Back to Journals » Clinical Interventions in Aging » Volume 12
Inappropriate pharmacological treatment in older adults affected by cardiovascular disease and other chronic comorbidities: a systematic literature review to identify potentially inappropriate prescription indicators
Authors Lucenteforte E, Lombardi N, Vetrano DL, La Carpia D, Mitrova Z, Kirchmayer U , Corrao G , Lapi F, Mugelli A, Vannacci A
Received 18 March 2017
Accepted for publication 28 July 2017
Published 19 October 2017 Volume 2017:12 Pages 1761—1778
DOI https://doi.org/10.2147/CIA.S137403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Ersilia Lucenteforte,1 Niccolò Lombardi,1,* Davide Liborio Vetrano,2,* Domenico La Carpia,2,* Zuzana Mitrova,3 Ursula Kirchmayer,3 Giovanni Corrao,4 Francesco Lapi,5 Alessandro Mugelli,1 Alfredo Vannacci1
On behalf of the Italian Group for Appropriate Drug prescription in the Elderly (I-GrADE)
1Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA), University of Florence, Florence, Italy; 2Department of Geriatrics Catholic University, Rome, Italy; 3Department of Epidemiology, ASL 1 Rome, Italy; 4Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milan, Italy; 5Epidemiology Unit, ARS Toscana, Florence, Italy
*These authors contributed equally to this work
Abstract: Avoiding medications in which the risks outweigh the benefits in the elderly patient is a challenge for physicians, and different criteria to identify inappropriate prescription (IP) exist to aid prescribers. Definition of IP indicators in the Italian geriatric population affected by cardiovascular disease and chronic comorbidities could be extremely useful for prescribers and could offer advantages from a public health perspective. The purpose of the present study was to identify IP indicators by means of a systematic literature review coupled with consensus criteria. A systematic search of PubMed, EMBASE, and CENTRAL databases was conducted, with the search structured around four themes and combining each with the Boolean operator “and”. The first regarded “prescriptions”, the second “adverse events”, the third “cardiovascular conditions”, and the last was planned to identify studies on “older people”. Two investigators independently reviewed titles, abstracts, full texts, and selected articles addressing IP in the elderly affected by cardiovascular condition using the following inclusion criteria: studies on people aged ≥65 years; studies on patients with no restriction on age but with data on subjects aged ≥65 years; and observational effectiveness studies. The database searches produced 5,742 citations. After removing duplicates, titles and abstracts of 3,880 records were reviewed, and 374 full texts were retrieved that met inclusion criteria. Thus, 49 studies reporting 32 potential IP indicators were included in the study. IP indicators regarded mainly drug–drug interactions, cardio- and cerebrovascular risk, bleeding risk, and gastrointestinal risk; among them, only 19 included at least one study that showed significant results, triggering a potential warning for a specific drug or class of drugs in a specific context. This systematic review demonstrates that both cardiovascular and non-cardiovascular drugs increase the risk of adverse drug reactions in older adults with cardiovascular diseases.
Keywords: inappropriate prescriptions, elderly, cardiovascular diseases, chronic diseases, systematic review
Introduction
The world population is aging at a rapid rate, in high- and low-income countries, challenging health care services from both the organizational and the economic point of view. Throughout the world, the number of people over 60 years doubled in the last century and in Europe, for example, the share of older population is expected to peak at up to 30% by 2050.1 Such epidemiological transition drives the pressing burden of the increasing prevalence of chronic diseases in this age group.2 In addition to the complexities related to the clinical management of older people suffering from multiple chronic diseases, one of the challenges physicians are facing is the consequent complication of complex pharmacological regimens.
Even if the potential benefits of pharmacological therapy are unquestionable, the hazards of negative drug-related outcomes often raise relevant concerns in older adults. Polypharmacy increases the risk of drug–drug and drug–disease interactions, and age-related changes in several physiological characteristics, as well as the presence of chronic illnesses (eg, chronic kidney or liver disease), may affect drugs’ pharmacokinetics and pharmacodynamics. Such issues potentially increase the risk of adverse drug reactions (ADRs) and explain the significant excess of morbidity, mortality rate, and health care costs within the older population.
In this context, what constitutes an appropriate or inappropriate prescription (IP) in the context of the geriatric population is still debated. Indeed, in order to identify inappropriate pharmacological prescriptions, different criteria have been proposed in recent years. The best known are the Beers criteria,3 the Screening Tool of Older People’s Prescriptions (STOPP), Screening Tool to Alert to Right Treatment (START),4 as well as the Medication Appropriateness Index (MAI),5 and the Assessing Care of Vulnerable Elderly (ACOVE)6 criteria. These criteria and tools are based on expert consensus and are not specifically tailored to any particular disease, even though stroke, myocardial infarction (MI), and other cardiovascular disorders constitute the most frequently treated clinical conditions by physicians in Western countries. Moreover, their impact has not been exhaustively validated toward “hard” end points, and they do not comprise an accurate selection and validation process of drug–drug interactions in light of overlying comorbidities.
Thus, the definition of IP indicators for older adults affected by cardiovascular disease and chronic comorbidities could be extremely useful for the prescriber and might offer advantages from a public health perspective. The aim of the present review was to identify and suggest to the scientific community a list of potential indicators for older adults suffering from cardiovascular diseases and other chronic comorbidities, to be subsequently validated in an ad hoc selected population sample, and eventually proposed as IP indicators. More specifically, we identified all the studies reporting a suspect of drug-related harm in the context of multimorbid older adults suffering from cardiovascular diseases and clustered them according to homogenous groups. Cardiovascular diseases are defined according to the World Health Organization as
a group of disorders of the heart and blood vessels and include coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, and deep vein thrombosis and pulmonary embolism.60
Methods
We performed this systematic review in keeping with the Cochrane Handbook for Systematic Reviews and reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol was registered a priori on PROSPERO (N CRD42017057795).
Data source and search strategy
We conducted a systematic search of PubMed, EMBASE, and CENTRAL databases up to October 2, 2014. A librarian (ZM) structured the search on free text and MESH terms with regard to four different domains: “prescriptions”, “adverse events”, “cardiovascular conditions”, and “older people”.
The PubMed search was ((“Drug Prescriptions”[MeSH] OR “Drug Utilization”[MeSH] OR “Adverse Drug Reactions”[tiab] OR “adverse drug events”[tiab] OR “drug safety”[tiab] OR “drug-drug interactions” OR ADRs[tiab] OR “Drug Interactions”[MeSH] OR ((inappropriate*[tiab] OR incorrect*[tiab] OR excess*[tiab] OR harmful*[tiab]) AND (medici*[tiab] OR prescrib*[tiab] OR prescription*[tiab] OR drug*[tiab] OR refill*[tiab] OR claim*[tiab])) OR “Drug-Related Side Effects and Adverse Reactions”[Mesh] OR ((“drug induced”[tiab] OR medication*[ti] OR prescription*[tiab]) AND (“adverse effects” [Subheading:NoExp] OR “adverse effects”[tiab] OR “adverse events”[tiab] OR mortality[sh])))) AND (“Cardiovascular Diseases”[Mesh:noexp] OR “Stroke”[MeSH] OR “Arrhythmias, Cardiac”[MeSH] OR “Hypertension”[MeSH] OR “Heart Diseases”[MeSH] OR “Brain Ischemia”[MeSH] OR “Brain Infarction”[MeSH] OR “Myocardial Ischemia”[MeSH] OR “Peripheral Arterial Disease”[MeSH] OR “Angina Pectoris”[MeSH] OR cardiovascular[tiab] OR “heart disease”[tiab] OR “heart diseases”[tiab] OR “coronary disease”[tiab] OR “coronary diseases”[tiab] OR “heart failure”[tiab] OR “cardiac failure”[tiab] OR “all cause mortality” OR cerebrovascular[tiab])) AND (Aged[Mesh] OR “old people”[tiab] OR “older people”[tiab] OR “old age”[tiab] OR “older age”[tiab] OR “older person”[tiab] OR “old person”[tiab] OR geriatric*[tiab] OR elder*[tiab] OR senior*[tiab]).
Identical searches were conducted in EMBASE and CENTRAL databases.
Study selection
Two trained investigators (NL and DLV) independently reviewed titles and abstracts, and excluded papers using the following criteria:
- Studies published in languages other than English
- Studies on pediatric population
- Studies regarding exposures other than drugs
- Studies on diseases other than cardiovascular ones (eg, patients with cancer without cardiovascular disease, with Parkinson without cardiovascular disease, and with diabetes without cardiovascular disease)
- Non-outcome studies.
The same investigators independently reviewed full texts and selected articles addressing inappropriate prescribing in elderly patients affected by cardiovascular condition using the following inclusion criteria:
- Studies on people aged ≥65 years
- Studies on patients with no restriction of age but with data on subjects aged ≥65 years
- Observational effectiveness studies.
We resolved disagreement by discussion and consensus with a third trained assessor (DLC). Additionally, we reviewed the reference lists of the included studies and previous reviews to identify additional papers that met inclusion criteria.
Data extraction and quality assessment
For each selected study, we extracted the following data: year of publication, study design, drugs, outcomes, country and setting, characteristic of the study population (eg, sample size, age, and gender), information on follow-up, and main results (ie., estimated with corresponding confidence intervals for each outcome) and additional results.
Two investigators (NL and DLC) independently assessed the methodological quality of included studies using the Newcastle–Ottawa Scale (NOS)7 for case–control and cohort study, the scale proposed by Jadad et al for randomized controlled trials,8 and the following criteria for case-crossover studies and self-controlled case series:
- Clearly stated aims
- Appropriate methods are used
- Well constituted context of the study
- Clearly described, valid, and reliable results
- Clearly described analysis
- Possible influences of the outcome are considered
- Conclusion is linked to the aim, analysis, and interpretation of results of the study
- Limitations on research are identified.
Results
The PRISMA flow diagram of study selection is shown in Figure 1. The database search produced 5,742 citations. After removal of duplicates, we reviewed titles and abstracts of 3880 records, among which 374 met the inclusion criteria and the corresponding full texts were retrieved and reviewed. Subsequently, 325 studies were removed because they did not present analysis for patients aged ≥65 years (258 papers) and had inappropriate study design (50), or for other reasons (6 were not original studies, 4 were on patients without cardiovascular disease, 2 with no safety outcomes, 2 with efficacy outcomes only, 2 were duplicate publications, and 1 was on exposure other than drug).
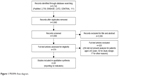  | Figure 1 PRISMA flow diagram. |
Supplementary material reports the characteristics of the 49 selected studies grouped according to 32 homogeneous potential IP indicators.9–57 Briefly, among the selected studies, two investigated bisphosphonates; seven investigated statins alone or in combination with ezetimibe and their interactions with other pharmacological agents (clopidogrel, vitamin K antagonists, and macrolides); eight investigated antipsychotics; one investigated long-acting beta-adrenoceptor agonist (LABAs) and long-acting anticholinergic drugs (LAAs); four regarded antidiabetics; one was on aspirin in association with clopidogrel and enoxaparin; two regarded anticholinergic drugs; one was on donepezil and its interactions with the antibacterial clarithromycin; two regarded calcium channel blockers (CCBs; short-acting nifedipine) and their interaction with Cytochrome P450 3A4 (CYP3A4) inhibitors; four regarded clopidogrel and its interactions with proton-pump inhibitors (PPIs); three regarded nonsteroidal anti-inflammatory drugs (NSAID); four regarded oral anticoagulants (OACs); one regarded postmenopausal hormones; one was on opioids; one investigated angiotensin-converting-enzyme (ACE) inhibitors; four investigated antidepressants; one investigated cholinesterase inhibitors; one was on benzodiazepines and benzodiazepine-related drugs; and one investigated the angiotensin receptor blocker olmesartan alone or in combination with other antihypertensive drugs.
Among the 32 homogeneous potential IP indicators, only 19 included at least one study that showed significant and direct association with adverse events (Table 1).
In greater detail, the potentially identified IP indicators were:
- Anticholinergics (No 1) were associated with cardiac arrhythmia, constipation, delirium, emergency visit, and hospitalization.
- Anticholinergics in cardiovascular patients (No 2) were associated with hospitalization.
- Antidepressants (No 3) were associated with attempted suicide/self-harm, epilepsy seizures, falls, fractures, hyponatremia, MI, mortality, stroke/transient ischaemic attack, upper gastrointestinal bleeding, and ventricular arrhythmia.
- Antidepressants in coronary artery disease (CAD) patients (No 4) were associated with cerebrovascular events.
- Antidiabetics (No 5) were associated with acute MI, atherosclerotic vascular heart disease, congestive heart failure (HF), and mortality.
- Antidiabetics in end-stage renal disease or disabled patients (No 6) were associated with HF, mortality, and stroke.
- Typical antipsychotics (No 7) were associated with cardiovascular death, cerebrovascular events, nervous system disorders, non-cancer death, respiratory disorders, and stroke. Atypical antipsychotics were also associated with mortality and MI.
- Typical antipsychotics in dementia patients (No 8) were associated with mortality, and MI.
- Bisphosphonates in fracture patients (No 12) were associated with atrial fibrillation (AF).
- CCBs in hypertensive patients (No 15) were associated with stroke.
- Cholinesterase inhibitors in dementia patients (No 16) were associated with bradycardia, hip fractures, permanent pacemaker insertion, and syncope.
- Clopidogrel + PPIs (No 17) were associated with MI, major cardiovascular events, and/or all-cause mortality.
- LABA and LAA in chronic obstructive pulmonary disease (COPD) patients (No 19) were associate with acute coronary syndrome and HF. LAA were also associated with cardiac arrhythmia.
- NSAIDs (No 21) were associated with mortality following upper gastrointestinal events, MI and cerebrovascular events, stroke, acute MI or stroke, or death from coronary heart disease.
- OACs in CAD patients (No 23) were associated with embolic and hemorrhagic events, gastrointestinal injuries, and mortality.
- Opioids (No 25) were associated with MI.
- Statins + Macrolides (No 28) were associated with acute kidney injury, mortality, and rhabdomyolysis.
- Statins in COPD patients (No 30) were associated with mortality.
- Warfarin + potentially interacting drugs (No 32) were associated with bleeding.
Studies had a good quality (NOS: 9 or 8/9, quality assessment: 7 or 8/8, Jadad: 4/5) in 14 out of 49 cases (29%), moderate (NOS: 7 or 6/9) in 30 cases (61%), and low (NOS: <6/9) in 5 cases (10%).
Discussion
The present systematic review led to the selection of 32 groups of studies indicating potential drug-related harm in older people with cardiovascular diseases. Among them, only 19 included at least one study that showed significant and direct association with adverse events, triggering a potential warning for a specific drug or class of drugs in a specific context. According to the authors of the present review, these 19 groups can be deemed as potential indicators of IP in multimorbid older adults affected by cardiovascular diseases.
The optimization of pharmacological therapy is an essential part of the process of care for an older person. In the past 20 years, several expert panels in Canada, the USA, and Europe have developed different sets of criteria useful for making quality assessments of prescribing practices and medication use in older adults and potentially helpful during the process of medication review. The most widely used criteria for inappropriate medications are the Beers criteria,3 initially developed in 1991 in the USA to target nursing home residents and then revised in 1997, 2003, 2012, and most recently in 2015. These criteria include more than 50 medications assigned to one of three possible categories: those that should always be avoided, those that are potentially inappropriate in older adults with particular health conditions or syndromes, and those that should be used with caution. It has been shown that potentially inappropriate medications included in the Beers criteria are associated with poor health outcomes such as confusion, falls, and mortality. Another important set of criteria is represented by START/STOPP4 which were first published in 2008 and last updated in 2014. STOPP criteria identify prescriptions that are potentially inappropriate to use in patients aged ≥65 years, while START criteria list drug therapies that should be considered where no contraindication to prescription exists in the same group of patients. Beers and START/STOPP criteria overlap in several areas, making them able to predict ADRs, but often with different reliability.58,59
The list of indicators provided in the present review is intended as a set of potential indicators of IP that need to be tested in the real world through a validation process based on tailored studies to explore health outcomes in different older populations and across different care settings. Eventually, these validation studies might lead to a structural proposal for a new set of criteria of IP in older adults suffering from multiple chronic conditions and affected by cardiovascular diseases. This systematic review represents the first step in the process of validation of new indicators, granted by the Italian Medicine Agency (AIFA) and carried out by the I-GrADE consortium.
Our list of potential indicators partially overlaps those proposed by the Beers and STOPP criteria. Several drugs highlighted in this review, including anticoagulants, antiplatelet, blood pressure lowering medications, and many psychotropic drugs, are listed by at least one of the aforementioned criteria. However, this can be no more than an indirect comparison, considering that this review specifically focuses on multimorbid older people suffering from cardiovascular diseases. However, when the attention of such criteria is focused on specific conditions, the agreement intensifies. For example, Beers criteria include a section of recommendations valid in specific contexts and make the case of HF. They point out NSAIDs, CCBs, thiazolidinediones, cilostazol, and dronedarone as potentially inappropriate medications in older adults suffering from HF. Interestingly, three out of five of these drugs have been included in our list. Several selection criteria beyond the specific selection of a population affected by cardiovascular diseases, and the decision-making process itself, might explain these and other discrepancies.
Several drugs not recommended for the treatment of cardiovascular diseases (but that have a potential role in determining ADRs in people with heart diseases) have been included in our list. Some of them are proposed here for the first time as potentially inappropriate. For example, in the study from Abrahamsen et al10 bisphosphonates showed a possible correlation with AF in patients with an underling cardiac disease. This finding, considering the high prevalence of both osteoporosis and cardiovascular diseases in the older population, represents an interesting area of future research, especially when considering the broad set of bisphosphonates with different pharmacokinetics and pharmacodynamics and the actual possibility of replacing these drugs with compounds recently developed for the treatment of osteoporosis, with a more favorable safety profile and good tolerance.
On the other hand, our research underlines the potential harm linked to drugs that have been synthesized and are recommended for the treatment of cardiovascular diseases. This is the case of statins whose toxicity, according to a research published in 2013 by Patel et al,40 may be exacerbated when co-prescripted with macrolides (especially clarithromycin and erythromycin). Considering the high frequency of use of both classes of drugs related to the prevalence of cardiovascular diseases in elders and the presence of macrolides in first-line therapy of community-acquired pneumonia – which is in turn a main cause of hospitalization in patients over 65 – it is very important to clarify the possible effect of such a co-prescription. In fact, the natural decline in renal function that accompanies aging may exaggerate the consequences of a rhabdomyolysis with a dramatic increase in the frequency of acute kidney failure and an excess of mortality.
As in the most recent 2015 version of Beers criteria, we took into account some drug–disease or drug–syndrome interactions. Some of them are well known and have been extensively explored in the literature, as is the case for antipsychotics and dementia, while others are completely new (ie., antidiabetics and stage renal disease or disability), thus opening the way to new and interesting knowledge acquisitions or future research areas.
To our knowledge, the present work is the first time a systematic review of studies has reported any kind of association between drug use and ADRs in multimorbid older adults suffering from cardiovascular diseases. However, the results we report should be read keeping in mind some limitations. First, we did not include any study assessing under use of medications, and it is now clear that underprescribing appropriate medications can be as great a concern as is overprescribing. Prescribing strategies that seek to simply limit the overall number of drugs prescribed to older adults in the name of improving quality of care may be seriously misdirected. Second, considering the broad and complex spectrum of scenarios existing when it comes to multimorbid older adults, and the heterogeneity of studies present in the literature, our search strategy might have missed some relevant hits. However, bibliographies of the selected papers were scrutinized in an attempt to reduce such occurrence. Third, the heterogeneity of study methodologies and care settings precludes the direct translation of our findings in definitive criteria of IP. However, this was an a priori assumption that suggests the setting up and running of ad hoc studies aimed at validating the criteria suggested here. Finally, a judgment of appropriateness cannot be issued on the basis of an all-or-nothing principle, but we should consider dose-dependent appropriateness of every drug for every target population. In this regard, none of the possible indicators relates to drug dosage, and we know that drug doses can be a main determinant for adverse drug events. Moreover, older patients often present an increased volume of distribution and a decreased drug clearance, which can prolong drug half-lives and lead to increased plasma drug concentrations. In addition, a decline in hepatic function with advancing age may account for significant variability in drug metabolism among older adults.
Other limitations were the exclusion of studies published in languages other than English and a lack of risk-of-bias assessment while quality of reporting was assumed to be directly related to quality of information.
Conclusion
The correct clinical and pharmacological management of complex older adults requires the availability of reliable tools of risk stratification, outcome prediction, and appropriateness of care. According to the present systematic review, both cardiovascular and non-cardiovascular drugs increase the risk of ADRs in older adults with cardiovascular diseases. As part of the I-GrADE consortium, the authors of the present study propose a list of potential indicators of IP for application in the context of multimorbid older adults suffering from cardiovascular diseases. It is worth passing such potential indicators through a validation process carried out in the real-world older population and across different care settings. This is part of the commitment of I-GrADE, and such a process will eventually lead to the publication of a reliable list of indicators of IP tailored to the aforementioned population. This and other efforts by the scientific community are required in the near future in order to cope with the emergency that stems from the rapid aging of the world population and to eventually provide better and more sustainable care to older adults.
Acknowledgment
*I-GrADE members: Alessandra Bettiol, Niccolò Lombardi, Ersilia Lucenteforte, Alessandro Mugelli, Alfredo Vannacci (University of Florence, Florence), Alessandro Chinellato (ULSS 9 Treviso, Treviso), Stefano Bonassi, Massimo Fini, Cristiana Vitale (IRCCS San Raffaele Pisana, Rome), Roberto Bernabei, Graziano Onder, Davide Liborio Vetrano (Catholic University, Rome), Claudia Bartolini, Rosa Gini, Francesco Lapi, Giuseppe Roberto (ARS Toscana, Florence), Nera Agabiti, Silvia Cascini, Marina Davoli, Ursula Kirchmayer, Chiara Sorge (ASL 1 Rome), Giovanni Corrao, Federico Rea (University of Milano-Bicocca, Milano), Achille Patrizio Caputi, Francesco Giorgianni, Michele Tari, and Gianluca Trifirò (University of Messina, Messina).
Disclosure
EL received research support from the Italian Agency of Drug (AIFA), which is not related to this study. AM received research support from the AIFA, the Italian Ministry for University and Research (MIUR), Gilead, and Menarini. In the past 2 years he has received personal fees as speaker/consultant from Menarini Group, IBSA, Molteni, Angelini, and Pfizer Alliance, none of which are related to this study. GC received research support from the European Community (EC), the European Medicine Agency (EMA), the Italian Agency of Drug (AIFA), and the Italian Ministry of Health, and of University and Research (MIUR). He has taken part in a variety of projects that were funded by pharmaceutical companies (ie, Novartis, GSK, Roche, AMGEN, and BMS). He has also received honoraria as member of the Advisory Board from Roche. None of these is related to this study.
AV, in the past 2 years, has received personal fees as consultant from Molteni, which is not related to this study. The authors report no other conflicts of interest in this work.
References
European Commision (2012). Global Europe 2050. Available from: https://ec.europa.eu/research/social-sciences/pdf/policy_reviews/global-europe-2050-report_en.pdf. Accessed July 24, 2017. | ||
Calderon-Larranaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. Epub 2016 Dec 21. | ||
By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. | ||
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. | ||
Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–1051. | ||
Wenger NS, Roth CP, Shekelle P; ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(Suppl 2):S247–S252. | ||
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 22, 2016. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Abraham NS, Castillo DL, Hartman C. National mortality following upper gastrointestinal or cardiovascular events in older veterans with recent nonsteroidal anti-inflammatory drug use. Aliment Pharmacol Ther. 2008;28(1):97–106. | ||
Abrahamsen B, Eiken P, Brixen K. Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med. 2009;265(5):581–592. | ||
Blagojevic A, Delaney JA, Levesque LE, Dendukuri N, Boivin JF, Brophy JM. Investigation of an interaction between statins and clopidogrel after percutaneous coronary intervention: a cohort study. Pharmacoepidemiol Drug Saf. 2009;18(5):362–369. | ||
Blanchette CM, Simoni-Wastila L, Zuckerman IH, Stuart B. A secondary analysis of a duration response association between selective serotonin reuptake inhibitor use and the risk of acute myocardial infarction in the aging population. Ann Epidemiol. 2008;18(4):316–321. | ||
Caughey GE, Roughead EE, Pratt N, Killer G, Gilbert AL. Stroke risk and NSAIDs: an Australian population-based study. Med J Aust. 2011;195(9):525–529. | ||
Chan MC, Chong CS, Wu AY, et al. Antipsychotics and risk of cerebrovascular events in treatment of behavioural and psychological symptoms of dementia in Hong Kong: a hospital-based, retrospective, cohort study. Int J Geriatr Psychiatry. 2010;25(4):362–370. | ||
Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. | ||
Enajat M, Teerenstra S, van Kuilenburg JT, et al. Safety of the combination of intensive cholesterol-lowering therapy with oral anticoagulation medication in elderly patients with atrial fibrillation: a randomized, double-blind, placebo-controlled study. Drugs Aging. 2009;26(7):585–593. | ||
Franchi C, Sequi M, Tettamanti M, et al. Antipsychotics prescription and cerebrovascular events in Italian older persons. J Clin Psychopharmacol. 2013;33(4):542–545. | ||
Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175–1185. | ||
Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–873. | ||
Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS. Mortality associated with benzodiazepines and benzodiazepine-related drugs among community-dwelling older people in Finland: a population-based retrospective cohort study. Can J Psychiatry. 2011;56(6):377–381. | ||
Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS. Effect of comorbidity on the risk of death associated with antipsychotic use among community-dwelling older adults. Int Psychogeriatr. 2012;24(7):1058–1064. | ||
Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304(4):411–418. | ||
Hartle JE, Tang X, Kirchner HL, et al. Bisphosphonate therapy, death, and cardiovascular events among female patients with CKD: a retrospective cohort study. Am J Kidney Dis. 2012;59(5):636–644. | ||
Heer T, Juenger C, Gitt AK, et al; Acute Coronary Syndromes (ACOS) Registry Investigators. Efficacy and safety of optimized antithrombotic therapy with aspirin, clopidogrel and enoxaparin in patients with non-ST segment elevation acute coronary syndromes in clinical practice. J Thromb Thrombolysis. 2009;28(3):325–332. | ||
Huang KH, Chan YF, Shih HC, Lee CY. Relationship between potentially inappropriate anticholinergic drugs (PIADs) and adverse outcomes among elderly patients in Taiwan. J Food Drug Anal. 2012;20(4):930–937+985. | ||
Hutson JR, Fischer HD, Wang X, et al. Use of clarithromycin and adverse cardiovascular events among older patients receiving donepezil: a population-based, nested case-control study. Drugs Aging. 2012;29(3):205–211. | ||
Jung SY, Choi NK, Kim JY, et al. Short-acting nifedipine and risk of stroke in elderly hypertensive patients. Neurology. 2011;77(13):1229–1234. | ||
Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180(7):713–718. | ||
Kulik A, Singh JP, Levin R, Avorn J, Choudhry NK. Association between statin use and the incidence of atrial fibrillation following hospitalization for coronary artery disease. Am J Cardiol. 2010;105(12):1655–1660. | ||
LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci. 2008;63(4):369–375. | ||
Lawes CM, Thornley S, Young R, et al. Statin use in COPD patients is associated with a reduction in mortality: a national cohort study. Prim Care Respir J. 2012;21(1):35–40. | ||
Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J Intern Med. 2013;273(5):511–526. | ||
Liperoti R, Onder G, Landi F, et al. All-cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia: a retrospective cohort study. J Clin Psychiatry. 2009;70(10):1340–1347. | ||
Lokkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard O. Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J. 2008;29(21):2660–2668. | ||
Macchia A, Romero M, D’Ettorre A, Tognoni G, Mariani J. Exploratory analysis on the use of statins with or without n-3 PUFA and major events in patients discharged for acute myocardial infarction: an observational retrospective study. PLoS One. 2013;8(5):e62772. | ||
Mahabaleshwarkar RK, Yang Y, Datar MV, et al. Risk of adverse cardiovascular outcomes and all-cause mortality associated with concomitant use of clopidogrel and proton pump inhibitors in elderly patients. Curr Med Res Opin. 2013;29(4):315–323. | ||
Margolis DJ, Hoffstad O, Strom BL. Association between serious ischemic cardiac outcomes and medications used to treat diabetes. Pharmacoepidemiol Drug Saf. 2008;17(8):753–759. | ||
Mujib M, Patel K, Fonarow GC, et al. Angiotensin-converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med. 2013;126(5):401–410. | ||
Pariente A, Fourrier-Reglat A, Ducruet T, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med. 2012;172(8):648–653; discussion 654–655. | ||
Patel AM, Shariff S, Bailey DG, et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann Intern Med. 2013;158(12):869–876. | ||
Poli D, Antonucci E, Testa S, et al. Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation. 2011;124(7):824–829. | ||
Pratt NL, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for stroke associated with antipsychotic use in the elderly: a self-controlled case series. Drugs Aging. 2010;27(11):885–893. | ||
Rassen JA, Choudhry NK, Avorn J, Schneeweiss S. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation. 2009;120(23):2322–2329. | ||
Rossini R, Capodanno D, Musumeci G, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis. 2011;22(3):199–205. | ||
Roumie CL, Choma NN, Kaltenbach L, Mitchel EF Jr, Arbogast PG, Griffin MR. Non-aspirin NSAIDs, cyclooxygenase-2 inhibitors and risk for cardiovascular events-stroke, acute myocardial infarction, and death from coronary heart disease. Pharmacoepidemiol Drug Saf. 2009;18(11):1053–1063. | ||
Ruiz Ortiz M, Romo E, Mesa D, et al. Outcomes and safety of antithrombotic treatment in patients aged 80 years or older with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107(10):1489–1493. | ||
Saito I, Kushiro T, Hirata K, et al. The use of olmesartan medoxomil as monotherapy or in combination with other antihypertensive agents in elderly hypertensive patients in Japan. J Clin Hypertens (Greenwich). 2008;10(4):272–279. | ||
Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56(9):1644–1650. | ||
Tanaka M, Tanaka A, Suemaru K, Araki H. The assessment of risk for gastrointestinal injury with anticoagulant and antiplatelet drugs: the possible beneficial effect of eicosapentaenoic Acid for the risk of gastrointestinal injury. Biol Pharm Bull. 2013;36(2):222–227. | ||
Uusvaara J, Pitkala KH, Kautiainen H, Tilvis RS, Strandberg TE. Association of anticholinergic drugs with hospitalization and mortality among older cardiovascular patients: a prospective study. Drugs Aging. 2011;28(2):131–138. | ||
Vanasse A, Carpentier AC, Courteau J, Asghari S. Stroke and cardiovascular morbidity and mortality associated with rosiglitazone use in elderly diabetic patients. Diab Vasc Dis Res. 2009;6(2):87–93. | ||
Vasilyeva I, Biscontri RG, Enns MW, Metge CJ, Alessi-Severini S. Adverse events in elderly users of antipsychotic pharmacotherapy in the province of Manitoba: a retrospective cohort study. J Clin Psychopharmacol. 2013;33(1):24–30. | ||
Vitry AI, Roughead EE, Ramsay EN, et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol Drug Saf. 2011;20(10):1057–1063. | ||
Winkelmayer WC, Setoguchi S, Levin R, Solomon DH. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch Intern Med. 2008;168(21):2368–2375. | ||
Wu CS, Wang SC, Cheng YC, Gau SS. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168(5):511–521. | ||
Yoshida M, Matsumoto T, Suzuki T, Kitamura S, Mayama T. Effect of concomitant treatment with a CYP3A4 inhibitor and a calcium channel blocker. Pharmacoepidemiol Drug Saf. 2008;17(1):70–75. | ||
Zivin K, Pfeiffer PN, Bohnert AS, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry. 2013;170(6):642–650. | ||
Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43(6):767–773. | ||
Wallace E, McDowell R, Bennett K, Fahey T, Smith SM. Impact of potentially inappropriate prescribing on adverse drug events, health related quality of life and emergency hospital attendance in older people attending general practice: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2017;72(2):271–277. | ||
Cardiovascular diseases (CVDs) [webpage on the Internet]. World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed September 21, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





