Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 11
Incidence and Predictors of Treatment Failure Among Children Receiving First-Line Antiretroviral Treatment in General Hospitals of Two Zones, Tigray, Ethiopia, 2019
Authors Sibhat M , Kassa M , Gebrehiwot H
Received 24 December 2019
Accepted for publication 2 March 2020
Published 6 March 2020 Volume 2020:11 Pages 85—94
DOI https://doi.org/10.2147/PHMT.S243656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Migbar Sibhat,1 Mekuria Kassa,2 Haftom Gebrehiwot2
1School of Nursing, College of Health Science and Medicine, Dilla University, Dilla, Ethiopia; 2School of Nursing, College of Health Science, Mekelle University, Mekelle, Ethiopia
Correspondence: Migbar Sibhat
School of Nursing, College of Health Science and Medicine, Dilla University, Dilla, Ethiopia
Email [email protected]
Background: Despite many efforts undertaken to control the human immunodeficiency virus epidemic, it remains to be the major global public health challenge. With expanding access to pediatric antiretroviral therapy, children are more likely to experience treatment failure. All previous studies conducted in Ethiopia estimated treatment failure using only clinical and CD4 criteria. Thus, the ART failure rate is expected to be underestimated in our country.
Objectives of the Study: To assess the incidence and predictors of treatment failure among children receiving first-line ART in general hospitals of Mekelle and Southern Zones of Tigray region, Ethiopia, 2019.
Methods: Retrospective follow up study was employed. The sample size was estimated based on a Log rank test using Stata V-13 and all 404 charts were taken for review. Data were collected by extraction tool; entered using Epi-data manager; cleaned and analyzed by Stata V-14. Data were described using the Kaplan-Meier curve, Log rank test, life table, and crude hazard ratios and analyzed using adjusted hazard ratios and p-value by Cox proportional hazard regression. Any variable at P < 0.05 in the bi–variable analysis was taken to multi–variate analysis and significance was declared at P≤ 0.05. Data were presented using tables, charts, and texts.
Results: The incidence rate of ART failure was 8.68 (95% CI 7.1 to 10.6) per 1000 person-month observations with a total of 11,061.5 person-month observations. Children who had tuberculosis at baseline [AHR=2.27; 95% CI 1.12– 4.57], advanced recent WHO stage [AHR=5.21; 95% CI 2.75– 9.88] and sub-optimal ART adherence [AHR=2.84, 95% CI 1.71– 4.72] were at higher hazard for first-line treatment failure. Besides this having a long duration of ART follow up [AHR=0.85; 95% CI 0.82– 0.87] was found to be protective against treatment failure.
Conclusion and Recommendation: The incidence of first-line ART failure was grown as a major public health concern. Treatment failure was predicted by the duration of follow up, advanced recent WHO stage, sub-optimal adherence, as well as the presence of tuberculosis at baseline. Hence, it is better to give priority for strengthening the focused evaluation of the WHO clinical stage and tuberculosis co-infection at baseline with continuous adherence monitoring.
Keywords: ART, children, Ethiopia, treatment failure, predictors, incidence
Introduction
Despite many efforts undertaken to control Human Immune-deficiency Virus (HIV) epidemic, it remains to be the major global public health challenge.1,2 Highly Active Anti-Retroviral Therapy (HAART) is those medications given to increase the life expectancy of children infected with HIV. Globally, scaling up Anti-Retroviral Therapy (ART) services has been increased markedly, particularly in sub-Saharan Africa.2–4
Treatment failure refers to sub-optimal response or a lack of sustained response to therapy excluding cases of Immune Reconstitution Inflammatory Syndrome (IRIS). It can be determined through the examination of clinical failure (clinical criteria), immunologic failure (CD4 criteria), virologic failure (viral load criteria), or a combination of all.5,6
With expanding access to pediatric ART, children are more likely to develop treatment failure, subsequently requiring 2nd line therapy.5,7–11 Of the 1.5 million children estimated to require ART by 2020, around 20% are expected to experience VF at some point.12
Recommending potent and effective second-line regimens for infants and children is difficult because treatment options are largely non-existent in most low-income countries.13–15 Consequently, this challenge emphasizes the significance of choosing potent first-line regimens and the necessity to make maximal efforts to ensure increased durability of first-line regimens.14
Ethiopia is one of the high HIV-burden countries and does not have appropriate ART drug formulations for children beyond the 2nd line.7 The diagnosis of ART failure is infrequent in most centers of Ethiopia.16–19 In addition to this, all available studies estimated treatment failure only by clinical and CD4 criteria.18,20 For this reason, the ART failure rate is expected to be underestimated in our country. Thus, unless we perform best to increase the durability and effectiveness of first-line regimens, we may end up with null or limited options to increase the survival of HIV–infected children and to control the epidemic plus short of hitting the UNAIDS 2020 targets.21–23 Furthermore, I could not find a single study conducted in the Tigray region with this topic. This implies that we need to research to determine the incidence of treatment failure and to identify factors that provoke treatment failure. Thereupon, this study intended to fill the aforementioned gap.
Methods
An institution-based retrospective follow-up study was employed at general hospitals of Mekelle and Southern zones of Tigray region, Ethiopia from December 2018–June 2019 by reviewing five years [2014–2018] follow up data of children below 18 years of age. The total population of Mekelle city was 423,172; of which 214,141 were males and 208,931 were females. Whereas, 756,515 people live in the Southern zone of the Tigray region; of those 371,692 were males and 384,823 were females.24 There are five general governmental hospitals in the two zones, all of which provide ART care services.
Study Population
All HIV/AIDS infected children below 18 years who were taking first-line ART at selected public general hospitals of Mekelle and Southern zones of Tigray region and who had a minimum of two follow-up visits with at least one visit six months post-initiation of ART. Those charts of children who have not at least one of the three key failure indicator variables (WHO clinical stage, CD4 count and VL) were excluded.
Sample Size Determination and Sampling Procedure
The sample size was calculated based on a Log rank test considering assumptions of the Cox proportional hazard model by using STATA version 13 software considering 95% CI and 80% power. Opportunistic infection at baseline which yields the largest sample size was considered as a significant predictor of failure, with an HR of 2.3 and proportion of failure among no OI group 6.4% (ie probability of failure among children without OI =0.064) and failure among OI group 11% from a study finding in Amhara region.25 Considering a 5% non-response rate, in favor of incomplete information, the total sample size required for the study was 400 with an expected number of events, E=47.
A cluster sampling technique was employed considering each hospital as a cluster. Among those clusters, three hospitals were selected by using the lottery method and all charts of children taking ART in the previous five years at all of the selected hospitals (clusters) were incorporated in the study for review.
Operational Definitions and Measurements
Events were HIV/AIDS infected children below 18 years who experienced treatment failure based on WHO criteria either clinical (new or repeated event indicative of WHO stage 3 or 4), immunologic (CD4 <200cells/L for older children and <100 cells/L for under 5), and/or virologic criteria (VL>1000copies/mL) after 6 months of effective ART treatment such that at least one criterion should be fulfilled.5
Censored were those who did not experienced treatment failure during follow-up including defaulters, transfer outs, died, exceed 18 years of age during follow up, on first-line at the end of follow-up and switchers due to factors other than first-line ART failure (eg primary non-response to ART, anti-TB treatment while on ART, drug resistance e.t.c.). The time to occurrence of an event or censored cases was measured in months.
Switch was considered when (i) commencement of ≥2 new drugs including a class-switch from PI to NNRTI or vice versa even without failure, (ii) class-switch from NNRTI to PI or vice versa only, with reason documented as treatment failure or (iii) change of both NRTIs and change from RTV to LPV/r with reason documented as treatment failure. A patient was considered a defaulter if no follow-up visit for ≥ 3 months and adherence was measured based on the 2017 national ART score cut-offs.5,7
Data Collection Instruments and Procedure
A data extraction checklist was developed from the national comprehensive HIV treatment guideline,7 ART registration booklet, ART monitoring multi-chart and reviewing related articles. The extraction tool was comprised of socio-demographic characteristics, clinical characteristics, treatment-related and other factors. The lists of participants were taken from the ART data clerk and MRN or unique ART numbers were used to find charts from the hospital card room. Four data collectors (BSc nurses) and one supervisor (ART trained BSc) were recruited and the data collection was accomplished from April 1–26/2019.
Data Processing, Analysis, Interpretation, and Presentation
At the end of data collection, data were entered into Epi-data manager version 4.4 and exported to and cleaned, edited, coded, and analyzed using STATA version 14. Exploratory data analysis was carried out to check the levels of missing values, the presence of influential outliers, and normality. Then the data were described using relative frequency, percent and median with interquartile range. Life-table was constructed to estimate probabilities of failure at different time intervals. Kaplan Meier’s failure curve was used to estimate median failure time during the treatment period and Log rank tests to compare survival curves for the presence of difference in the incidence of failure among the groups.
The bi–variable analysis was done to identify associations between dependent and each independent variable. Variables, significant at P ≤0.05 level in the bi–variable analysis, were included in multivariable analysis, to identify independent predictors of failure. This was to maintain the balance between the amount of available information (96 events) and the number of parameters (variables) to be estimated. Moreover, it helps to decrease the junk effect as there were significant numbers of candidate variables with this cut-off point which were eligible to be recruited in the final model and therefore no need to consider a p-value of below 0.25 in this case.
Multi co-linearity was checked using the VIF command (mean vif=1.73). The Cox regression model for its fitness to the data was checked using the Cox-Snell residuals. Furthermore, proportional hazard assumptions were checked using the global test with a value of p> χ2 =0.3208. The Log-likelihood test was significant at prob > chi2 = 0.0000. Generally, we could conclude that the final model fits the data successfully. In the multi–variable analysis, any statistical test was considered significant at P ≤0.05. Then, the association was summarized by using adjusted hazard ratio and statistical significances tested at 95% CI. Finally, the findings were presented using tables, graphs, and texts.
Data Quality Assurance
To assure the quality of data, data collectors and supervisors were trained about how and what information they should collect from the medical records for one day. The checklist was pretested on 5% (21) of randomly selected charts, which were not included in the actual study. Completeness of each collected data was audited at the end of each day by the principal investigator and supervisor. Whenever there appear incompleteness and uncertainty of recording, the filled information formats were cross-checked with source data soon. Individual records with incomplete data during data collection were excluded.
Ethical Consideration
The study was carried out after getting ethical approval (ERC 1272/2019) from the institutional review board (IRB) of Mekelle University, college of health sciences. After approval by IRB, an official letter of co-operation was written to each selected hospital from the school of nursing and permission was received. Then, data were collected after getting permission from selected hospitals on behalf of patients and respective units of each hospital since the study was conducted through a review of medical records. Data coding and aggregate reporting were used so that names and other personal identification of respondents were not stated throughout the study process to ensure anonymity and confidentiality.
Results
From the beginning of 2014 to the end of 2018, a total of 502 children below 18 years of age were started ART. One hundred ninety-eight (198) cases were from Alamata General Hospital, 165 were from Mekelle General Hospital and the rest 139 were from Lemlem Karl hospital. Of those, 98 cases (41 from Alamata hospital, 33 from Mekelle hospital and 24 from Lemlem Karl) were excluded from analysis because of missing data on at least one of the 3 failure indicator variables (WHO stage, CD4 count and viral load) and due to short duration spent on ART (below 6 months). This is because first it is impossible to detect failure without one of the 3 criteria and secondly 6 months of effective ART follow-up is mandatory to diagnose treatment failure for a certain ART regimen. Finally, four hundred four (n=404) children aged between 6 months and 17 years who fulfilled the eligibility criteria were incorporated for analysis from three general hospitals of Mekelle and Southern zone of Tigray region.
Socio-Demographic Characteristics
The study finding showed that from two hundred three (50.25%) female participants, 11.4% experience treatment failure. Of 106 (26%) children started ART below the age of five years, 6% failed to respond for first-line ART whereas 8% of those who started ART after 10 years old developed treatment failure. The result also notified that 149 (42%) study subjects were orphan children who lost either one or both parents; of those 41 (10.15%) were failed to respond to ART (Table 1). Children followed for a minimum of 6 months and a maximum of 60 months with a median follow up of 24.3 months starting from January 2014 to the end of 2018.
 |
Table 1 Distribution of Socio-Demographic Characteristics Among HIV/AIDS Infected Children in Public General Hospitals of Mekelle and Southern Zones of Tigray Region, Northern Ethiopia, 2019 (n=404) |
Clinical Characteristics
Among those who started ART with advanced WHO stage, 44.3% developed treatment failure. Meanwhile, 16.4% of children experienced treatment failure after initiating ART with either WHO stage one or two. The finding also revealed that 185 (46%) children did not have CD4 count after ART initiation and only 221 (55%) of them had access to a viral load of which only 64 (15.8%) had at baseline (Table 2). Considerably, children acquired opportunistic infections at baseline as well as after initiation of ART were 39% and 24% respectively. Forty (47%) of children who had failed first-line treatment developed OI after started ART and 17% of the censored group acquired OI after treatment initiation (Table 3).
 |
Table 2 Clinical Characteristics of HIV/AIDS Infected Children in Public General Hospitals of Mekelle and Southern Zones of Tigray Region, Northern Ethiopia, 2019 (N=404) |
 |
Table 3 Distribution of Opportunistic Infections Among HIV/AIDS Infected Children in Public General Hospitals of Mekelle and Southern Zones of Tigray Region, Northern Ethiopia, 2019 (n=404) |
Treatment-Related and Other Factors
Three hundred twenty-nine (81.4%) participants started with NNRTIs based ART regimen with NVP-based dominating with 191 (47.3%) whereas 33 (8.2%) started with boosted PI (LPV/r) based regimen. Sixty (15%) children exposed previously to ART for PMTCT or PEP and 375 (93%) had taken cotrimoxazole and or INH as prophylaxis. One hundred fourteen children, (28%) started ART without knowing their serostatus; 110 (27%) had suboptimal adherence as well as 126 (31.2%) and 94 (23.3%) developed side effects and substituted their initial first-line regimen during follow up respectively. Moreover, 8 (2%) children died during follow up, 61 (15.1%) transferred out to other facilities, 37 (9.2%) lost as defaulters, and 31 (7.7%) switched to second-line ART regimen because of first-line regimen failure and other reasons.
Among the total 404 children in the study, 308 (76.24%) children were censored and 96 (23.8%; 95% CI 19.6, 27.9) experienced first-line treatment failure during the follow-up period. Of those 56 (58.3%), 19 (19.8%), and 18 (18.8%) were virological, immunological, and clinical failure respectively whereas the rest 3 (3.1%) found as combined failure fulfilling the diagnostic criteria for all the three types of failures. Alarmingly, 29 (30.2%) failures were reported within the first 12 months of the follow-up period. It is also awful that, from 96 children who failed for first-line, only 23 (24%) children were switched to second-line drugs after first-line treatment failure.
Comparison of Survival Status Using Kaplan Meier
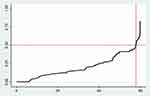
The Kaplan Meier failure curve increases stepwise and it crosses the failure function at failure probability of 0.5 (Figure 1).
Survival Function and an Incidence Density Rate of ART Failure
The total person-time observation was 11,061.5 person-months. The incidence rate of first-line ART regimen treatment failure was 8.7 (95% CI 7.10 to 10.60) per 1000 person-months of observation. The incidence of virological, immunological clinical and combined failure were 5.1 (95% CI 3.9 to 6.6), 1.7 (95% CI 1.1 to 2.7), 1.63 (95% CI 1.03 to 2.6) and 0.3 (95% CI 0.1 to 0.8) per 1000 person-months respectively. The median survival time was found to be 57.7 months (95% CI 54.8, 59.8). The cumulative probabilities of failure at the end of 12, 24, 36, 48 and 60 months were 0.08, 0.12, 0.20, 0.35 and 0.61 respectively.
Predictors of First-Line Treatment Failure
In the final Cox proportional hazard model, duration of follow up, advanced WHO stage, ART adherence and TB at baseline were found to be independent predictors of first-line ART treatment failure in HIV/AIDS infected children at 95% confidence level (Table 4).
 |
Table 4 Bi–Variable and Multi–Variable Analysis Output for HIV/AIDS Infected Children in Public General Hospitals of Mekelle and Southern Zones of Tigray Region, Northern Ethiopia, 2019 (n=404) |
Children who started ART with sub-optimal adherence were observed to have a 3 times higher hazard of first-line treatment failure [AHR=2.84; 95% CI 1.71–4.72] than their counterparts. In addition to this, children who diagnosed as advanced WHO stage at last visit had 5.2 times higher hazard than those who defined as in the early WHO stages [AHR=5.21; 95% CI 2.75–9.88].
The other factor that showed significant association was the duration of ART follow-up. Children who had been on ART for a long period were less likely to develop treatment failure. For each month increase in the duration of ART follow up, the hazard of developing treatment failure will decrease by 15% [AHR=0.85; 95% CI 0.82–0.87]. Moreover, those children who had tuberculosis infection at baseline were also 2.3 times at higher hazard of experiencing treatment failure than those with no TB infection at ART initiation [AHR=2.27, 95% CI 1.12–4.57].
Discussion
This study was conducted to determine the incidence and predictors of first-line ART failure among HIV/AIDS infected children receiving first-line ART. The incidence rate of first-line ART regimen treatment failure was 8.7 (95% CI 7.10 to 10.60) per 1000 person-months of observation. The median survival time was found to be 57.7 months (95% CI 54.8, 59.8) with 0.61 cumulative probability of failure at the end of 60 months. Having a short duration of follow-up, advanced WHO stage at follow up visit, ART non-adherence, and TB at baseline was found to be independent predictors of first-line ART treatment failure.
In this study, the median survival time was 57.7 months (95% CI 54.83, 59.77) which is longer than the findings of studies in India; 43 months,26 a combined study in Mozambique and Uganda; 12.6 months27 and Uganda; 26.4 months.28 This could be explained by the improvements in the therapeutic and diagnostic measures in current visits from then and the increase in access and variety of more potent drugs nowadays than the past periods.7 The other reason may be the Ugandan study considered treatment failures which occur before six months of follow up which is not the case in this study.
The incidence rate of first-line ART failure was 8.7 (95% CI 7.10 to 10.60) per 1000 person-months which is found to be higher than the finding in the Amhara region, 2.2 (95% CI 1.72 to 2.82) per 1000 person-months observations.25 This may be due to the difference in person-time observations. The total person-time observation was 28, 562.5 person-months which is much more than the current study, 11, 061 person-months. The other possible reason might be that they determine failure rate only by immunologic and clinical criteria which could further lengthen the time to detection than viral load criteria.6
Children who started ART regimens while having tuberculosis had 2.3 times more hazard of failure than those with no TB at ART initiation [AHR=2.27; 95% CI 1.12–4.56]. This finding was supported by the report of a combined study conducted in Mozambique and Uganda (24). This might be because TB increases HIV replication through the process of immune activation leading to increased viral load. This results in a more rapid progression of HIV disease which counteracts with the action of ART drugs.7
This study revealed that the WHO clinical stage at last visit was significantly associated with first-line ART failure (AHR=5.21; 95% CI 2.75–9.88). No other earlier studies examined the WHO stage at the last follow up visit. However, baseline WHO stage was stated as a predictor of treatment failure in findings of Mozambique and Uganda study27 and the study at Fiche and Kuyu hospitals29 which is not significant in the current study. This difference could be rationalized because the current guideline-recommended is more advanced management and short follow up visits;7 so that those children who had advanced WHO stage at ART initiation might receive strict and close intervention in terms of treatment regimen selection, laboratory investigations, and repeated follow up visits.
The current study also showed that children those having sub-optimal adherence for ART regimens were 3 times at higher hazard of experiencing first-line ART failure compared to their counterparts (AHR=2.84 95% CI 1.71–4.72). This finding was conformable with the results of previous studies at Fiche and Kuyu hospitals, Oromia,29 Rwanda,30 Uganda28 and Tanzania.31 This is because a high level of sustained adherence is necessary to suppress viral replication and improve immunological and clinical outcomes which in turn decrease the risk of developing ARV drug resistance and reduce the risk of transmitting HIV. To the contrary, poor adherence to ART drugs is commonly encountered in the treatment of children and adolescents living with HIV due to a variety of factors including regimens for children often require taking multiple pills with frequent dosing requirements each with potential adverse effects and drug interaction, limited choice of pediatric formulations and poor palatability of liquid formulations. A child’s age and developmental stage may also influence adherence since this age group needs support from others to take medication timely and also may face difficulties in swallowing tablets.6,7
A long duration of follow up was also reported as having a protective effect on first-line ART failure (AHR=0.85; 95% CI 0.82–0.87). This finding is similar to the finding of a study done in Amhara region hospitals.25 Amazingly, 29 (30.2%) of failures had occurred in the first twelve months of follow up after ART initiation. Several reasons could be stated for this. For example; different rapid effects like IRIS were very common in the early months after ART start, adherence level of children might increase with time and might be considerably lower in the first few months, and the need for adaptation to daily ART drug intake may hinder the patient’s response to ART regimen and increase the chance of failure.6
Conclusion
The incidence rate of ART failure remains unacceptably high in this study since it is higher than the maximum expected level of the virological failure rate of 20% and far less than 10% of failure rate sated by UNAIDS projection for 2020. Even though efforts are taken to increase ART access, the issue of inadequate viral suppression and increased treatment failure rate becomes a burning issue these days. Moreover, children who had TB at ART initiation, advanced WHO clinical stage after ART initiation, non-adherence to ART regimen, and those who were on ART for a short period were at higher hazard of treatment failure.
Ethical Approval and Consent to Participate
Ethical clearance and ethical approval were obtained from the Intuitional Review Board (IRB) of Mekelle University. As the study was retrospective, the IRB waived that the research could be done based on record review without contacting patients. A cooperation letter was obtained from Mekelle University, College of Health Science, and School of Nursing. Permission letters were obtained from each hospital administration (Mekelle general hospital, Alamata general hospital, and Lemlem Karl general hospital) and respective hospital ART coordinators. All information was kept confidential and no individual identifiers were collected.
Data Sharing Statement
Extra data that support the findings of this study are available from the corresponding author upon reasonable request and can be shared upon legal request via email to [email protected] or [email protected].
Acknowledgments
The authors would like to thank data collectors, supervisors, hospital staffs and administrators for their unreserved efforts and commitment. The authors would also like to acknowledge Dilla University for funding this study and Mekelle University to pursue this chance.
Author Contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. However, the financial backing of this research was provided by Dilla University. The funder had no role in study design, data collection, analysis, preparation of the manuscript and decision to publish.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS Statistics – 2018 Fact Sheet. UNAIDS; December 2018.
2. UNICEF. HIV Epidemiology Among Children and Adolescents: UNICEF; 2017.
3. UNAIDS. Report on the Global AIDS Epidemic: World Health Organization; 2010.
4. U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). PEPFAR Strategy for Accelerating HIV/AIDS Epidemic Control (2017-2020). U.S.A; September 2018.
5. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. WHO; 2016.
6. Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection: AIDS Info; 2018.
7. Federal Ministry Of Health. National Guidelines for Comprehensive HIV Prevention, Care, and Treatment. Vol. 5. Addis Ababa, Ethiopia; 2017:256.
8. World Health Organization. Pocketbook of Hospital Care for Children.
9. World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach-2010 Revision. WHO; 2010.
10. WHO. Manual on Pediatrics HIV Care and Treatment for District Hospitals. Geneva, Switzerland; 2011.
11. Davies M-A, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa-the IeDEA Southern Africa Collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270. doi:10.1097/QAI.0b013e3182060610
12. WHO. Paediatric ARV Drug Optimization 2 Meeting Report. Geneva, Switzerland: WHO;
13. Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30(1):52–56. doi:10.1097/INF.0b013e3181ed2af3
14. Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother. 2009;65(1):118–124. doi:10.1093/jac/dkp412
15. Salou M, Dagnra AY, Butel C, et al. High rates of virological failure and drug resistance in perinatally HIV‐1‐infected children and adolescents receiving lifelong antiretroviral therapy in routine clinics in Togo. J Int AIDS Soc. 2016;19(1):20683. doi:10.7448/IAS.19.1.20683
16. Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first-line antiretroviral therapy. BMC Infect Dis. 2012;12(1):197. doi:10.1186/1471-2334-12-197
17. Boender TS, Kityo CM, Boerma RS, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016;71(10):2918–2927. doi:10.1093/jac/dkw218
18. Reepalu A. Antiretroviral Treatment at Ethiopian Health Centers. Lund University; 2017.
19. Rossouw TM, Nieuwoudt M, Manasa J, et al. HIV drug resistance levels in adults failing first‐line antiretroviral therapy in an urban and a rural setting in South Africa. HIV Med. 2017;18(2):104–114. doi:10.1111/hiv.2017.18.issue-2
20. Assefa Y, Gilks CF, Lynen L, et al. Performance of the antiretroviral treatment program in Ethiopia, 2005–2015: strengths and weaknesses toward ending AIDS. Int J Infect Dis. 2017;60:70–76. doi:10.1016/j.ijid.2017.05.012
21. Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programs in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10(3):155–166. doi:10.1016/S1473-3099(09)70328-7
22. Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review. Clin Infect Dis. 2009;49(12):1915–1927. doi:10.1086/648079
23. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90. An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland; October 2014.
24. Tigray Regional Health Bureau. Regional Estimates of the Population with the Conversion Factor. Unpublished resources: Tigray Regional Health Bureau; 2018.
25. Sisay MM, Ayele TA, Gelaw YA, Tsegaye AT, Gelaye KA, Melak MF. Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: a retrospective follow-up study. BMJ Open. 2018;8(4):e019181. doi:10.1136/bmjopen-2017-019181
26. Mukherjee A, Shah N, Singh R, Vajpayee M, Kabra SK, Lodha R. Outcome of highly active antiretroviral therapy in HIV-infected Indian children. BMC Infect Dis. 2014;14(1):701. doi:10.1186/s12879-014-0701-2
27. Costenaro P, Penazzato M, Lundin R, et al. Predictors of treatment failure in HIV-positive children receiving combination antiretroviral therapy: cohort data from Mozambique and Uganda. J Pediatric Infect Dis Soc. 2014;4(1):39–48. doi:10.1093/jpids/piu032
28. Sebunya R, Musiime V, Kitaka SB, Ndeezi G. Incidence and risk factors for first-line antiretroviral treatment failure among Ugandan children attending an urban HIV clinic. AIDS Res Ther. 2013;10(1):25. doi:10.1186/1742-6405-10-25
29. Yassin S, Gebretekle GB. Magnitude and predictors of antiretroviral treatment failure among HIV‐infected children in Fiche and Kuyu hospitals, Oromia region, Ethiopia: a retrospective cohort study. Pharmacol Res Perspect. 2017;5(1):e00296. doi:10.1002/prp2.296
30. Mutwa PR, Boer KR, Asiimwe-kateera B, et al. Safety and effectiveness of combination antiretroviral therapy during the first year of treatment in HIV-1 infected Rwandan children: a prospective study. PLoS One. 2014;9(11):e111948. doi:10.1371/journal.pone.0111948
31. Emmett SD, Cunningham CK, Mmbaga BT, et al. Predicting virologic failure among HIV-1-infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acquir Immune Defic Syndr. 2010;54(4):368. doi:10.1097/QAI.0b013e3181cf4882
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.