Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Incidence and Predictors of Hypertension Among HIV Patients Receiving ART at Public Health Facilities, Northwest Ethiopia: A One-Year Multicenter Prospective Follow-Up Study
Authors Mulugeta H , Afenigus AD , Haile D , Amha H, Kassa GM , Wubetu M, Abebaw E, Jara D
Received 19 July 2021
Accepted for publication 26 August 2021
Published 7 September 2021 Volume 2021:13 Pages 889—901
DOI https://doi.org/10.2147/HIV.S329838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Henok Mulugeta,1 Abebe Dilie Afenigus,1 Dessalegn Haile,1 Haile Amha,1 Getachew Mullu Kassa,2 Muluken Wubetu,3 Ermias Abebaw,4 Dube Jara2
1Department of Nursing, College of Health Science, Debre Markos University, Debre Markos, Ethiopia; 2Department of Public Health, College of Health Science, Debre Markos University, Debre Markos, Ethiopia; 3Department of Pharmacy, College of Health Science, Debre Markos University, Debre Markos, Ethiopia; 4Department of Pediatrics and Child Health, School of Medicine, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Henok Mulugeta
Department of Nursing, College of Health Science, Debre Markos University, P.O. Box: 269, Debre Markos, Ethiopia
Tel +251913645701
Fax +0587711764
Email [email protected]
Background: The introduction of highly active retroviral therapy has dramatically reduced mortality and improved survival among HIV patients. However, there is a possible risk of comorbid complications such as hypertension. Little evidence is available regarding the incidence of hypertension among HIV patients receiving anti-retroviral therapy in Ethiopia.
Purpose: To assess the incidence and predictors of hypertension among HIV positive patients receiving ART at Public Health Facilities, Northwest Ethiopia.
Patients and Methods: A one-year prospective follow-up study was conducted among a cohort of 302 new adult individuals initiating on a standard anti-retroviral therapy regimen with a median (IQR) age of 35 years (IQR=30– 41). A pretested data extraction checklist was used to extract baseline patient records. The collected data were entered into Epi-Data version 3.1 and exported to STATA version 14 for analysis. The incidence rate was calculated, and a Kaplan–Meier survival curve was used to estimate the survival probabilities of developing hypertension. Cox proportional hazards model was fitted to identify the predictors of hypertension.
Results: About 40 (13.25) new hypertensive cases were observed during the follow-up period, and the remaining 262 (86.75%) were censored. The overall incidence rate of hypertension was 16.35 per 1000 person-month with 2447 patient-month observations. Male sex (AHR = 2.45, 95% CI: 1.02, 6.14), old age (AHR = 2.83, 95% CI: 1.08, 7.45), high BMI (AHR = 6.54, 95% CI: 2.03, 21.13), diabetic comorbidity (AHR = 2.36, 95% CI: 1.07, 5.22), and patients who were on Zidovudine (AZT)-based ART regimen (AHR =3.47, 95% CI: 1.10, 10.94) were significant predictors for the development of hypertension.
Conclusion: The findings of this study revealed that incident hypertension is a common problem among HIV patients receiving ART. Routine monitoring of blood pressure and screening and treating high blood pressure should be an integral part of follow-up for HIV patients in ART clinics.
Keywords: anti-retroviral therapy, HIV/AIDS, hypertension, Ethiopia
Introduction
The growing burden of HIV/AIDS remains one of the significant challenging health problems affecting many people worldwide, and its impact has been most marked in sub-Saharan Africa. Globally, about 37 million people are infected with HIV,1 and an estimated 20 million people have already died.2 In Ethiopia, about 1.2 million people are living with HIV/AIDS, and adult HIV prevalence in 2016 was estimated to be 1.1%.3,4 The number of individuals with access to anti-retroviral therapy (ART) has significantly increased from 700,000 to >16 million since the year 2000.5
A high prevalence of cardiovascular complications such as hypertension and other comorbidities have been reported in HIV positive individuals with or without anti-retroviral therapy (ART) and have emerged as a challenging problem in post-antiretroviral therapy (ART) era.6–8 Dyslipidemia and lipodystrophy syndrome can cause hypertension, a global public health concern that causes ischemic heart disease, stroke, and heart failure and is linked to non-AIDS-related mortality.9,10 The burden of hypertension is higher among HIV-infected adults on ART as compared with HIV-uninfected individuals.11 It is responsible for an estimated 80% of global mortality in low- and middle-income countries12,13 and 13–14% of global mortality.14 HIV related mortality and morbidity have been reduced radically after introducing highly active anti-retroviral therapy to treat HIV infection.15,16
Globally, few studies have provided evidence on the incidence and predictors of hypertension among the HIV-positive population. For instance, the incidence of hypertension was 4.6 per 100 person-year in Korea,17 72/1000 person-years in the USA18 and 64.1/1000 person-years in Norway.19 In Africa, a study conducted in Uganda and South Africa revealed that the incidence of hypertension among HIV patients receiving ART was 111.5/1000 person-years and 5.4 per 100 person-years.20,21
The risk of high blood pressure in HIV positive individuals might be due to a combination of HIV-associated immune dysregulation, microbial translocation to the systemic circulation, altered lipid metabolism, and chronic systemic inflammation.22,23 Moreover, the commonly known risk factors such as family history, older age, excess body fat, physical inactivity, excessive alcohol consumption, and a high salt-rich diet can be associated with the development of hypertension in HIV-positive populations.24,25 In addition, duration of HIV infection, CD4 count, plasma viral load, the class of anti-retroviral drugs, length of ART utilization, body mass index (BMI), and adherence to ART are important predictors of hypertension among HIV infected individuals.17–19
Although hypertension is a major contributor to cardiovascular diseases among HIV patients taking ART,9 very little research has been conducted in Ethiopia to assess hypertension among HIV positive patients on ARV treatment, especially in the selected study area. Therefore, this study aims to determine the incidence and identify hypertension among HIV patients receiving ART at public health facilities, Northwest Ethiopia.
Methods
Study Area
The study was conducted at East Gojjam Zone Health Facilities (Hospitals and Health centers providing ART services), located northwest of Addis Ababa, Ethiopia’s capital city.
Study Design
An institution-based prospective follow-up study.
Study Population
All adult HIV positive patients who initiated ART at ART clinic of East Gojjam Zone Health Facilities during the study period (between October and December 2019).
Eligibility Criteria
We included adult people with HIV initiating ART and screened to be free of hypertension between October and December 2019.
Sample Size
A three-month cohort of all the available newly ART initiated HIV patients (302 patients) at East Gojjam zone health facilities were followed for one year (48 weeks).
Variables
Dependent Variable
- Incidence of hypertension: time to event (hypertension).
Independent Variables
- Socio-demographic characteristics (age, sex, marital status, residence, educational status)
- Clinical characteristics (family history of hypertension, body mass index (BMI), Baseline blood pressure, World health organization (WHO) staging)
- Comorbidities (Renal diseases, Heart diseases, Diabetes mellitus, Hepatitis B infection)
- ART and other related factors (Duration of HIV infection, non-ART medications history, ART regimen at the time of initiation)
- Substance use (alcohol, cigarette smoking, khat chewing)
Operational Definitions
- Incidence of hypertension: the development of hypertension after initiation of ART within the follow-up period.
- Hypertension: high blood pressure of 130 systolic or higher, or 80 diastolic or higher at least on two or more consecutive visits.26
- Event: new occurrence of hypertension during the follow-up period at any time after initiation of ART.
- Time to Event: the time from initiation of ART to the development of hypertension.
- Censor: HIV positive who were lost, transferred, died, or does not develop the events until the last visit.
- Body mass index (BMI): Physical status was classified based on the BMI as: underweight for BMI < 18.5 kg/m2; normal for BMI 18.5–24.9 kg/m2; overweight for BMI 25.0–29.9 kg/m2, and obesity for BMI > 30 kg/m2.21,27
Data Collection Procedure and Instruments
A standard checklist prepared after reviewing different literature was used to collect relevant data from the patient, ART registration book and patient medical charts. The checklist consists of baseline (at the time of ART initiation) variables such as socio-demographic characteristics, clinical characteristics, ART regimen, WHO clinical stage, and other variables. The data collection tool was pretested for its completeness, consistency, and accuracy before the actual data collection. A mercury sphygmomanometer was used to take blood pressure measurements while the patients were sitting. Before the measurement, the patients were given 5 minutes to sit. The cuff was put at the level of the heart during the measurement. Two blood pressure measures were performed within five minutes to define incident hypertension. The data were collected by trained health professionals who are working in ART clinics. Supervisors closely supervised the process of data collection.
Data Processing and Analysis
The collected data were cleaned, entered Epi-Data version 3.1, and exported to STATA version 14 for analysis. Descriptive analysis (frequency distribution, mean with standard deviation, median with interquartile range) was performed to describe the sample’s baseline socio-demographic characteristics and other variables. A Kaplan-Meier (KM) survival curve was used to estimate the survival probabilities of developing hypertension, and Log rank tests were used to compare the probabilities of survival curves between different categories. Both bivariable and multivariable Cox proportional hazards model were fitted to identify predictors of time to hypertension. Independent variables having a P-value ≤0.25 in the bivariable analysis were fitted into the final multivariable model for further analysis. The assumptions of Cox proportional hazard regression model were checked based on Schoenfeld residual global test and graphically using the log-Log plot test. Adjusted Hazard Ratio (AHR) with 95% confidence intervals (CI) was used to assess the strength of association and statistical significance.
Results
Socio-Demographic and clinical Characteristics of the Study Participants
A total of 302 HIV patients who initiated a standard ART regimen were eligible and included in this prospective study. The median age was 35 (Interquartile range IQR=30–41), and 51.32% of the participants were female. Most of the participants (36.1%) were married and Orthodox Christians by religion (89.4). Among all respondents, 109 (36.1%) did not attend formal education. The occupational status of the participants showed that most of the participants (41.1%) were private workers, and almost half of the total respondents (52%) came from urban areas. Regarding the baseline clinical presentation, 190 (62.9%) had baseline BMI between 18.5–24 kg/m2, and most of the participants, 147 (48.7%), started ART at WHO clinical stage I. The baseline mean SBP of participants was 105.62±9.75mmHg, and the mean DBP was 70.08±7.89mmHg. Most of the participants had less than a one-year duration of HIV infection. About 75 (24.8%) of the patient has a history of taking non-ART medications, and only 46 (15.23%) of the participant has a family history of hypertension. About 18.9% of patients consumed alcohol, 7% chewed khat, and 5.6% smoked cigarettes. Regarding ART regimen at the time of initiation, most (85.10%) of the HIV patients received a DTG-based ART regimen (TDF+3TC+DTG). Comorbidities such as diabetes mellitus, heart disease, and renal diseases were observed in 18.5%, 3.3%, and 4.3% of the patients. Additionally, the prevalence of participants co-infected with hepatitis B was 5% (Table 1).
 |
Table 1 Baseline Socio-Demographic and Clinical Characteristics of Patients Receiving ART in East Gojjam Zone Health Facilities, Northwest Ethiopia (N = 302) |
Incidence of Hypertension
The patients were followed for a minimum and maximum follow-up time of 1 month and 12 months, respectively. The median follow-up time was nine months (IQR: 4–12 months). During the follow-up period, 40 (13.25%) new hypertensive cases were observed, and the remaining 262 (86.75%) were censored. Of these censored patients, 58 (22.14%) were lost to follow up, 62 (23.66%) were transferred out, 8 (3.05%) were dead, the rest 134 (51.15%) were normotensive at the end of the follow-up. The median survival time to develop hypertension was seven months with an interquartile range (IQR: 5.00–9.00 months). The overall incidence rate of hypertension was 16.35 per 1000 person-month (95% CI:11.99–22.28) with a total of 2447 patient-month observations (Figure 1).
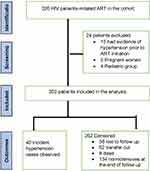 |
Figure 1 Flowchart diagram of the study profile. |
Kaplan-Meier Survival Analysis
The Kaplan–Meier survival curve showed that the probability of developing hypertension among HIV patients receiving ART increased over time. Many hypertensive cases were observed after five months of the follow-up period (Figure 2). Besides, the estimated cumulative probability of developing hypertension over time was significantly higher among male patients (Figure 3), patients whose age is ≥40 years (Figure 4), patients with BMI >25 kg/m2 (Figure 5), and among patient with diabetes mellitus (Figure 6). Furthermore, patients on a Zidovudine (AZT)-based ART regimen had a significantly higher estimated cumulative probability of developing hypertension over time (Figure 7).
 |
Figure 2 The Kaplan–Meier curve showing the survival probability of developing hypertension. |
 |
Figure 3 The Kaplan–Meier curve showing the survival probability of developing hypertension among HIV patients receiving ART based on sex. |
 |
Figure 4 The Kaplan–Meier curve showing the survival probability of developing hypertension among HIV patients receiving ART based on age groups. |
 |
Figure 5 The Kaplan–Meier curve showing the survival probability of developing hypertension among HIV patients receiving ART based on body mass index. |
 |
Figure 6 The Kaplan–Meier curve showing the survival probability of developing hypertension among HIV patients receiving ART based on DM comorbidity. |
 |
Figure 7 The Kaplan–Meier curve showing the survival probability of developing hypertension among HIV patients receiving ART based on ART regimen. |
Predictors of Hypertension
In the bivariate Cox regression model, factors with a p-value of less than 0.25 were sex, age, BMI, marital status, residence, WHO staging, ART regimen, heart disease comorbidity, DM comorbidity, HBV co-infection and alcohol intake. However, only six variables became statistically significant in the final Multivariable Cox regression model: sex (male), age (old age), WHO Staging (stage 3 and 4), diabetes mellitus comorbidity, ART regimen (AZT containing) and BMI (overweight and obesity). The Schoenfeld residual test showed all the covariates, and the overall model satisfies the proportional hazard assumption (global test, P=0.47). Males compared with females had significantly 2.45 times increased risk of hypertension (adjusted hazard ratio (AHR): 2.45; 95% confidence interval (CI):1.02, 6.14). These HIV patients who had a baseline BMI >25kg/m2 were 6.54 times (AHR: 6.54;95% CI: 2.03, 21.13) higher risk of developing hypertension as compared to these HIV patients who had BMI < 18 (kg/m2). Similarly, patients who had diabetes at baseline were 2.36 times (AHR: 2.36;95% CI: 1.07, 5.22) higher risk of developing hypertension at any time as compared to the counterpart. Finally, the hazard of developing hypertension among patients who had taken Zidovudine (AZT)-based regimen (AZT+3TC+EFV) were 3.47 (AHR:3.47;95% CI: 1.10, 10.94) times as compared to those who had taken Dolutegravir (DTG) based ART regimen (TDF+3TC+DTG) (Table 2).
 |
Table 2 The Bivariable and Multivariable Cox-Regression Analysis for Predictors of Hypertension Among HIV Patients Receiving ART, Northwest Ethiopia (N = 302) |
Discussion
This is the first prospective follow up study in Ethiopia to investigate the new-onset (incidence) of hypertension and its predictors among initially normotensive HIV patients initiating ART. Thus, the incidence rate was found to be 16.35 per 1000 person-month with a median follow-up time of 9 months (IQR: 4–12 months). The incidence of hypertension was higher as compared to other similar studies conducted in Uganda,21 Tanzania27 and South Africa.20 In addition, the incidence of hypertension among HIV patients receiving ART in our study is also much higher than in another study conducted in North America28 and China.29 The higher incident in this study might be attributed to the lower cut off value (>130/80mmhg) to diagnose incident hypertension. Differences in sample size, study design (prospective Vs retrospective cohort), study setting, and socio-demographic characteristics of study participants might be the additional reasons for incidence rate variations.
In the current study, male patients, patients over the age of 40, patients with WHO stages 3 and 4, patients with a high (>25kg/m2) BMI, patients with diabetes mellitus comorbidity, and patients on an AZT-based ART regimen all had a significantly higher risk of developing hypertension. This outcome is consistent with findings from other similar studies.20,21 The gender difference in the incidence of hypertension could be explained by hormonal differences that protect women from hypertension,30 and more males were overweight, drank alcohol, and smoked in this study than females. One study showed that hypertension was highly prevalent among female HIV patients.31 High blood pressure in elderly patients could be caused by reduced arterial distension and vasoconstriction.32 In addition, diabetes mellitus comorbidity was revealed to be an independent predictor of hypertension in HIV patients on ART. Comorbidity with diabetes mellitus was also found to be a significant predictor of hypertension in another study.33 Increased blood pressure in diabetes patients could be due to increased peripheral vascular resistance.34 Hypertension was linked to patients on the AZT-based ART regimen (AZT+3TC+EFV). Another research20,35 found a similar conclusion. The effects of Zidovudine (AZT) on vascular smooth muscle and the rise in triglyceride levels may be linked to an increase in blood pressure.36,37 However, a similar cohort study in China revealed that zidovudine exposure was a protective factor for hypertension among HIV patients.29
Controlling high blood pressure is critical in HIV positive individuals to prevent organ damage and ultimately reduce mortality.38,39 The burden of non-communicable diseases like hypertension in HIV positive patients can be minimized by implementing risk reduction interventions and strategies. For example, health education about lifestyle changes, regular risk factor assessments, appropriate ART regimen and subsequent modification with a primary focus on viral suppression, regular blood pressure monitoring during ART follow-up, and the development of hypertension monitoring and treatment protocols are all important in reducing the incidence of high blood pressure among HIV patients.40–42
The findings of this study have implications for clinical practice. This prospective study monitors blood pressure changes among HIV patients receiving ART for 12 months. Determining the incidence rate of hypertension during the follow-up period provides up-to-date evidence to understand how incidence rates change over time. It is also critical to develop guidelines for regular blood pressure monitoring for patients receiving ART to reduce further complications and mortality. Moreover, identifying the predictors could help clinicians prioritize and consider their routine clinical practice for HIV positive patients. However, future research should consider some limitations. The short follow up period in this study is the primary limitation of this study. Given the significant increase in blood pressure during the first year on ART, it would be interesting to investigate the longer-term incidence of hypertension with large sample size, despite the fact that we cannot predict what changes in blood pressure would occur later from this study. Moreover, baseline laboratory tests, CD4 and HIV viral loads were not done to control potential confounders.
Conclusions
The findings of this study revealed that incident hypertension is a common problem among HIV patients receiving ART. About 13.25% of normotensive HIV patients at ART initiation develop hypertension during the follow-up period (12 months) with an incidence rate of 16.35 per 1000 person-month. Sex (being male), old age (≥40 years), high BMI (>25), Diabetic comorbidity and Zidovudine (AZT)-based ART regimen were significant predictors for the development of hypertension. HIV care in Ethiopia needs to develop guidelines and strategies for routine monitoring, screening, and treatment of hypertension (when necessary) among HIV patients. Regular monitoring, counselling, and high blood pressure therapy should also be a part of HIV patients’ follow-up in ART clinics. Moreover, a large-scale prospective study with a long follow-up period is needed to understand better how incidence rates change over time and identify additional predictors.
Abbreviations
AHR, Adjusted Hazard Ratio; AIDS, Acquired Immune Deficiency Virus; ART, Anti-Retroviral Therapy; AZT, Zidovudine; BMI, Body Mass Index; COR, Crude Hazard Ratio; DM, Diabetes Mellitus; DTG, Dolutegravir; EFV, Efavirenz; HAART, Highly Active Antiretroviral Therapy; HIV, Human Immune Virus; NNRTIs, Non-Nucleoside Reverse Transcriptase Inhibitors; PLWH, People Living With HIV/AIDS; 3TC, Lamivudine; TDF, Tenofovir; WHO, World Health Organization.
Data Sharing Statement
The data analyzed in this prospective follow up study is available from the corresponding author (Principal investigator) on reasonable request.
Ethical Considerations
The study was conducted after the approval of the proposal by the Ethical committee of Debre Markos University College of Health Sciences on the 05th of October 2019. Since this study involves human participants, the patient’s dignity was ensured and was respected. Before data collection, verbal informed consent was obtained from each participant after informing them about the aims, data collection methods, and the study purpose. The Ethical committee of Debre Markos University College of Health Sciences approved the verbal informed consent. Further, patient’s privacy was protected, and confidentiality of their personal information was maintained.
Acknowledgments
The authors would like to thank Debre Markos University for funding the study. Our gratitude extends to all staff members of East Gojjam Zone Health Bureau administrative offices who cooperated to give important information about the study areas during this study. We would also like to extend our appreciation to the data collectors and the respected respondents who took part in this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final draft of the report.
Funding
Debre Markos University funded this study.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. HIV/AIDS: Global Situation and Trends. Global Health Observatory; 2013.
2. Piot P, Bartos M, Ghys PD, Walker N, Schwartländer B. The global impact of HIV/AIDS. Nature. 2001;410(6831):968. doi:10.1038/35073639
3. De Cock KM, Weiss HA. The global epidemiology of HIV/AIDS. Trop Med Int Health. 2000;5(7):A3–A9. doi:10.1046/j.1365-3156.2000.00590.x
4. Mulugeta H, Dessie G, Wagnew F, Jara D, Leshargie CT, Negesse A. Seroprevalence and trend of human immunodeficiency virus among blood donors in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):383. doi:10.1186/s12879-019-4012-5
5. World Health Organization. Global Health Sector Response to HIV, 2000–2015: Focus on Innovations in Africa: Progress Report. World Health Organization; 2015.
6. Currier JS, Lundgren JD, Carr A, et al.; Working Group 2. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active anti-retroviral therapy. Circulation. 2008;118(2):e29–e35. doi:10.1161/CIRCULATIONAHA.107.189624
7. Barnes RP, Lacson JCA, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep. 2017;19(5):20. doi:10.1007/s11883-017-0652-3
8. McDonald CL, Kaltman JR. Cardiovascular disease in adult and pediatric HIV/AIDS. J Am Coll Cardiol. 2009;54(13):1185–1188. doi:10.1016/j.jacc.2009.05.055
9. Palacios R, Santos J, Garcia A, et al. Impact of highly active anti-retroviral therapy on blood pressure in HIV‐infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7(1):10–15. doi:10.1111/j.1468-1293.2005.00333.x
10. Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active anti-retroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi:10.7326/0003-4819-145-6-200609190-00003
11. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797. doi:10.1093/cid/ciu701
12. Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21(7):1377–1382. doi:10.1097/00004872-200307000-00028
13. World Health Organization. Noncommunicable Diseases Progress Monitor 2015. World Health Organization; 2015.
14. Nguyen KA, Peer N, Mills EJ, Kengne AP. Burden, determinants, and pharmacological management of hypertension in HIV-positive patients and populations: a systematic narrative review. AIDS Rev. 2015;17(2):83–95.
15. Montaner JS, Lima VD, Barrios R, et al. Expanded HAART coverage is associated with decreased population-level HIV-1-RNA and annual new HIV diagnoses in British Columbia, Canada. Lancet. 2010;376(9740):532. doi:10.1016/S0140-6736(10)60936-1
16. Morison L. The global epidemiology of HIV/AIDS. Br Med Bull. 2001;58(1):7–18. doi:10.1093/bmb/58.1.7
17. Kim J, Bang JH, Shin J-Y, Yang BR, Lee J, Park B-J. Hypertension risk with abacavir use among HIV-infected individuals: a Nationwide Cohort Study. Yonsei Med J. 2018;59(10):1245–1252. doi:10.3349/ymj.2018.59.10.1245
18. Thiébaut R, El-Sadr WM, Friis-Moller N, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10(7):811.
19. Krauskopf K, Natta MLV, Danis RP, et al. Correlates of hypertension in patients with AIDS in the era of highly active anti-retroviral therapy. J Int Assoc Provid AIDS Care. 2013;12(5):325–333. doi:10.1177/2325957413491432
20. Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on anti-retroviral treatment in public sector treatment programs in South Africa. PLoS One. 2018;13(10):e0204020. doi:10.1371/journal.pone.0204020
21. Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV initiating anti-retroviral therapy in Southwestern Uganda. J Hypertens. 2015;33(10):2039. doi:10.1097/HJH.0000000000000657
22. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(suppl_3):S375–S382. doi:10.1093/infdis/jis200
23. Mateen FJ, Kanters S, Kalyesubula R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31(7):1372–1378. doi:10.1097/HJH.0b013e328360de1c
24. Cahn P, Leite O, Rosales A, et al. Metabolic profile and cardiovascular risk factors among Latin American HIV-infected patients receiving HAART. Braz J Infect Dis. 2010;14(2):158–166. doi:10.1016/S1413-8670(10)70030-1
25. Kannel WB. Risk factors in hypertension. J Cardiovasc Pharmacol. 1989;13:S4–10. doi:10.1097/00005344-198900131-00003
26. American Heart Association. What is High Blood Pressure? South Carolina State Documents Depository; 2017.
27. Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania–A prospective cohort study. PLoS One. 2017;12(3):e0172089. doi:10.1371/journal.pone.0172089
28. Wong C, Gange SJ, Buchacz K, et al. First occurrence of diabetes, chronic kidney disease, and hypertension among North American HIV-infected Adults, 2000–2013. Clin Infect Dis. 2017;64(4):459–467.
29. Fan H, Guo F, Hsieh E, et al. Incidence of hypertension among persons living with HIV in China: a multicenter cohort study. BMC Public Health. 2020;20(1):1–11. doi:10.1186/s12889-020-08586-9
30. Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61(1):1–17. doi:10.1080/19485565.2014.929488
31. Berhane T, Yami A, Alemseged F, et al. Prevalence of lipodystrophy and metabolic syndrome among HIV positive individuals on Highly Active Anti-Retroviral treatment in Jimma, South West Ethiopia. Pan Afri Med J. 2012;13(1).
32. Avolio A, Deng F-Q, Li W-Q, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–210. doi:10.1161/01.CIR.71.2.202
33. Krauskopf K, Van natta ML, Danis RP, et al. Correlates of hypertension in patients with AIDS in the era of highly active anti-retroviral therapy. J Int Assoc Provid AIDS Care. 2013;12(5):325–333.
34. Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension. 1992;19(5):403–418. doi:10.1161/01.HYP.19.5.403
35. Pangmekeh PJ, Awolu MM, Gustave S, Gladys T, Cumber SN. Association between highly active anti-retroviral therapy (HAART) and hypertension in persons living with HIV/AIDS at the Bamenda regional hospital, Cameroon. Pan Afr Med J. 2019;33. doi:10.11604/pamj.2019.33.87.15574
36. de Arruda Junior ER, Lacerda HR, Moura LCRV, et al. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14(3):281–287. doi:10.1016/S1413-8670(10)70057-X
37. Ruga E, Bova S, Nussdorfer G, et al. Zidovudine-induced alterations in the heart and vascular smooth muscle of the rat. Cardiovasc Res. 2003;60(1):147–155. doi:10.1016/S0008-6363(03)00364-X
38. Remais JV, Zeng G, Li G, Tian L, Engelgau MM. Convergence of non-communicable and infectious diseases in low-and middle-income countries. Int J Epidemiol. 2012;42(1):221–227. doi:10.1093/ije/dys135
39. Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antivir Ther. 2011;16(5):639. doi:10.3851/IMP1809
40. Patel P, Speight C, Maida A, et al. Integrating HIV and hypertension management in low-resource settings: lessons from Malawi. PLoS Med. 2018;15(3):e1002523. doi:10.1371/journal.pmed.1002523
41. Pereira M, Lunet N, Paulo C, Severo M, Azevedo A, Barros H. Incidence of hypertension in a prospective cohort study of adults from Porto, Portugal. BMC Cardiovasc Disord. 2012;12(1):1–8. doi:10.1186/1471-2261-12-114
42. Stein JH, Hadigan CM, Brown TT, et al. Prevention strategies for cardiovascular disease in HIV-infected patients. Circulation. 2008;118(2):e54–e60. doi:10.1161/CIRCULATIONAHA.107.189628
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
