Back to Journals » Risk Management and Healthcare Policy » Volume 14
Incidence and Burden of Acute Kidney Injury among Traumatic Brain-Injury Patients
Authors Wang R, Zhang J, Xu J, He M, Xu J
Received 20 August 2021
Accepted for publication 31 October 2021
Published 11 November 2021 Volume 2021:14 Pages 4571—4580
DOI https://doi.org/10.2147/RMHP.S335150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mecit Can Emre Simsekler
Ruoran Wang,1 Jing Zhang,1 Jing Xu,2 Min He,2 Jianguo Xu1
1Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China
Correspondence: Min He
Department of Critical Care Medicine, West China Hospital, Sichuan University, 37 Guoxue Alley, Chengdu, 610041, People’s Republic of China
Email [email protected]
Jianguo Xu
Department of Neurosurgery, West China Hospital, Sichuan University, 37 Guoxue Alley, Chengdu, 610041, People’s Republic of China
Email [email protected]
Background: Acute kidney injury (AKI) has been occurs commonly in the clinical management of traumatic brain injury (TBI) patients and is correlated with outcomes in these patients. We designed this study to investigate the incidence, duration, stage, and burden of AKI among these patients.
Methods: A total of 419 TBI inpatients at our hospital were included in the study. We calculated the AKI burden, reflecting both stage and duration, and then analyzed associations among AKI occurrence, highest AKI stage, AKI duration, AKI burden, and outcomes with logistic regression analysis.
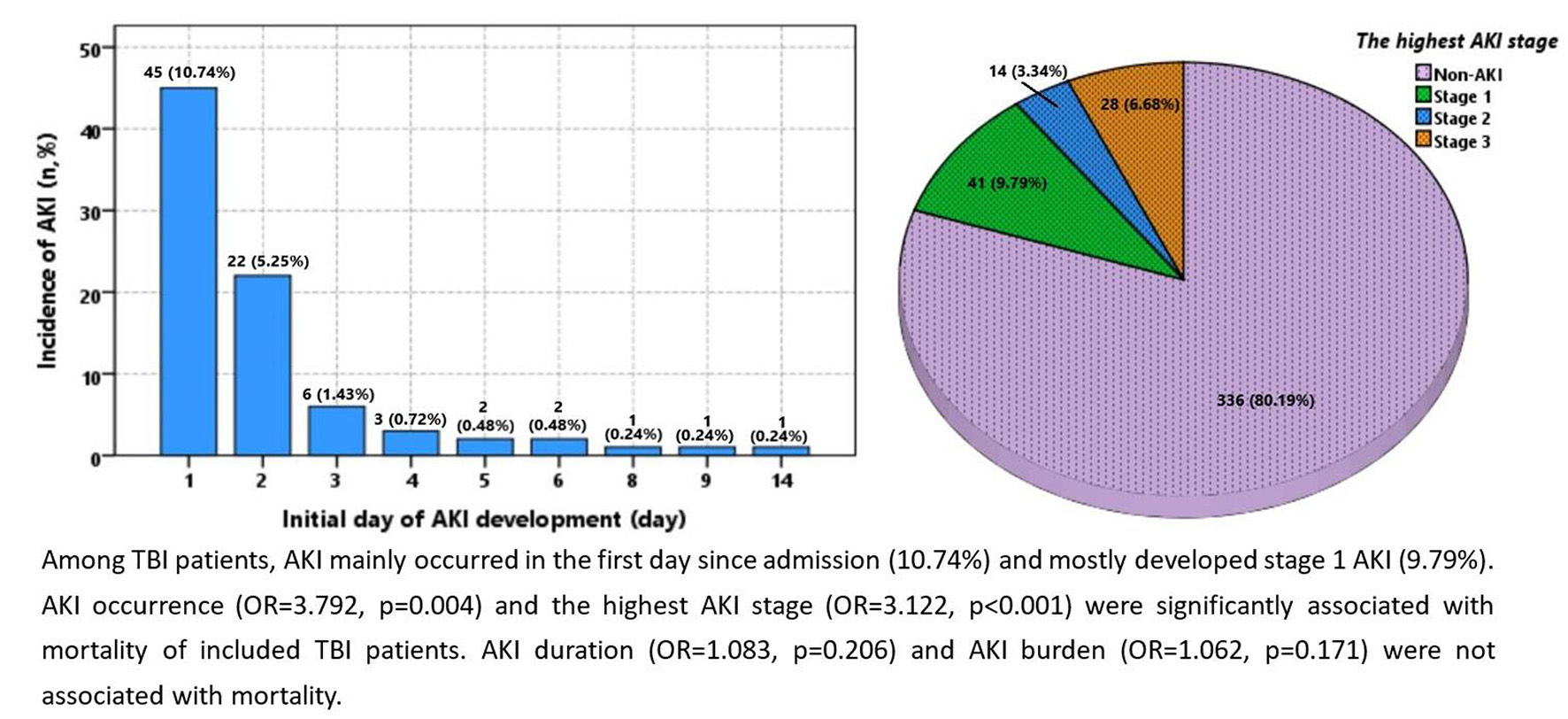
Results: Incidence of AKI among TBI patients was 19.8%. These patients’ AKIs occurred mainly on the first day from admission (10.74%), and mostly developed stage 1 AKI (9.79%). Modes of AKI duration and burden in those with AKI were both 1. Multivariate logistic regression showed AKI occurrence (OR 3.792, p=0.004) and the highest AKI stage (OR 3.122, p< 0.001) was significantly associated with mortality. Neither AKI duration (OR 1.083, p=0.206) nor AKI burden (OR 1.062, p=0.171) were associated with mortality. Incorporating AKI occurrence or highest AKI stage did not improve the predictive value of the constructed prognostic model.
Conclusion: The high-incidence period of AKI in TBI patients was the first 3 days after admission. AKI occurrence and highest AKI stage were associated with mortality, while AKI duration and AKI burden were not associated with mortality.
Keywords: acute kidney-injury duration, acute kidney-injury burden, traumatic brain injury, mortality
Graphical Abstract:
Introduction
Traumatic brain injury (TBI) is a severe public health issue involved a series of pathophysiological processes following the initial injury. Both initial head injury and subsequent secondary brain injury can aggravate extracranial organ dysfunction through complex mechanisms. The incidence of nonneurological organ dysfunction developing in TBI patients is 33%–89%.1–4 Among various kinds of organ dysfunction, acute kidney injury (AKI) has attracted wide attention, due to its unfavorable effects on cerebral edema and prognosis of TBI patients. Both injury severity and inappropriate medical strategy can promote the development of AKI in TBI patients. Many factors play complex roles in the development of AKI after TBI, such as massive release of catecholamine transmitters, systemic inflammation, and iatrogenic factors, including massive drug doses reducing intracranial pressure and nephrotoxic antibiotics.5–7
The incidence of AKI in TBI patients is 9.2%–24%.8–11 There have been few studies to investigate the details of AKI duration and AKI stage and their correlation with mortality in TBI patients. Joint or additive effects of AKI duration and AKI stage on prognosis of TBI patients is worth exploring. In one study, the authors designed a novel index, the AKI burden, to indicate both AKI duration and AKI stage in aspecific critically ill patients.12 We designed this study to investigate the incidence, timing, duration, stage, and burden of AKI among hospitalized TBI patients in detail and verify their association with prognosis of TBI patients.
Methods
Patients
Patients admitted to West China Hospital directly after a TBI between January 2015 and June 2019 were eligible for this study. Exclusion criteria were admission ≥6 hours after the injury, lacking relevant clinical variables, and existing chronic kidney disease. The flowchart for the inclusion process is presented in Figure 1. In sum, 419 patients were finally included. Thtudy was approved by the ethics committee of West China Hospital and performed in accordance with the Declaration of Helsinki. Informed-consent forms were regularly signed by patients themselves or their legally authorized representatives once admitted.
 |
Figure 1 Flowchart of patient inclusion. |
Data Collection
Demographic and clinical information — injury cause, vital signs on admission, Glasgow Coma Scale (GCS) score on admission, injury severity score (ISS), laboratory tests, and radiology — were collected. Results of laboratory tests were obtained by analyzing the first blood sample collected once patients had been admitted to the emergency department. Five AKI-related variables were included in analysis: AKI development, initial day of AKI development, AKI duration, highest AKI stage, and AKI burden. AKI development was evaluated every day as per KDIGO guidelines using creatinine criteria.13 As part of blood biochemistry testing, serum creatinine was measured and recorded every day. Initial day of AKI development was defined as the first day on which changes in serum creatinine met the KDIGO criteria. Day of admission was regarded as the first day. AKI duration was defined as the sum of days on which AKI lasted or progressed during the whole hospitalization. The end of AKI was defined as not meeting the KDIGO criteria. Highest AKI stage was defined as the most severe stage of AKI patients developed according to the KDIGO criteria. If patients did not develop AKI, their highest AKI stage was regarded as 0. AKI burden was calculated by multiplying AKI-stage score by the number of days in each stage. Non-AKI, stage 1, stage 2, and stage 3 were assigned scores of 0, 1, 2 and 3, respectively. The primary outcome of this study was mortality in TBI patients.
Statistical Analysis
The normality of variables was confirmed utilizing the Kolmogorov–Smirnov test. Normally distributed and abnormally distributed variables are shown in the form of means ± SD and medians (IQR), respectively. Categorical variables are presented as numbers and percentages. Independent Student’s t-tests and Mann–Whitney U tests were performed to analyze the difference between groups of normally and abnormally distributed variables, respectively. Fisher’s or χ2 tests were used to compare differences among categorical variables. Univariate logistic regression was conducted to discover potential risk factors of mortality. Then, multivariate logistic regression was conducted to analyze the relationship between AKI-related variables and mortality through adjusting the confounding effects of factors significant on univariate regression (factors with p<0.05). To construct prognostic models incorporating AKI-related variables, stepwise forward multivariate logistic regression was applied. Receiver-operating characteristic (ROC) curves were drawn and area under the ROC curve (AUC) calculated to assess the prognostic value of single AKI-related variables and constructed models. Z tests were conducted to compare AUC differences among models. Two-sided p<0.05 was considered statistically significant. SPSS 22.0 and GraphPad (GraphPad Software, La Jolla, CA, USA) were used for all statistical analyses and drawing of figures.
Results
Baseline Characteristics
AKI incidence was 19.8% (Table 1). Compared with the non-AKI group, AKI group were older (47 years vs 41 years, p=0.004) and had higher ISSs (25 vs 22, p<0.001) and lower GCS scores (5 vs 6, p<0.001). Results of laboratory tests indicated that the AKI group had higher levels of glucose (12.24 mmol/L vs 8.97 mmol/L, p<0.001) and serum creatinine (128 µmol/L vs 65 µmol/L, p<0.001) than the non-AKI group. Platelets (74×109/L vs 116×109/L, p<0.001) and hemoglobin (80 g/L vs 94 g/L, p<0.001) were both lower in the AKI group. Median initial AKI development was 1 (IQR 1–2) day since admission. Median AKI duration and AKI burden was 1 (IQR 1–3) and 2 (IQR 1–6), respectively. Median highest AKI stage was 2 (IQR 1–3). The AKI group had significantly higher mortality (84.3% vs 40.2%, p<0.001) and shorter hospital stay (7 vs 15 days, p<0.001) than the non-AKI group.
 |
Table 1 Baseline characteristics of TBI patients with and without AKI |
Distribution of AKI-Related Variables
Figure 2A showes the initial day of AKI development during hospitalization was mainly the first (10.74%) following admission. Incidence for the second and third days was 5.25% and 1.43%, respectively. As such, AKI following a TBI mainly developed in the 3 days following admission. Figure 2B shows that AKI duration during hospitalization for TBI was mostly 1 day (10%). Duration of 2 days and 3 days ranked second and third: 4.3% and 2.1%, respectively. Incidence of stage 1, 2, 3 AKI was 9.79%, 3.34%, and 6.68%, respectively (Figure 2C). Figure 2D shows AKI burden of 1 (7.2%), 2 (3.1%), and 3 (2.4%) patients with AKI.
Univariate Logistic Regression Analysis of Risk Factors of Mortality
Univariate logistic regression indicated that heart rate (OR 1.013, p<0.001), ISS (OR 1.108, p<0.001), glucose (OR 1.279, p<0.001), serum creatinine (OR 1.013, p<0.001), subdural hematoma (OR 2.886, p<0.001), subarachnoid hemorrhage (OR 1.825, p=0.002), and diffuse axonal injury (OR 1.829, p=0.009) were potential risk factors of mortality in TBI patients (Table 2). GCS (OR 0.597, p<0.001), platelet count (OR 0.991, p<0.001), and hemoglobin (OR 0.975, p<0.001) were negatively associated with increased mortality, and injury cause (p=0.017) was related withto mortality.
 |
Table 2 Univariate logistic regression analysis for risk factors of mortality in TBI patients |
Association Between AKI-Related Variables and Mortality
Through multivariate logistic regression (enter method), we analyzed the association between four AKI related variables and mortality of TBI patients by adjusting the confounding effects of significant factors on univariate logistic regression. Results showed AKI occurrence (OR 3.792, p=0.004) and highest AKI stage (OR 3.122, p<0.001) were significantly associated with mortality, while AKI duration (OR 1.083, p=0.206) and AKI burden (OR 1.062, p=0.171) were not (Table 3). Figure 3A–D showed detailed mortality differences regarding AKI occurrence, AKI duration, AKI stage, and AKI burden. The cutoff point for grouping of AKI duration and AKI burden was based on quartiles of these two variables in the AKI group.
 |
Table 3 Characteristics of AKI-related variables and their association with mortality on univariate and multivariate logistic regression analysis |
Prognostic Value of AKI-Related Variables and Constructed Logistic Models
From the ROC curve, we evaluated the prognostic value of AKI-related variables and found the AUC of single AKI-related variables did not differ from one other. As shown in Table 4, the AUC of AKI occurrence, AKI duration, the highest AKI stage, and AKI burden was 0.640, 0.643, 0.643, and0.644, respectively. Due to the insignificant association between AKI duration, AKI burden, and mortality, we constructed only prognostic models incorporating AKI occurrence or highest AKI stage. The forward stepwise multivariate logistic regression indicated GCS, glucose, SDH (subdural hematoma), AKI occurrence, or highest AKI stage were independently associated with mortality. We constructed a prognostic logistic model incorporating these factors. Comprising GCS, glucose, and SDH, model 1 had an AUC of 0.860 (Table 4) (Figure 4). Adding AKI occurrence or highest AKI stage to Model 1, the model 2 and model 3 had AUCs of 0.880 and 0.885, respectively. Z tests indicated that the AUCs of model 2 and model 3 were not significantkly higher than model 1 (0.880 vs 0.860, Z=0.8305, p>0.05; 0.885 vs 0.860, Z=1.0381, p>0.05).
 |
Table 4 Predictive value of single lactate and the logistic model |
Discussion
TBI patients commonly develop nonneurological dysfunctions during hospitalization, due to a series of pathophysiological processes, including catecholamine surge and local and systemic inflammation.6,7 Characterized as massive releases of adrenergic transmitters, the catecholamine surge after TBIs excessively activates the renin–angiotensin–aldosterone system, which in turn promotes renal dysfunction.6,14 Many inflammatory mediators released from damaged cerebral tissue, such as IL6 and CRP, promote systemic inflammation involved in extracranial organ damage, including renal dysfunction.15,16 Some iatrogenic factors may also play significant roles in the development of AKI after TBI. Massive doses of mannitol and hypertonic saline can both increase the risk of AKI development.17–19 Nephrotoxic antibiotics, such as vancomycin, are sometimes used in medical centers for treating nosocomial infections during hospitalization.
AKI incidence among our TBI patients was 19.8%, consistent with previous studies.8–11 Variations in incidence of AKI in previous studies on TBI may be attributable to the severity of brain injury, complicated extracranial injuries, history of underlying diseases, and diversity of clinical practice at different medical centers. Few studies have specifically investigated the distribution of initial day of AKI development. We found that 17.42% of patients had AKI onset TBI within the first 3 days after admission, which accounted for 87.95% (73 of 83) of patients with AKI. The median initial day was 1 (IQR 1–2), similar to a study in which reported AKI developed early after ICU admission, with a median of 2 (IQR 1–4) days.20 AKI is an early nonneurological complication that mainly occurs within 7 days after brain injury. The special early phase of AKI development may be due to concomitant pathophysiological processes in the early phase following injury, including massive bleeding–induced hypoperfusion, systemic inflammation caused by initial and secondary brain injury, and rapid and heavy use of hyperosmotic drugs once admitted.5,21–23 This fact underlines the need to evaluate risk of developing AKI and consequently adjust treatment decisions for TBI patients as early as possible. Future studies need to be conducted to discover risk factors of AKI in the prehospital period and construct valuable risk scores based on these factors. Among the three AKI stages, stage 1 accounted for a majority of TBI patients with AKI, consistent with previous studies.10,11,20 Additionally, AKI stage was significantly associated with mortality in patients in our study. A higher AKI stage, indicating worse renal function, may affect prognosis by reflecting injury severity and aggravating brain edema and and hence prescription of nephrotoxic antibiotics to prevent infection.
In our study, AKI duration of 1 day accounted for 50.60% (42 of 83) of TBI patients with AKI, which indicated most AKI events in TBI were transient. This may partially be due to close attention to serum-creatinine levels and subsequent treatment adjustment, such as changing hypertonic drugs and avoidance of nephrotoxic antibiotics. In addition, AKI duration was not correlated with mortality after adjusting for confounding effects. Most of previous studies have confirmed the association between prolonged AKI duration and poorer prognosis in various kinds of patients, including diabetics, those undergoing cardiac surgery, and the critically ill patients.20,24–27 However, another two studies verified that AKI duration was not associated with mortality in critically ill patients.28,29 We considered that the correlation between AKI duration and outcome may be confounded by illness severity, demographic characteristics, underlying diseases, and AKI stage. Compared with transient AKI, persistent AKI may more likely present as high-stage AKI due to the ongoing process of high-stage AKI development. Therefore, the association between AKI duration and outcomes should be viewed with cautioun, taking into account the aforementioned confounding factors, especially AKI stage.
Calculated by the sum of each AKI stage multiplied by duration at each stage, AKI burden could collectively reflect the degree and duration of AKI. One study found that a prognostic model including AKI burden outperformed models incorporating AKI presence, severity, or duration in critically ill patients.12 Another study revealed that the AUC of a prognostic model incorporating AKI duration and stage together was higher than one including AKI stage alone, but not including AKI duration alone in critically ill patients after non–cardiac surgery.30 Similarly to findings of that study, our results indicated AKI duration and burden were not significantly associated with mortality in TBI patients. The AUC of AKI burden in our study was 0.644, higher than the 0.643 for AKI duration or highest AKI stage alone, yet without statistical significance. Perhaps studies with larger samples could further verify differences in AUCs among AKI burden, duration, or highest stage. Due to the insignificance of association between AKI duration and mortality in our study, the AKI burden calculated by multiplying AKI stage with AKI duration showed no statistical significance in relation to mortality on multivariate logistic regression either.
The prognostic model composed of GCS, SDH, and glucose had an AUC of 0.860, though AKI occurrence and highest AKI stage were associated with mortality on multivariate logistic regression. Incorporating AKI occurrence or highest AKI stage did not improve the predictive value of the original three-factor model, which means AKI did not provide additive prognostic value for outcomes. The insignificant statistical difference in AUC between the three factors model and AKI-included models may be due to the insufficient sample size in our study. Further studies with larger samples could be done to verify the additive prognostic value of AKI on outcomes of TBI patients.
This study has several limitations. Firstly, it was a single-center study with moderate sample size. Therefore, selection bias was inevitable and the generalization of conclusions is limited. Future studies conducted in multiple centers with larger samples need to be conducted to verify our conclusions. Secondly, we could not obtain the baseline value of serum creatinine before TBI, due to the retrospective nature of the study and characteristics of acute illness. This limitation is hard to avoid in research on TBI. The inclusion criterion of the time limit in this study was 6 hours after injury to include generalized and broad TBI patients as much as possible. However, the interval between suffering initial injury and obtaining blood samples could influence the baseline serum-creatinine level. Thirdly, AKI was diagnosed based on KDIGO guidelines using only creatinine criteria. Due to the lacke of records on urine output in the electronic system in our hospital, we could not evaluate AKI according to urine output and thus may have underestimated the prevalence of AKI among our patients.
Conclusion
The first three days after admission was the high-incidence period of AKI in TBI patients. AKI occurrence and highest AKI stage were associated with mortality in TBI patients, while AKI duration and AKI burden were not associated with mortality. Future studies with larger samples are needed to verify our conclusions.
Ethics
This study was approved by the ethics committee of West China Hospital (approval ID 2018-359) and performed based on the Declaration of Helsinki. Informed-consent forms were signed by the patients themselves or their legally authorized representatives before study commencement.
Funding
This study was funded by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18007), Key Research and Development Project of the Science and Technology Department of Sichuan Province (2019YFS0392), Popularization and Project Scientific Research Project of the Sichuan Health Commission (20PJ041), the Postdoctoral Research Project, Sichuan University (2021SCU12027), and the Postdoctoral Research Project, West China Hospital, Sichuan University (2019HXBH094).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Astarabadi M, Khurrum M, Asmar S, et al. The impact of non-neurological organ dysfunction on outcomes in severe isolated traumatic brain injury. J Trauma Acute Care Surg. 2020;89(2):405–410. doi:10.1097/TA.0000000000002771
2. Krishnamoorthy V, Temkin N, Barber J, et al. Association of early multiple organ dysfunction with clinical and functional outcomes over the year following traumatic brain injury: a transforming research and clinical knowledge in traumatic brain injury study. Crit Care Med. 2021;49(10):1769–1778. doi:10.1097/CCM.0000000000005055
3. Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33(3):654–660. doi:10.1097/01.CCM.0000155911.01844.54
4. Zygun D, Berthiaume L, Laupland K, Kortbeek J, Doig C. SOFA is superior to MOD score for the determination of non-neurologic organ dysfunction in patients with severe traumatic brain injury: a cohort study. Critical Care. 2006;10(4):R115. doi:10.1186/cc5007
5. An S, Luo H, Wang J, et al. An acute kidney injury prediction nomogram based on neurosurgical intensive care unit profiles. Ann Transl Med. 2020;8(5):194. doi:10.21037/atm.2020.01.60
6. Zygun D. Non-neurological organ dysfunction in neurocritical care: impact on outcome and etiological considerations. Curr Opin Crit Care. 2005;11(2):139–143. doi:10.1097/01.ccx.0000155356.86241.c0
7. Lim HB, Smith M. Systemic complications after head injury: a clinical review. Anaesthesia. 2007;62(5):474–482. doi:10.1111/j.1365-2044.2007.04998.x
8. Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010;32(9):1060–1065. doi:10.3109/0886022X.2010.510234
9. Li N, Zhao WG, Xu FL, Zhang WF, Gu WT. Neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury in patients with traumatic brain injury. J Nephrol. 2013;26(6):1083–1088. doi:10.5301/jn.5000282
10. Ahmed M, Sriganesh K, Vinay B, Umamaheswara Rao GS. Acute kidney injury in survivors of surgery for severe traumatic brain injury: incidence, risk factors, and outcome from a tertiary neuroscience center in India. Br J Neurosurg. 2015;29(4):544–548. doi:10.3109/02688697.2015.1016892
11. Li N, Zhao WG, Zhang WF. Acute kidney injury in patients with severe traumatic brain injury: implementation of the acute kidney injury network stage system. Neurocrit Care. 2011;14(3):377–381. doi:10.1007/s12028-011-9511-1
12. Wiersema R, Eck RJ, Haapio M, et al. Burden of acute kidney injury and 90-day mortality in critically ill patients. BMC Nephrol. 2019;21(1):1. doi:10.1186/s12882-019-1645-y
13. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184.
14. Chen YT, Chan CK, Li WY, et al. Renin-angiotensin-aldosterone system inhibition decreased contrast-associated acute kidney injury in chronic kidney disease patients. J Formos Med Assoc. 2021;120:641–650.
15. Giannoudis PV, Harwood PJ, Loughenbury P, Van Griensven M, Krettek C, Pape HC. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff predict a SIRS state? J Trauma. 2008;65(3):646–652.
16. Staubli SM, Schäfer J, Rosenthal R, Zeindler J, Oertli D, Nebiker CA. The role of CRP and Pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci Rep. 2019;9(1):18340. doi:10.1038/s41598-019-54910-8
17. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18–24. doi:10.1097/SLA.0b013e318256be72
18. Kumar AB, Shi Y, Shotwell MS, Richards J, Ehrenfeld JM. Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: a retrospective analysis. Neurocrit Care. 2015;22(2):184–191. doi:10.1007/s12028-014-0067-8
19. Kim MY, Park JH, Kang NR, et al. Increased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial hemorrhage. J Neurosurg. 2014;120(6):1340–1348. doi:10.3171/2013.12.JNS13888
20. Robba C, Banzato E, Rebora P, et al. Acute kidney injury in traumatic brain injury patients: results from the collaborative European neurotrauma effectiveness research in traumatic brain injury study. Crit Care Med. 2021;49(1):112–126. doi:10.1097/CCM.0000000000004673
21. Sadan O, Singbartl K, Kraft J, et al. Low-chloride- versus high-chloride-containing hypertonic solution for the treatment of subarachnoid hemorrhage-related complications: the ACETatE (A low ChloriE hyperTonic solution for brain Edema) randomized trial. J Intensive Care. 2020;8:32. doi:10.1186/s40560-020-00449-0
22. Büttner S, Stadler A, Mayer C, et al. Incidence, risk factors, and outcome of acute kidney injury in neurocritical care. J Intensive Care Med. 2020;35(4):338–346. doi:10.1177/0885066617748596
23. Deng Y, Yuan J, Chi R, et al. The incidence, risk factors and outcomes of postoperative acute kidney injury in neurosurgical critically ill patients. Sci Rep. 2017;7(1):4245. doi:10.1038/s41598-017-04627-3
24. Hatton GE, Harvin JA, Wade CE, Kao LS. Importance of duration of acute kidney injury after severe trauma: a cohort study. J Trauma Acute Care Surg. 2021;6(1):e000689. doi:10.1136/tsaco-2021-000689
25. Alobaidi R, Anton N, Burkholder S, et al. Association between acute kidney injury duration and outcomes in critically ill children. Pediatr Crit Care Med. 2021;22(7):642–650. doi:10.1097/PCC.0000000000002679
26. Han SS, Kim S, Ahn SY, et al. Duration of acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol. 2013;14:133. doi:10.1186/1471-2369-14-133
27. Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90(4):1142–1148. doi:10.1016/j.athoracsur.2010.04.039
28. Perinel S, Vincent F, Lautrette A, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43(8):e269–e275. doi:10.1097/CCM.0000000000001077
29. Federspiel CK, Itenov TS, Mehta K, Hsu RK, Bestle MH, Liu KD. Duration of acute kidney injury in critically ill patients. Ann Intensive Care. 2018;8(1):30. doi:10.1186/s13613-018-0374-x
30. Wu HC, Wang WJ, Chen YW, Chen HH. The association between the duration of postoperative acute kidney injury and in-hospital mortality in critically ill patients after non-cardiac surgery: an observational cohort study. Ren Fail. 2015;37(6):985–993. doi:10.3109/0886022X.2015.1044755
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



