Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Incidence and Predictors of Virological Failure Among Adult HIV/AIDS Patients on Second-Line Anti-Retroviral Therapy, in Selected Public Hospital of Addis Ababa, Ethiopia: Retrospective Follow-Up Study
Authors Zakaria HF , Raru TB , Hassen FA, Ayana GM , Merga BT , Debele GR , Kiflemariam G, Kebede SA , Ayele TA
Received 24 March 2022
Accepted for publication 29 June 2022
Published 8 July 2022 Volume 2022:14 Pages 319—329
DOI https://doi.org/10.2147/HIV.S367677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Hamdi Fekredin Zakaria,1 Temam Beshir Raru,1 Fila Ahmed Hassen,2 Galana Mamo Ayana,1 Bedasa Taye Merga,2 Gebiso Roba Debele,3 Genet Kiflemariam,4 Sewnet Adem Kebede,5 Tadesse Awoke Ayele5
1Department of Epidemiology and Biostatistics, School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2Department of Public Health and Health Policy, School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3Department of Public Health, College of Health Sciences, Metu University, Metu, Ethiopia; 4International Institute for Primary Health Care, Addis Ababa, Ethiopia; 5Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medical and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Temam Beshir Raru, Department of Epidemiology and Biostatistics, School of Public Health, College of Health and Medical Sciences, Haramaya University, P.O. Box: 235, Harar, Ethiopia, Email [email protected]
Introduction: Virological suppression for persons living with HIV (PLHIV) on antiretroviral therapy (ART) reached 85% at the end of 2018, still falling short of the UNAIDS target of 95%. In Ethiopia, there were studies on treatment failure focusing on viral suppression and immunological failure of ART users, but none of them have addressed virological failure for second-line regimens.
Objective: This study was aimed to estimate the incidence and predictors of virological failure among HIV patients who were switched to second-line ART at the selected public hospitals in Addis Ababa.
Methods: An institutional-based retrospective follow-up study was conducted from September 2018 to January 2021 at public hospitals in Addis Ababa. The sample size was determined by using the Schoenfeld formula. Data entry were done by Epi Data version-4.6.0.0 and exported to R-software version-4.1.0 for analysis. Kaplan–Meier methods were used to compare the survival estimates. Cox proportional hazard model was used to identify predictors of virological failure and model adequacy was checked by using the Cox–Snell residuals plot.
Results: Overall 44 (12.22%) HIV/AIDS patients developed virological failure with incidence density of 3.57/1000 Person-Month (PM) with 95% CI of [2.65– 4.79]. Age > 45 years (AHR=0.36, 95% CI: 0.12– 0.99), CD4 count < 100cell/mm3 (AHR=3.02, 95% CI: 1.17– 7.78), TB co-infection (AHR=2.48, 95% CI: 1.10– 6.33), ATV/r-based second-line regimen (AHR=0.27, 95% CI: 0.11– 0.70), and poor adherence at the start of second-line ART (AHR=6.18, 95% CI: 1.93– 19.76) were the significant predictors of virological failure.
Conclusion: A high incidence of virological failure was noticed. The rate of virological failure was higher for patients who had poor ART adherence, small CD4count, and TB co-infection. Therefore, targeted HIV care interventions shall be provided to young ages and efforts stepped up to improve adherence to ART, which helps to increase immunity and suppress viral replication. In addition, prevention and early detection of TB co-infection are crucial to the patients.
Keywords: incidence, second-line antiretroviral therapy, predictors, virological failure, HIV/AIDS
Introduction
There is a global commitment to end the AIDS epidemic by 2030, and the global HIV response has improved HIV-positive people’s access to care and survival.1,2 By the end of 2018, global efforts to increase ART coverage for all persons living with HIV (PLHIV) have resulted in an estimated 23.3 million PLHIV on ART.2 Even though virological suppression for PLHIV on ART had reached 85% at the end of 2018, still falls short of the UNAIDS target of 95%. The cost and complexity of viral load (VL) testing, as well as a lack of awareness about the benefits of frequent VL monitoring among health-care providers and patients, have hampered the scale-up of routine HIV VL testing in resource-limited settings.3
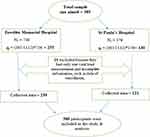 |
Figure 1 Proportional allocation of sample size among selected public hospitals in Addis Ababa, Ethiopia. |
 |
Figure 2 Kaplan–Meier survival function of the virological failure of HIV/AIDS patients on second-line ART at public hospitals of Addis Ababa Ethiopia, September 2018 to January 2021. |
Ethiopia is one of the most impacted countries in Sub-Saharan Africa, with an estimated 610,335 PLHIV in 2018.4 ART reduced HIV-related morbidity and death associated with HIV infection while also increasing patient survival at a low cost of drug toxicity.5 Even though ART reduces morbidity and mortality, treatment failure, drug resistance, and non-adherence are becoming increasingly difficult to overcome to improve treatment outcomes. It can be an immunological, clinical, or virological failure if an ART regimen fails to control viral replication, and the preferred monitoring approach for diagnosing and verifying treatment failure is viral load.6
First-line ART is the initial regimen prescribed for an ART naive patient when the patient fulfills national clinical and laboratory criteria to be initiated on ART. Second-line ART is the subsequent regimen used in sequence after First-line therapy has failed. To ensure long-term HIV treatment success in Sub-Saharan Africa, there is a clear and increasing need for expanded access to third-line drug options. However, in resource-constrained environments, HIV treatment failure involving second-line regimens has very limited opportunities for further switching, which is a serious concern.7,8
First-line treatment failures have become more common as the number of PLHIV on ART has increased, as has the duration of medication. As a result, a growing proportion of PLHIV has begun second-line ART. Although VL is the gold standard method, clinical and immunological criteria are the most commonly employed monitoring tools for ART in Ethiopia for patient health status.9 These could lead to late diagnosis of virological failure, jeopardizing the long-term effectiveness and treatment success of second-line regimens. As the increased prevalence of antiretroviral resistance threatens the maintenance of virally suppressive ART, a broader understanding of the durability of second-line would facilitate advocacy efforts around optimizing lifelong ART.
The finding of a systematic review and meta-analysis shows that high baseline viral load, advanced clinical stage of HIV at baseline, low peak CD4 cell counts at baseline (<100 cells/mm3) and suboptimal adherence to second-line therapy were factors associated with the significantly increased occurrence of second-line ART virological failures.10 The national routine viral load program data from Ethiopia Public Health Institute (EPHI) showed that 40 out of 198 (20%) patients on second-line had a virological failure.8
Even though second-line ART was first offered in Ethiopia ten years ago, there are few studies on the therapeutic outcomes of second-line therapy. Although there has been researching on treatment failure concentrating on viral suppression and immunological failure in ART users, none of these studies have looked into virological failure in second-line regimens in Ethiopia. Therefore, this study was aimed estimate the incidence of virological failure and its predictors among adult HIV patients who were switched to second-line therapy at the selected public hospital in Addis Ababa, Ethiopia.
Methods
Study Design and Setting
An institutional-based retrospective follow-up study was conducted from September 2018 to January 2021 at public hospitals in Addis Ababa. There are around ten public hospitals in Addis Ababa.11,12
Zewditu Memorial Hospital was established in 1925. It is the first ART service delivery site (since 2003) and has grown to become the largest HIV care and treatment site in the country. St. Paul’s hospital Millennium Medical College was established in 1968 by the late Emperor Haile Selassie, although the medical school opened in 2007. It has also provided comprehensive HIV/AIDS care for the past 16 years. It was initially payment based but after two years, it was transitioned to free service with the support of the center for disease control (CDC).
Population and Eligibility Criteria
The source population in the study were all adult HIV/AIDS patients ≥15 years old on second-line ART enrolled in ART clinics at selected public hospitals in Addis Ababa and the study population were patients on second-line ART enrolled in ART clinics at selected public hospitals in Addis Ababa during the study period, ie, from September 2018 to January 2021.
All enrolled adult HIV patients on second-line treatment during the study period, followed for at least six months were included. Patients with unknown dates of second-line ART initiation and unknown date of virological failure were excluded from the study.
Sample and Sampling Procedure
The sample size was determined based on the power method by considering predictors significantly associated with time to virological treatment failure from previous studies using the Schoenfeld formula13 using STATA 15 software with Cox proportional hazard assumptions. The sample size was calculated for the two predictor variables including CD4 cell count at the switch, and WHO stages at the switch from the study conducted in Northwest Ethiopia.14 Accordingly, the minimum sample size for this study is the maximum of the two, which is 385. Thus, ART patients who meet inclusion criteria were enrolled from selected hospitals.
Out of ten public hospitals in Addis Ababa, Zewditu Memorial hospital and St. Paul’s hospital Millennium Medical College were selected purposely for this study. A total of 1112 HIV patients on second-line ART between September 2018 to January 2021 had a follow-up in these facilities. From these, there are 736 and 376 HIV patients on second-line ART at Zewditu Memorial Hospital and St. Paul’s Hospital Millennium Medical College, respectively.
By using the proportional allocation method, from 385-study sample size 255 and 130 HIV patients on second-line ART was selected from Zewditu Memorial Hospital and St. Paul’s Hospital Millennium Medical College, respectively. However, twenty-five records of patients were excluded because they had only 3 months’ follow-up, their date of enrollment, and the date event happened were unknown. The final analysis was done on 360 patients. Among these 239 of them were from Zewditu Memorial Hospital and 121 were from St. Paul’s Hospital Millennium Medical College. In addition, study subjects from each hospital were selected using simple random sampling after gathering HIV patient identification number from the registration book and using it as a sampling frame (Figure 1).
Measurements
The dependent variable for this study was Incidence of virological failure, whereas the independent variable was Socio-Demographic variables (Sex, Age, Educational level, Marital status, Occupational status, religion), Clinical and immunological variables (Functional status, CD4 count, TB co-infection, Adherence, BMI, WHO stage at the start of the second line (at the switch), Second-line regimen, Opportunistic infection, Number of times first-line regimen changed, and Duration on first-line ART).
Time to event (survival time) was defined as a time in a month from the start of second-line ART treatment to the development of virological failure. The event was defined as patients who developed virological failure during the follow-up time. Virological failure is defined as a persistently detectable viral load exceeding 1000 copies/mL (two consecutive viral load measurements within a 3-month interval with adherence support between measurements) after at least 6 months of using ART.6 Censored was defined as when the patient was transferred out or being event-free at the end of the study, death, and lost to follow-up.
ART adherence was measured by considering patient’s clinical compliance in scheduled visits. It was classified as good, fair, and poor. Poor (Drug adherence of <85% or ≥5 ART drug doses missed out of 30 doses or >9 ART drug doses missed out of 60 doses), Fair (Drug adherence of 85–94% or 2–4 missed drug doses missed out of 30 doses or 4–9 missed drug doses out of 60 doses), and Good (Drug adherence of <95% and above or < 2 missed drug doses missed out of 30 doses or ≤3 missed drug doses out of 60 doses) (Supplementary File-1). Normal BMI: based on the WHO classification normal weight was from 18.5 to 24.99 kg/m2, mild from 17 to 18.49 kg/m2, moderate from 16 to 16.99 kg/m2, and BMI less than 16 and greater than 25 is severe and overweight, respectively (Supplementary File-1).
Data Collection Procedures and Quality Control
A standardized checklist was prepared and used to collect relevant data by reviewing patient charts. Secondary data was regularly recorded from patients’ charts followed up at each selected hospital. As data collectors, health professionals working at the ART clinic were assigned and data was collected by analyzing patient follow-up charts and cards. Individual patient cards were identified using the Health Management Information System (HMIS) card number. Then, socio-demographic, baseline, and follow-up clinical as well as immunological data were collected from the date patient started to follow up until the end of the study.
To confirm the quality of the data, a preliminary test was conducted among 5% of the sample size of the selected hospitals. Then, adequacy of the checklist was evaluated and unclear questions were modified and variables on which data are unavailable were removed before actual data collection. To ensure consistency, one-day training was given to data collectors and supervisors before data collection. Every day, the assigned supervisors were monitor and supervise the data collection process. In addition, the collected data were checked for completeness and consistency.
Data Processing and Management
Before the data was entered, it was checked for inconsistencies, completeness, coding error, clarity, and missing values. The data entry was performed using Epi-data version 4.6.0.0 and then exported to R statistical software Version 4.1.0 for further analysis.
Statistical Analysis
To describe the study, population descriptive measures such as means, median, standard deviation, IQRs, percentages, and frequencies were used. Besides, graphical methods and a frequency table were used for descriptive data. Kaplan–Meier (KM) method was used to estimate the survival time and the Log rank test was used to compare survival time between groups of categorical variables. Before fitting the survival model, proportional hazard assumptions were checked using Schoenfeld residual test statistically.
Cox proportional hazard model was fitted to identify predictors of virological failure. The factors that were significantly associated with virological failure in the uni-variable analysis at p-values less than 0.2 were included in the multivariable model. Variables with a p-value <0.05 and an adjusted hazard ratio (AHR) with a 95% confidence interval (CI) were considered statistically significant predictors of virological failure. Model adequacy was checked using cox Snell residuals.
Results
Socio-Demographic Characteristics
The data of 385 patients who were on second-line ART were reviewed and collected. Twenty-five records (6.49%) of patients were excluded because they had incomplete information, such as date of enrollment, and date of outcome of interest. The final analysis was done on 360 patients.
The mean age of the patients was 40.59 with an SD of 10.96 years and most 185 (51.39%) of the participants were between 30 and 45 years of age at the start of second-line ART. The majority of study participants 281 (78.06%) were Orthodox religious followers and 181 (50.28%) were non-government employed (Table 1).
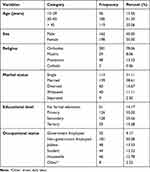 |
Table 1 Sociodemographic Characteristics of Patients on Second-Line ART at Public Hospitals of Addis Ababa Ethiopia, September 2018 to January 2021 |
Baseline Clinical and Anti-Retroviral Medication-Related Characteristics
The majority, 247 (68.61%) of the patients had no regimen change. At the beginning of second-line ART, 330 (91.67%) of the patients were started on atazanavir (ATV/r) boosted protease inhibitor second-line regimen and 30 (8.33%) received lopinavir (LPV/r) based regimen. More than two-fifth (42.78%) of the patients had a CD4 count below 100 cells/mm3. Majority, 302 (83.89%) of the patients had a good/fair clinical adherence. In addition, at the start of second-line ART, 271 (75.28%) of the patients were found in WHO clinical stage I/II (Table 2).
 |
Table 2 Characteristics of Patients During Switching and After Switched to Second-Line ART at Public Hospitals of Addis Ababa Ethiopia, September 2018 to January 2021 |
The Incidence of Virological Failure
Patients were followed for a minimum of 11.8 and a maximum of 59.83 months; 44 (12.22%) of patients developed virological failure during the follow-up period (95% CI: 9.21–16.05). Hence, the overall incidence density of virological failure in this follow-up study was 3.57 (95% CI, 2.65–4.79) per 1000 PM of observations.
A graph of the Kaplan–Meier (KM) failure function was used to describe the cumulative incidence rate (IR) of virological failure over the follow-up period. The cumulative probability of surviving or being free from the event of interest at the end of 20, 30, 40, and 50 months was 91.74%, 87.64%, 87.10%, and 85.02%, respectively (Figure 2).
Predictors of Second-Line Virological Failure
As variable selection precedes model diagnostics, factors significantly associated with time to virological failure in the univariate analysis at p values less than 0.2 were included in the multivariable survival model. The multicollinearity of the variables was assessed using pseudo-VIF and its values range from 1.04 to 1.61 which indicates the absence of multicollinearity among the independent variables. The proportional hazards assumption was checked by using a cumulative log hazard plot, logrank test and tested globally using Schoenfeld residuals, and it was revealed that all covariates and the complete model satisfied the proportional hazard assumption (Chi-square = 20.86, p-value = 0.53). The goodness of fitness of the model was satisfied after being checked by using the Nelson-Aalen cumulative hazard function against Cox–Snell residual plot (Figure 3).
The results from the multi-variable Cox proportional hazard model revealed that baseline age, CD4 count, TB co-infection, second-line regimen, and ART adherence were found to be significant predictors of second-line virological failure.
The hazard of virological failure for patients who were >45 years old at the start of second-line ART (AHR: 0.36, 95% CI: 0.12–0.99) was lower by 64% as compared to patients who were 15–29 years old at the start of second-line ART. The risk of virological failure was 2.48 times higher (AHR=2.48, 95% CI: 1.10–6.33) among patients who started second-line ART with TB co-morbidity as compared to those who have no TB co-morbidity. Likewise, the hazard of experiencing Virological failure was 6.18 times higher (AHR=6.18, 95% CI: 1.93–19.76) for patients who started second-line ART with poor adherence as compared to those with good/fair adherence levels (Table 3).
Discussion
The incidence and predictors of virological failure among adult HIV/AIDS patients on second-line ART in selected public hospitals in Addis Ababa were explored in this study. The overall incidence of virological failure in this follow-up was 3.57 per 1000 PM observations (95% CI, 2.65–4.79). Cox proportional hazard model was performed and significant predictors of virological failure were age, ART adherence, CD4 count, TB co-infection, and types of second-line drug regimen.
In the follow-up period, participants contributed a total of 12,339.567 person-months of observations, and the overall incidence rate of virological failure was 3.57 (95% CI, 2.65–4.79) per 1000 PM. This is higher than the study conducted in Northern Ethiopia.15 The possible reason for this might be due to the difference in the duration of time taken for the studies. Since in most studies virological failures on second-line ART occurred during the first year of follow-up period. However, our result was lower than study conducted in Amhara region.16 The difference might be as a result of the fact that the patients of the study participated in Amhara region were only under the first-line ART regimens, but our study patients were under second-line of ART regimen and the difference in follow-up period may contribution for this.
In this study, being older (>45 years’ age) at the start of second-line ART was found to be significantly associated with second-line virological failure. There is a lower incidence of virological failure among patients above 45 years old, compared to those in the age group of 15–29 years at the start of second-line ART. This is supported by similar findings in Ethiopia, Rwanda, and Malawi, where the odds of developing virological failure on second-line ART adult patients were high in the age category of 15–29 years.17–19 This could be related to several distinct behavioral and psychosocial characteristics among the young age groups, such as anxiety, stigma, lack of disclosure, low social-economic position, and poor adherence to ART treatment.
The incidence of virological failure among patients with a CD4 count below 100 cells/mm3 at the start of second-line ART was higher compared to patients with CD4 count 100 cells/mm3 or above at the start of second-line ART. This is comparable with previous studies from Tanzania.20,21 This could be due to a patient’s immunological status deteriorating, resulting in a higher rate of viral replication than in patients with a good immune status.22
Furthermore, the study identified that those patients who had TB co-infection were more likely to develop virological failure as compared to those patients who had no TB co-infection. This is in congruent with previous studies conducted in Northern Ethiopia15 and South Africa.23 This is because protease inhibitors and rifampicin have pharmacological interactions. Rifampicin is a powerful liver enzyme inducer that lowers serum levels of protease inhibitors, resulting in virological failure if used concurrently.24
A lower incidence of virological failure was observed in patients with ATV/r-based second-line regimen as compared to patients with LPV/r-based second-line regimen. In the study conducted in Amhara Region Ethiopia, Second-line ART regimen based on tenofovir (TDF) and zidovudine (AZT) have been demonstrated to be more associated with treatment failure than drug regimens based on abacavir (ABC).25 Our finding is not in keeping with results found from a Kenyan study, which reported better physical and mental scores for patients who were on AZT than those on TDF.26 We are not sure of the reasons behind these differences; however, we urge that more research will be needed. Our postulation is that different sample sizes among the studies could have played a role.
The incidence of virological failure in patients with poor adherence was higher compared to patients with good/fair adherence at the initiation of second-line ART. This is in line with previous studies conducted in Ethiopia, Tanzania, South Africa, and Kenya.17,20,27,28 This might be because, if individuals miss doses on a daily dose of ART per month it may increase the risk of virological failure, resulting in lowered immunity. As a result, they may be vulnerable to opportunistic infections. This implies that strict adherence to ART plays a crucial role in the success of therapy for peoples with HIV.
Limitation of the Study
Despite the fact that we did our best to estimate the incidence of second-line virological failure and its predictors, there are still some limitations. Because of the retrospective nature of the study, it was unable to include all conceivable factors that could influence the incidence of virological failure. Some of the potential parameters that were not evaluated in this study included hemoglobin level and adverse effects. Consideration of pill count as adherence to ART is also the limitation of the study. In addition, purposively selecting the study population was also limits the generalizability of this study.
Conclusion
The incidence rate of virological failure was high among HIV patients on the second-line ART. Age >45 years, CD4count below 100 cells/mm3, TB co-infection, ATV/r-based second-line regimen, and poor adherence were independent predictors of virological failure. The rate of virological failure was higher for patients who had poor ART adherence, small CD4count, and who had TB co-infection. However, the rate of virological failure was lower among patients above 45 years’ old and among those who are taking ATV/r-based second-line regimen. Therefore, targeted HIV care interventions shall be provided to young ages and efforts stepped up to improve adherence to ART, which helps to increase immunity and suppress viral replication. In addition, prevention and early detection of TB co-infection are crucial to the patients.
Abbreviations
AHR, adjusted hazard ratio; ART, antiretroviral therapy; CI, confidence interval; EPHI, Ethiopian Public Health Institute; KM, Kaplan–Meir; PLHIV, person living with HIV; PM, person month; SD, standard deviation; VL, viral load.
Data Sharing Statement
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Ethics Approval
Ethical clearance and a Letter of cooperation for all selected hospitals were obtained from the Institutional Review Board of the University of Gondar with Reference Number: IPH/2135/2013. Support letters were obtained from the medical director of the Zewditu Memorial hospitals and St. Paul’s Hospital Millennium Medical College to access the medical records of patients. Confidentiality was maintained at all levels of study and data was held on a secured password-protected system. All the procedures were based on the principles of the Helsinki declaration.
Acknowledgments
We would like to thank all data collectors for their cooperation and support during the study period. In addition, we would like to than University of Gondar for financial support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported financially by the University of Gondar. However, the University had no role in study design, data collections, analysis, the decision to publish, and/or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. UNAIDS. Fast track strategy to end the AIDS epidemic by 2030; 2014.
2. UNAIDS. UNAIDS data 2019; 2018.
3. Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis. 2016;62(8):1043–1048. doi:10.1093/cid/ciw001
4. EthiopiaCountry Operational Plan. Strategic direction summary; 2018.
5. Churchill D, Waters L, Ahmed N, et al. British HIV association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV Med. 2016;17(Suppl 4):s2–s104. doi:10.1111/hiv.12426
6. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization; 2016.
7. Boender TS, Hamers RL, Ondoa P, et al. Protease inhibitor resistance in the first 3 years of second-line antiretroviral therapy for HIV-1 in Sub-Saharan Africa. J Infect Dis. 2016;214(6):873–883. doi:10.1093/infdis/jiw219
8. FMOH Ethiopia. National consolidated guidelines for comprehensive HIV prevention, care and treatment; 2018.
9. Assefa Y, Gilks CF, Lynen L, et al. Performance of the Antiretroviral Treatment Program in Ethiopia, 2005–2015: strengths and weaknesses toward ending AIDS. Int J Infect Dis. 2017;60:70–76. doi:10.1016/j.ijid.2017.05.012
10. Edessa D, Sisay M, Asefa F. Second-line HIV treatment failure in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2019;14(7):e0220159. doi:10.1371/journal.pone.0220159
11. Mulugeta A, Assefa H, Tewelde T, Dube L. Determinants of survival among HIV positive children on antiretroviral therapy in public hospitals, Addis Ababa, Ethiopia. Qual Prim Care. 2017;25(4):235–241.
12. Ayana GM, Akalu TY, Ayele TA. Joint modeling of of tuberculosis and change in viral load over time among adult HIV/AIDS patients on anti-retroviral therapy at Zewditu Memorial Hospital in Addis Ababa, Ethiopia. HIV/AIDS. 2021;13:239. doi:10.2147/HIV.S291872
13. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. doi:10.2307/2531021
14. Tsegaye AT, Wubshet M, Awoke T, Addis Alene K. Predictors of treatment failure on second-line antiretroviral therapy among adults in northwest Ethiopia: a multicentre retrospective follow-up study. BMJ Open. 2016;6(12):e012537. doi:10.1136/bmjopen-2016-012537
15. Zenebe Haftu ADA, Bezabih NM, Bayray Kahsay A, et al. Incidence and factors associated with treatment failure among HIV infected adolescent and adult patients on second-line antiretroviral therapy in public hospitals of Northern Ethiopia: multicenter retrospective study. PLoS One. 2020;15(9):e0239191. doi:10.1371/journal.pone.0239191
16. Agegnehu CD, Merid MW, Yenit MK. Incidence and predictors of virological failure among adult HIV patients on first-line antiretroviral therapy in Amhara regional referral hospitals; Ethiopia: a retrospective follow-up study. BMC Infect Dis. 2020;20(1):460. doi:10.1186/s12879-020-05177-2
17. Seid A, Cherie N, Ahmed K. Determinants of virologic failure among adults on second line antiretroviral therapy in Wollo, Amhara Regional State, Northeast Ethiopia. HIV/AIDS. 2020;12:697–706.
18. Nsanzimana S, Semakula M, Ndahindwa V, et al. Retention in care and virological failure among adult HIV+ patients on second-line ART in Rwanda: a national representative study. BMC Infect Dis. 2019;19(1):312. doi:10.1186/s12879-019-3934-2
19. Ongubo DM, Lim R, Tweya H, et al. A cross-sectional study to evaluate second line virological failure and elevated bilirubin as a surrogate for adherence to atazanavir/ritonavir in two urban HIV clinics in Lilongwe, Malawi. BMC Infect Dis. 2017;17(1):461. doi:10.1186/s12879-017-2528-0
20. Gunda DW, Kilonzo SB, Mtaki T, Bernard DM, Kalluvya SE, Shao ER. Magnitude and correlates of virological failure among adult HIV patients receiving PI based second line ART regimens in north western Tanzania; a case control study. BMC Infect Dis. 2019;19(1):235. doi:10.1186/s12879-019-3852-3
21. Gunda DWKC, Kidenya BR, Kabangila R, et al. Plasma concentrations of efavirenz and nevirapine among HIV-infected patients with immunological failure attending a tertiary hospital in North-Western Tanzania. PLoS One. 2013;8(9):e75118. doi:10.1371/journal.pone.0075118
22. Alexander G. Chapter 34 Liver and biliary tract. In: Bennett PN, Brown MJ, Sharma P, editors. Clinical Pharmacology
23. Collier D, Iwuji C, Derache A, et al. Virological outcomes of second-line protease inhibitor-based treatment for human immunodeficiency virus type 1 in a high-prevalence rural South African setting: a competing-risks prospective cohort analysis. Clin Infect Dis. 2017;64(8):1006–1016. doi:10.1093/cid/cix015
24. Antagonists TN. LiverTox: clinical and research information on drug-induced liver injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases, Ed. Rifampin. Bethesda, MD, USA; 2012.
25. Alene M, Awoke T, Yenit MK, Tsegaye AT. Incidence and predictors of second-line antiretroviral treatment failure among adults living with HIV in Amhara region: a multi-centered retrospective follow-up study. BMC Infect Dis. 2019;19(1):599. doi:10.1186/s12879-019-4243-5
26. Etenyi JO, Okalebo FA, Oluka M, et al. Comparison of zidovudine and tenofovir based regimens with regard to health-related quality of life and prevalence of symptoms in HIV patients in a Kenyan Referral Hospital. Front Pharmacol. 2018;9:984. doi:10.3389/fphar.2018.00984
27. Onoya D, Nattey C, Budgell E, et al. Predicting the need for third-line antiretroviral therapy by identifying patients at high risk for failing second-line antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2017;31(5):205–212. doi:10.1089/apc.2016.0291
28. Mwangi A, van Wyk B. Factors associated with viral suppression among adolescents on antiretroviral therapy in Homa Bay County, Kenya: a retrospective cross-sectional study. HIV/AIDS. 2021;13:1111–1118.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


