:10.1186/s13018-014-0097-0
24. Kim HJ, Kang KT, Chun HJ, et al. The influence of intrinsic disc degeneration of the adjacent segments on its stress distribution after one-level lumbar fusion. Eur Spine J. 2015;24(4):827–837. doi:10.1007/s00586-014-3462-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.