Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Incidence and Predictors of Initial Antiretroviral Therapy Regimen Change Among HIV-Infected Adults Receiving Antiretroviral Therapy at Arba Minch General Hospital, Southern Ethiopia
Authors Gebremichael MA , Gurara MK , Weldehawaryat HN
Received 19 March 2020
Accepted for publication 27 June 2020
Published 3 August 2020 Volume 2020:12 Pages 315—329
DOI https://doi.org/10.2147/HIV.S254386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Mathewos Alemu Gebremichael, Mekdes Kondale Gurara, Haymanot Nigussie Weldehawaryat
Department of Public Health, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Haymanot Nigussie Weldehawaryat Tel +251921875816
Email [email protected]
Background: The effectiveness of the initial antiretroviral therapy regimen is the key to treatment success. However, regimen change affects this treatment success. Yet, evidence on the incidence and predictors of regimen change is scarce. Therefore, the aim of this study was to assess the incidence and predictors of initial antiretroviral therapy regimen change among HIV-infected adults receiving antiretroviral therapy.
Methods: An institutional-based retrospective cohort study was conducted. Data were collected from patients’ charts selected by simple random sampling. Data entered into EpiData version 4.43 and analyzed by STATA version 13. The life table was used to estimate cumulative survival. Kaplan–Meir curve and Log-rank test were used to compare the survival experience of explanatory variables. Cox proportional hazard model was used to identify predictors.
Results: Five hundred and eight patients were followed for 871.87 person-years of observation with the median follow-up period of 16.12 months. The incidence rate of initial ART regimen change was 11.36 (95% CI: 9.32– 13.83) per 100 person-year and the median survival time is 54 months. Not disclosing HIV status (adjusted hazard ratio (AHR) = 5.41, 95% CI = 2.38– 12.27), co-medication with ART (AHR = 4.64, 95% CI = 1.43– 15.10), occurrence of side effect on initial regimen (AHR = 7.32, 95% CI = 4.43– 12.10), baseline CD4 count < 200 cells/mm3 (AHR = 2.18, 95% CI = 1.37– 3.47), ambulatory/bedridden baseline functional status (AHR = 3.55, 95% CI = 2.30– 5.48) were significant predictors of initial regimen change.
Conclusion: The incidence rate of initial ART regimen change was found to be low. HIV disclosure status, co-medication with ART, the occurrence of side effects on an initial regimen, low baseline CD4 count, ambulatory and bedridden baseline functional status were found to be predictors.
Keywords: initial antiretroviral therapy, regimen change, incidence, survival analysis, Arba Minch
Background
Human immunodeficiency virus (HIV) is the cause of acquired immune deficiency syndrome (AIDS) which is responsible for world pandemic. It continues to be a major global public health issue with an estimated 36.9 million people living with it and with a global prevalence of 0.8% among adults. The vast majority of people living with HIV are located in low and middle-income countries with an estimated 66% living in sub-Saharan Africa. Among these 19.6 million are living in East Africa.1 Globally in 2017, 21.7 million People Living with HIV (PLWHIV) were receiving Antiretroviral Therapy (ART).1 In Ethiopia, one of the East African countries, a linear increase has been observed in the number of PLWHIV. Currently, 738,976 people are living with HIV and all of them require ART however only 426,000 are currently taking ART2 and about 98.6% of adults in urban Ethiopia are using ART from 2017 to 2018.3
Antiretroviral Therapy is using three or more antiretroviral drugs combining from different classes which include: entry inhibitors, nucleoside and non-nucleoside analog inhibitors of reverse transcriptase, integrase inhibitors, and protease inhibitors, either taken individually or in fixed-dose combinations for the treatment of HIV.4
According to the 2017 consolidated ART guideline of Ethiopia, ART should be initiated for all individuals living with HIV immediately after confirming HIV diagnosis regardless of the World Health Organization (WHO) clinical staging and Cluster of Differentiation 4(CD4) count.2 Preferred first-line regimens as fixed-dose combinations (FDC) for adults are Tenofovir (TDF) + Lamivudine (3TC) + Efavirenz (EFV) and Zidovudine (AZT) + Lamivudine (3TC) + Efavirenz (EFV)/Zidovudine (AZT) + Lamivudine (3TC) + Nevirapine (NVP)/Tenofovir (TDF) + Lamivudine (3TC) + Nevirapine (NVP) as alternative regimens and preferred second-line regimens are TDF + 3TC +LPV/r (Lopinavir) or ATV/r (Atazanavir), if AZT was used in first-line ART, AZT + 3TC + LPV/r or ATV/r, if TDF was used in first-line ART.2
Access to Highly Active Antiretroviral Therapy (HAART) has increased the survival of HIV patients. However, change in treatment limits the sustainability of the HIV treatment program and the therapeutic success of the original regimen.5 Besides; regimen change results in several challenges, it affects both the duration and the chance of viral control due to cross resistance between possible alternatives and overlapping toxicity between and within classes of Antiretroviral (ARV) drugs.
Regimen change also affects the success of the treatments to achieve Joint United Nation program on HIV/AIDS (UNAIDS) 90-90-90 goals.6
To improve the long-term sustainability and success of HIV treatment program: optimizing the limited available ARV regimen is very vital and needed in the public health approach to ART.7
However, frequent changes in the initial regimen increased now a day. Especially the rate of initial modification and interruption of treatment were high with in the first year after combination of Antiretroviral Therapy (cART) initiation.8
Evidence shows that globally from 14.1% up to 47.7% patients changed their initial ART regimen from 2013 to 20189,10 and the rate of initial regimen change varies from 0.9 per 100 person-years up to 28.3 per 100 person-years of observations.11,12 Different studies conducted in Ethiopia showed that initial regimen change among patients taking ART was 21–21.8% from 2014 to 2016.13,14 The proportion of regimen change increases from year to year, which accounts for 20–47.7% from 2017 to 2018 with the incidence rate of 10.11–16.08 per 100 person-years of observation. Among those who changed their initial regimen, about 60% changed early within first 6 months and 71.6% within a year of ART initiation.9,13,15
Like other developing countries, treatment options and resources are limited in Ethiopia, so that the strategy to maximize drug tolerability, the benefit of the original treatment regimen and increasing the durability on these limited drugs are essential. To achieve this goal it is important to determine the incidence and predictors of initial antiretroviral therapy regimen change among adult patients receiving antiretroviral therapy.
Methods
Study Setting
Arba Minch General Hospital is situated 495km south of Addis Ababa in Arba Minch town, Gamo zone.16 A total of 1699 adult HIV patients are active ART followers. From 1st January 2014 to 31st December 2018 about 730 adult HIV patients enrolled. The treatment protocol implemented using WHO ART treatment guideline for HIV infections in adults and adolescents.2 A baseline assessment is done at week 0 followed by visits on week 1, week 2, week 4, week 8, week12, week16, and week 24. After the 24th week of initiation of antiretroviral therapy patients are scheduled to return every twelve weeks.2
Study Design
An institutional-based retrospective cohort study design.
Source Population
All HIV-infected adults being on ART and registered for chronic care in the Arba Minch hospital ART clinic.
Study Population
All HIV-infected adults being on ART and registered for chronic care in the Arba Minch hospital ART clinic from 1st January 2014 to 31st December 2018.
Inclusion and Exclusion Criteria
All patients aged 15 and above who enrolled in Arba Minch hospital ART clinic from 1st January 2014 to 31st December 2018 were included but patients who started ART but did not attend at least one follow up, patients with an incomplete card, patients who are transferred in were excluded.
Sample Size Determination
The sample size was determined by using single population proportion formula through Open Epi, Version 3, open-source considering the following assumptions: 95% level of confidence, 3.5% margin of error, and taking incidence of regimen change 16.08%.9 This made sample size of 423 and by adding 20% of the loss to follow up, the final sample size was 508.
Sampling Technique and Procedure
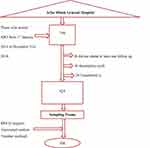
Simple random sampling was used to select the predetermined sample size. Card number/registration number of patients enrolled obtained from an electronic database. Then using the card number patients’ charts were drawn. From the total 730 charts of adults’ from 1st January 2014 to 31st December; patients who started ART but did not attend at least one follow up, transferred in and patients with incomplete baseline information were excluded. Then sampling frame was developed for the rest of 624 records by assigning id.numbers from 1 to 624 for all MRNs and a computer-generated random number was used to recruit the 508 records of study participants within five years of follow-up period (Figure 1).
Study Variable
Dependent Variable
Incidence of initial regimen change.
Independent Variables
Age, sex, level of education, marital status, occupation, Substance use, residence, and disclosure status (disclosed HIV status to their partner, family or other relatives/Not).
Eligibility criteria (initiated by test –start criteria/Not), initial regimen, co-medication, treatment duration, drug side effects, no. of pills/day; cotrimoxazole prophylactic treatment (CPT) at the start of ART.
Baseline weight, baseline WHO clinical stage, baseline CD4 count, Tuberculosis (TB) on the initial regimen, opportunistic infections (OIs) other than TB on the initial regimen, Comorbidity other than OIs, baseline ALS, baseline HGB, baseline Creatinine, baseline functional status.
Operational Definitions
Time
Was calculated by subtracting the date of antiretroviral therapy (ART) regimen initiation from the date of the event occurred or censored.9
Event
Initial ART regimen change, which was defined as either first-time substitution of at least one drug from the original regimen or complete switch of the entire regimen.13
Censored
Patients with the first date of lost to follow up, defaulters, transfer out, death before the end of the follow-up period and completed the follow-up period without developing the event was considered as censored.13
Incomplete card: was considered when the indicator of the dependent variable and/or 20% of the independent variable is not registered.
Side Effects
Defined as the occurrence of diarrhea, nausea, vomiting, anemia, rash, fatigue, lipodystrophy, metabolic disturbances or any other in relation to antiretroviral therapy.2
Functional Status
Defined as working (ie, the ability to perform usual work in and out of the house), ambulatory (ie, the ability to perform activities of daily living), and bedridden (ie, not able to perform activities).9
Data Collection Procedure
The data were collected by reviewing the patient’s medical cards and follow-up charts by 3 BSc nurses who had ART training. Two data clerks were selected to support them by identifying the charts. The 3 data collectors and one MPH supervisor in public health were trained for one day on objectives of the study, selection of study participants card, how to keep confidentiality of information, the contents of the questionnaire, how to fill the data collection format and data quality management by the principal investigator. The principal investigator and supervisor conducted a day-to-day follow up during the whole period of data collection. Every day after data collection, each questionnaire was reviewed and checked for completeness and consistency by the supervisor and the principal investigator and the necessary feedback was given to the data collectors to the next day. The overall activity of the study was supervised by the principal investigator.
Data Quality Management
Data quality was assured by caring out a careful design of data extraction formats and appropriate modification, appropriate recruitment and by giving adequate training and follow-up for data collectors and supervisor. A pretest was performed on 5% of the populations in Arba Minch General hospital from records of adult ART patients. Intensive supervision was done by the principal investigator and supervisor during the whole period of data collection. A random sample of registration forms was reviewed by the principal investigator to confirm the reliability of data before data collection and the investigator made random cross-checking for their completeness, accuracy, and consistency at the end of each day and corrective discussion was undertaken with all data collectors. Remarks were given during morning times on how to minimize errors and take corrective actions. The data were checked for completeness and consistency and then coded, entered and stored into the computer using EpiData version 4.43. The data collectors were individuals who had training on ART this also assured the quality of data.
Data Process and Analysis
After data collection, each questionnaire was checked for completeness and consistency by the principal investigator and supervisor. Data were cleaned, edited, coded and entered into EpiData version 4.43 and exported to STATA version 13. Exploratory data analysis was carried out to check the levels of missing values and the missed values were handled by multiple imputation method. Descriptive statistics such as mean, standard deviation, median and interquartile range were used after checking the distribution of variables. Frequencies and proportions were used to describe the characteristics of the cohort. The incidence rate of the initial regimen change was calculated, dividing the numbers who changed their initial regimen in the follow-up period by person-time at risk contributed throughout the observation period. Life table was used to estimate the cumulative survival. Kaplan–Meier survival curve together with Log rank test was used to estimate median time and to compare the overall survival experience of two or more groups. Cox proportional hazards model was fitted after checking the reasonability of assumptions through the Shoenfeld residuals test and graphically. The bivariable analysis was done to identify associations between dependent and each independent variable. Variables with P ≤ 0.25 levels in the bivariable analysis were entered into the multivariable analysis.17,18 Besides, context and findings of previous studies were considered in the identification of candidate variables for multivariable analysis. The backward variable selection procedure was applied to get a list of best predictors and adjusted hazard ratio, 95% CI and P-value were used to assess the strength of association and statistical significance. Any statistical test was considered significant at P-value ≤ 0.05.
Ethical Consideration
Ethical clearance was obtained from Arba Minch University, Medicine and Health Sciences College, Institution review Board (IRB) with approval number CMHS/12,033,792/11. The administration of Arba Minch general hospital was informed about the objective of the study through a support letter from department of Public Health and permission was obtained before the data collection. The study used the routine existing patient record data. The confidentiality of the information was assured by using an anonymous questionnaire and keeping the data in a secure place.
Result
Socio-Demographic Characteristics
A total of 508 HIV patients’ files were selected and analyzed. The mean ± SD (standard deviation) of age at initiation of ART was 35.12 ± 8.21 years and the majority 219 (43.11%) were in the age groups between 25 and 34 years. More than half of patients 289 (56.89%) were females and about 308 (60.63%) were Orthodox Christian followers. Regarding the educational status of the patients about 195 (38.39%) completed the primary level. A total of 375 (73.82%) were urban dwellers but the rest were rural. A total of 485 (95.47%) disclosed their HIV status to either their family or other relatives (Table 1).
 |
Table 1 Baseline Socio-Demographic Characteristics of HIV-Positive Adults at the Initiation of ART at Arba Minch General Hospital, January 2014 to December 2018 (n=508) |
Baseline Clinical, Immunological and Treatment-Related Characteristics
The majority of patients, 270 (53.15%) were eligible to their ART not by test and start whereas, 238 (46.85%) started by test and start eligibility criteria. The predominant HAART regimen initially prescribed for them were a combination of Tenofovir (TDF) + Lamivudine (3TC) + Efavirenz (EFV), 498 (98.03%) followed by Tenofovir (TDF) + Lamivudine (3TC) + Nevirapine (NVP), Zidovudine (AZT) + Lamivudine (3TC) + Efavirenz (EFV) and Zidovudine (AZT) + Lamivudine (3TC) + Nevirapine (NVP). The majority of patients, 491 (96.65%) started by taking one ART pills/day and 284 (55.91%) of the patients were taking CPT prophylaxis (Table 2).
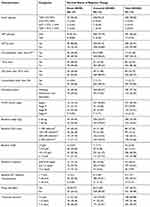 |
Table 2 Baseline Clinical, Immunological and Treatment-Related Characteristics of HIV-Positive Adults on ART in Arba Minch General Hospital, January 2014 to December 2018 (N=508) |
Two hundred seventy-one (53.35%) were WHO clinical stage I at the initiation of ART. The majority, 395 (77.76%) of the participant were on working functional status at a baseline. The median weight of the participants at the initiation of ART and at the end of the follow-up period were 55.00 kg [interquartile range (IQR): 48–60] and 57.627 kg [IQR: 52–60], respectively. The median CD4 count at initiation of ART was 281.50 cell/mm3 [IQR: 144.50–407.00] and it was 457.343 cell/mm3 [IQR: 400–485.750] at the end of follow-up period and above half, 306 (60.2%) of study subjects started ART at HGB level of between 10 and 12.9 g/dl. Eighty-four (16.54%) of the study participants were diagnosed TB at the initiation of ART and about 85 (16.73%) had opportunistic infection other than TB (Table 2).
The Incidence Rate of Initial ART Regimen Change
The minimum follow up period was 0.47 month and the maximum was 54.08 months and gave a total of 10,462.49 person-months (871.87 person-years) of observation.
The median follow-up period was 16.1150 months [IQR; 5.848–33.59], a total of 99 (19.49%) of patients changed their initial regimen, 16 (3.15%) died, 46 (9.06%) loss to follow up and defaulters, 55 (10.83%) transfer out, 292 (57.48%) keep on initial regimen. Hence, the overall incidence rate of initial ART regimen change was 11.36 (95% CI: 9.32, 13.83) per 100 person-years of observation. Regarding to the rate of initial regimen change in different treatment duration, 51 (64.78, 95% CI: 49.09, 85.47) per 100 PYs in <1 year, 18 (11.88, 95% CI: 7.48, 18.85) per 100 PYs between 1 and 2 years and 30 (4.82, 95% CI: 3.38, 6.85) per 100 PYs changed after 2 years of follow-up.
Among the reasons for regimen change, toxicity was the commonest reason for initial regimen change which accounts for 71.72% of cases and contributes for the 69.02 (95% CI: 54.69–87.09) per 100 PYs.
The cumulative probability of surviving on an initial regimen to the end of 6 months 88%; to the end of 1year was 85%; to the end of 3 years was 70% and to the end of follow-up period was 52%. The median survival time from initiation of HAART to initial regimen change was 54 (95% CI: 47.614–59.819) months (Table 3).
 |
Table 3 Life Table on the Incidence of Initial ART Regimen Change and Predictors Among Adult HIV Patients at Arba Minch General Hospital, January 2014 to December 2018 (N=508) |
The overall Kaplan–Meir survival function estimate showed that most of the initial ART regimen changes occurred in earlier months of ART initiation, which declined in the later months of follow up (Figure 2).
 |
Figure 2 Kaplan–Meir curve of surviving on initial regimen for HIV-positive adults on initial HAART at Arba Minch General Hospital, January 2014 to December 2018 (n=508). |
Comparison of Survival Probability Among Categories of Covariates
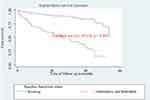
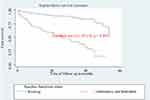
Kaplan–Meier curve together with the Log-rank (Mantel-Cox) test was used to check for the existence of any significant differences in survival probability between different categories of explanatory variables for the incidence of initial regimen change. Accordingly, the median survival time on the initial regimen for patients who disclosed their status on initial regimen was 53.72 months but it was 14.72 months for patients who do not disclose on initial regimen and the difference was significant (log-rank, p-value<0.001) (Table 4, Figures 3–7).
 |
Figure 3 Kaplan–Meier curve of surviving on an initial regimen based on the disclosure status on initial regimen in Arba Minch General Hospital from January 2014 to December 2018 (n=508). |
Predictors of Initial ART Regimen Change
Bi variable Cox-regression analysis showed that disclosure status, type of initial regimen, CPT prophylaxis, the occurrence of side effect, numbers of pills per a day, baseline WHO stage, baseline CD4 cells count, baseline HGB level, the occurrence of TB at the initiation of ART, co-medication other than CPT, the occurrence of OIs other than TB at the initiation of ART and baseline functional status were associated with the incidence rate of initial regimen change. In addition to significantly associated variables in bivariable cox-regression sex, marital status, occupation, and comorbidity condition other than opportunistic infections were with p-values of ≤ 25% and candidate variables for multivariable Cox regression. However, in the Multivariable Cox-regression analysis, only disclosure status, co-medication with ART, the occurrence of side effects on the initial regimen, baseline CD4 count and baseline functional status remained statistically significant predictors of the incidence rate of initial regimen change (Table 5).
Accordingly, the HIV patients on ART who did not disclose their status increased the rate of initial regimen change by 5.41 times when compared to those who disclosed (AHR = 5.41, 95% CI: 2.38–12.27). Those Patients on ART who were taking other medication with ART had about 4.64 times higher rate of changing their initial regimen at any time as compared to those who did not take other medication (AHR = 4.64, 95% CI 1.43–15.10). Patients who had ART drug side effects on initial regimen were 7.32 times at a higher rate of changing their initial regimen as compared to those who had no side effects (AHR = 7.32 95% CI: 4.43–12.10). Those ART patients whose baseline CD4 cell count <200 cells/mm3 had 2.18 times higher rate of initial regimen change when compared with those whose baseline CD4 cell count ≥200 cells/mm3(AHR = 2.18, 95% CI:1.37–3.47). Those patients who had ambulatory and bedridden baseline functional status had 3.55 times higher rate of initial regimen change (AHR = 3.55, 95% CI: 2.30–5.48) when compared with patients who had working baseline functional status.
Discussion
In low-income countries, the choice of a good regimen is limited so that well-managed first-line ART is very important. An assessment of the rate of initial regimen changes continuously and its predictor helps to keep patients on the first ART regimen as much as possible.19
In this study, the incidence rate of initial ART regimen change was 11.36 (95% CI: 9.32, 13.83) per 100 person-years and the median time to initial regimen change was found to be 54 months (95% CI: 47.614, 59.819). The cumulative probability of surviving on the initial regimen at the end of the follow-up period was 52%. The major factors that affect the incidence rate of initial ART regimen change were disclosure status, co-medication with ART, the occurrence of side effects on the initial regimen, baseline CD4 count and baseline functional status.
In this study the incidence rate of initial ART regimen change was 11.36 (95% CI: 9.32–13.83) per 100 person-years follow up. This is in line with studies conducted in Thailand 13.8/100 PYs,19 in Kenya 11.1/100PYs,20 in Senegal 10.5/100 PYs,21 in Gondar 10.11/100 PYs.13 This is lower than a study conducted in Brazil 28.3/100 PYs and 1.42/100 person-months,12,22 in Cameroon 14.6/100 PYs,23 in Kenya 18.6/100 PYs,24 in South West Ethiopia 16.08/100 PYs.9 This lower rate might be due to the difference in median follow-up period which was 22 months in Brazil, 37 months in Cameroon, and 54.20 months (95% CI: 51.012–57.388) in South West Ethiopia but 16.115 months in this study. In addition to this, our study included none of participants who started stavudine (D4T) based and PI-based ART regimen but in Brazil, 5.5% who started with stavudine (D4T) based which was responsible for toxicity like Lipodystropy, peripheral neuropathy, 61% PI-based ART regimen which was less tolerable and induces metabolic alteration in adults.12 1.8% patients started stavudine (D4T) based in Cameroon,23 59.7% in Kenya,24 61.4% in South West Ethiopia.9 Another possible reason might be the measurement of viral load and the availability of subsequent ARV treatment options are limited when compared with resource-rich settings.25 In this study setting regular monitoring of viral load which enables the identification of virological failure early and calls the need for regimen change is not routine. The finding of this study is higher than the study done in Swaziland 0.9 per 100 person-years of observation.11 The difference might be due to a difference in the study period of cohorts, which was two years in Swaziland but five years in the current study.
The median survival time in this cohort was 54 months (95% CI: 47.61, 59.82). This finding was higher when compared with studies conducted in Brazil 14 months,12 in Spain 31 months,26 in Portugal 12.1 months,27 in Australia 11.6 months,5 in South West Ethiopia 24.37.9 This higher durability might be due to the difference in the type of initial regimen. This study only included patients who started with FDC and after the phase-out of stavudine (D4T) after 2010 by WHO recommendation. This might also be because of the 90 90 90 UNAIDS policy which advocates sustained use of HIV treatment and ongoing virologic monitoring to verify treatment success. This enables to detect treatment failure early before the patients reached compelling condition to change the regimen.28
The finding of this study showed that disclosure status was found to be significant predictors of incidence of initial regimen change in which those patients who did not disclose their status at initiation had 5.41 times higher rate of regimen change when compared with patients who disclosed their status. This might be due to patients who disclosed their status were more aware of the proper utilization of ART medication because they might have the chance to get social network for advice, emotional support, information, and other social resources but patients who did not disclose might lack these advantages.
Those who had co-medication with ART were 4.64 times higher rate of changing their initial regimen as compared to those who did not take other medication. This finding is supported by a study conducted at the University of Gondar Referral Hospital, Ethiopia and Mekele Hospital, Ethiopia.13,15 This might be due to drug to drug interaction and overlapping toxicity between ART drugs and other medication. The other possible explanation might be polypharmacy which could lead to poor adherence due to pill burden which in turn resulted in poor efficacy of treatment result in drug change secondary to treatment failure.
Patients on ART who had side effects on the initial regimen had 7.32 times higher rate of changing their initial regimen at any given time as compared to those who did not develop side effects. This study is supported by a study done in Mekele Hospital, Ethiopia and Gondar, Ethiopia.13,15 This might be because the occurrence of side effect alters the quality of life. Especially for grade III and IV toxicity can result in the death of a patient and it is a short-term predictor for regimen change but WHO recommend following the side effect cautiously for grade I and II toxicity before regimen change.2
Those patients whose baseline CD4 count <200 cells/mm3 were 2.18 times at higher rate of changing their initial regimen at any time as compared to those whose baseline CD4 count ≥200 cells/mm3. This study is supported by studies done in Brazil, in Swaziland and Kenya.11,12,24 The possible explanation might be those patients who started ART treatment with the advanced stage are sicker patients and are more likely to have side effects and more regimen changes and possibly due to the interaction of other medications for opportunistic infections. Slower virologic and immunologic responses than those with higher CD4 count. But this study is contrary to finding from Europe and America.8 This might be due to the cessation of short-term treatment that was commenced during pregnancy in Europe and America. The other possible explanation might be the death of a patient with lower CD4 count in Europe and America, about 427 patients died before developing events.8
Patients on ART with ambulatory and bedridden baseline functional status had a 3.55 times higher rate of changing their initial regimen at any time as compared to those with working baseline functional status. The possible reason may be patients with ambulatory and bedridden baseline functional status may have advanced disease stage, low CD4 count, OIs and other chronic diseases. This might increase the chance of taking additional medication and this also can lead to a drug to drug interaction, occurrences of side effects and poor treatment success. Contrary to other studies, the WHO clinical Stage III/IV was not associated with the initial regimen change rate. This might be most likely because HIV patients at WHO clinical stage III/IV generally have a low survival rate and may die earlier before the occurrence of possible ART regimen change.
Limitations of the Study
The limitations of this study: since the data were collected from a secondary source, some missed variables like BMI, the status of mental illness and stigma might be predictors. The other limitation of this study was increased risk for misclassification bias because the information that was not previously recorded appropriately during the patient follow up may be misinterpreted or coded. Selection bias is also likely to have occurred in the case of participants who were lost to follow-up who had only one follow up visit after treatment initiation. This may lead to underestimation of the magnitude of ART regimen change as well as bias the conclusion about factors associated with ART regimen change.
Conclusion
The incidence rate of initial regimen change was found to be low and most of the change occurred within a year after initiation of ART. Not disclosing the HIV serostatus, co-medication other than CPT with ART, the occurrence of side effect on the initial regimen, baseline CD4 count <200 cells/mm3, ambulatory and bedridden baseline functional status were found to be independent predictors of initial ART regimen change.
Recommendations
To Health-Care Providers
- In collaboration with the hospital, it is important to have Close follow-up and screening of patients for side effects and give proactive management.
- It is needed to encourage patients who started ART to disclose their HIV status.
- It is recommended to Collaborate with the health office for Early HIV diagnosis and ART initiation.
- It is better give special attention to patients who have baseline CD4 count <200 cells/mm3 and with ambulatory and bedridden baseline functional status.
- It would be better to give special attention while the health professionals prescribe additional medication for ART patients.
To Patients
- Patients need to disclose their HIV status to their family and/or relatives.
To Researchers
- In collaboration with stake holders It is recommended to conduct Prospective follow-up studies by including socioeconomic, nutritional, mental status and stigma-related factors.
- It is also better to study Reason specific predictors of regimen change rate.
Abbreviations
3TC, lamivudine; AHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; ALT, alanine transaminase; ART, antiretroviral therapy; ATV, Atazanavir; AZT¸ Zidovudine; CD4, cluster of differentiation; CPT, cotrimoxazole prophylactic treatment; EFV, Efavirenz; EPHIA, Ethiopia population-based HIV impact assessment; FDC, fixed-dose combination; HIV, human immunodeficiency virus; IRB, Institution Review Board; IQR, interquartile range; LPV, Lopinavir; LTF, loss to follow up; NNRTI, None Nucleoside reverse transcriptase inhibiter; NRTI, nucleoside reverses transcriptase inhibiter; NVP, Nevirapine; OI, opportunistic infection; PLWHIV, People Living With HIV; PYs, person-years; TB, tuberculosis; TDF, Tenofovir; UNAIDS, Joint United Nation program on HIV/AIDS; WHO, World Health Organization.
Data Sharing Statement
All relevant data are available on the paper. The STATA data of individual patients are not permitted to be provided to other bodies as indicated in the ethical clearance. However, researchers who need further clarification can get anonymized data from the corresponding author on reasonable request.
Acknowledgment
The authors acknowledge the Arba Minch University, College of Medicine and Health Sciences for funding and Arba Minch general hospital for providing access to the data. We also would like to acknowledge data collectors and supervisors for accomplishing their tasks.
Author Contributions
All authors participated in the conception of the study, data collection, data analysis and interpretation; took part in drafting or revising the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. USAIDS. Global HIV & AIDS Statistics — 2018 Fact Sheet. USAIDS; 2018. Available from: http://www.unaids.org/en/resources/fact-sheet.
2. FMOH. National guidelines for comprehensive HIV prevention, care and treatment 2017. Available from: https://aidsfree.usaid.gov/sites/default/files/resources/ethiopia_art_guidelines_2017.pdf.
3. EPHIA. Ethiopia population-based HIV impact assessment EPHIA 2017–2018, summary sheet: preliminary findings Ethiopia. December, 2018 Available from: https://phia.icap.columbia.edu/wpcontent/uploads/2018/12/3511%E2%80%A2EPHIA-Summary-Sheet.
4. Blanco J, Clotet B. Learning from drug changes in antiretroviral therapy. Aids. 2013;27(5):833–834. doi:10.1097/QAD.0b013e32835c1213
5. Rappold M, Rieger A, Steuer A, et al. Treatment modification in HIV-infected individuals starting antiretroviral therapy between 2011 and 2014. J Int AIDS Soc. 2014;17(4):19768. doi:10.7448/IAS.17.4.19768
6. USAIDS. 90-90-90 on the Right Track Towards the Global Target. USAIDS; 2016. Available from: https://reliefweb.int/sites/reliefweb.int/files/resources/90_90_90_Progress_ReportFINAL.pdf.
7. Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83.
8. Abgrall S, Ingle SM, May MT; Collaboration ATC. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS (London, England). 2013;27(5):803–813. doi:10.1097/QAD.0b013e32835cb997
9. Mekonnen E, Workicho A, Hussein N, Feyera T. Reasons and predictors for antiretroviral therapy change among HIV-infected adults at South West Ethiopia. BMC Res Notes. 2018;11(1):351. doi:10.1186/s13104-018-3470-y
10. Ohene S-A, Addo NA, Zigah F, et al. Evaluation of antiretroviral therapy (ART) provision in an early cohort of patients initiating ART in Ghana. Pan Afr Med J. 2013;16.
11. Takuva S, Louwagie G, Zuma K, Okello V. Durability of first line antiretroviral therapy: reasons and predictive factors for modifications in a Swaziland cohort. J Antivir Antiretrovir. 2012;4(1):14–20. doi:10.4172/jaa.1000040
12. Cardoso SW, Grinsztejn B, Velasque L, et al. Incidence of modifying or discontinuing first HAART regimen and its determinants in a cohort of HIV-infected patients from Rio de Janeiro, Brazil. AIDS Res Hum Retroviruses. 2010;26(8):865–874. doi:10.1089/aid.2009.0274
13. Anlay DZ, Alemayehu ZA, Dachew BA. Rate of initial highly active anti-retroviral therapy regimen change and its predictors among adult HIV patients at University of Gondar Referral Hospital, Northwest Ethiopia: a retrospective follow up study. AIDS Res Ther. 2016;13(1):10. doi:10.1186/s12981-016-0095-x
14. Gebremedhin L, Birhane A. Reasons for anti-retroviral regimen changes in HIV/AIDS patients of Ayder Referral Hospital ART clinic, Mekelle, Ethiopia. Int J Pharma Sci Res. 2014;5(0975–9492):693–700.
15. Bayou T, Woldu M, Meskel GG, Mezgebe H. Factors determinant for change of initial antiretroviral treatment regimen among patients on ART follow-up clinic of Mekelle Hospital, Mekelle, Ethiopia. Int J Basic Clin Physiol Pharmacol. 2017;3(1):44–49. doi:10.5455/2319-2003.ijbcp20140201
16. Chisha Y, Terefe W, Assefa H, Lakew S, Reboldi G. Prevalence and factors associated with diabetic retinopathy among diabetic patients at Arbaminch General Hospital, Ethiopia: cross sectional study. PLoS One. 2017;12(3):e0171987. doi:10.1371/journal.pone.0171987
17. Tolosie K, Sharma MK. Application of Cox proportional hazards model in case of tuberculosis patients in selected Addis Ababa health centres, Ethiopia. Tuberc Res Treat. 2014;2014.
18. Ayele T, Jarso H, Mamo G. Clinical outcomes of tenofovir versus zidovudine-based regimens among people living with HIV/AIDS: a two years retrospective cohort study. Open AIDS J. 2017;11(1):1. doi:10.2174/1874613601711010001
19. Tsuchiya N, Pathipvanich P, Wichukchinda N, et al. Incidence and predictors of regimen-modification from first-line antiretroviral therapy in Thailand: a cohort study. BMC Infect Dis. 2014;14(1):565. doi:10.1186/s12879-014-0565-5
20. Ndakala FN, Oyugi JO, Oluka MN, Kimani J, Behrens GMN. The incidence of first-line antiretroviral treatment changes and related factors among HIV-infected sex workers in Nairobi, Kenya. Pan Afr Med J. 2017;28(1). doi:10.11604/pamj.2017.28.7.10885
21. Diouf A, Youbong T, Dieye F, Ciss V, Diallo Mbaye K. First antiretroviral therapy changes in people living with HIV in senegal: incidence, causes and predictive factors. J AIDS Clin Res. 2017;8(07):2. doi:10.4172/2155-6113.1000709
22. Penna Braga L, Pinto Mendicino CC, Reis EA, Carmo RA, Menezes de Pádua C. Incidence and predictors of antiretroviral treatment modification in HIV-infected adults: a brazilian historical cohort from 2001 to 2010. J Trop Med. 2017;2017:1–10. doi:10.1155/2017/9612653
23. Amin ET, Ngu RC, Abanda MH, Kika BT, Mvilongo TN, Fon PN. Modification of antiretroviral therapy regimen: incidence and predictors in two major HIV/AIDS treatment centers in the Southwest Region of Cameroon. Int J Adv Med Health Res. 2018;5(1):7. doi:10.4103/IJAMR.IJAMR_69_17
24. Inzaule S, Otieno J, Kalyango J, et al. Incidence and predictors of first line antiretroviral regimen modification in western Kenya. PLoS One. 2014;9(4):e93106. doi:10.1371/journal.pone.0093106
25. Keiser OAK, Schechter M, Balestre E, et al. Antiretroviral therapy in resource-limited settings 1996–2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13(7):870–879.
26. De La Torre-lima J, Aguilar A, Santos J, et al. Durability of the first antiretroviral treatment regimen and reasons for change in patients with HIV infection. HIV Clin Trials. 2014;15(1):27–35. doi:10.1310/hct1501-27
27. Moniz P, Alçada F, Peres S, et al. Durability of first antiretroviral treatment in HIV chronically infected patients: why change and what are the outcomes? J Int AIDS Soc. 2014;17:19797. doi:10.7448/IAS.17.4.19797
28. Joint United Nations Programme on HIV/AIDS, Joint United Nations Programme on HIV/Aids. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: Unaids; October 16, 2014.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.