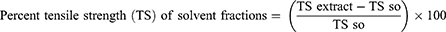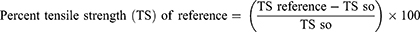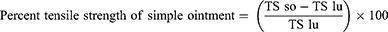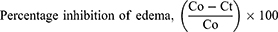Back to Journals » Journal of Experimental Pharmacology » Volume 14
In-vivo Wound Healing and Anti-Inflammatory Activity of the Solvent Fraction of Zehneria scabra L.F. Sond (Cucurbitaceae) Leaves
Authors Fisseha N , Hammeso WW , Nureye D , Tesfaye T, Yimer T
Received 31 August 2022
Accepted for publication 29 November 2022
Published 2 December 2022 Volume 2022:14 Pages 367—377
DOI https://doi.org/10.2147/JEP.S387364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Nebeyi Fisseha,1 Workineh Woldeselassie Hammeso,2 Dejen Nureye,1 Tarekegn Tesfaye,1 Tesfaye Yimer3
1Department of Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, South West Region, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Dilla University, Dilla, Ethiopia; 3Department of Pharmacy, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Nebeyi Fisseha, PO Box: 260, Mizan-Aman, Ethiopia, Tel +251913214835, Email [email protected]
Background: Wounds continue to be a difficult clinical problem, with early and late consequences causing significant morbidity and death. As a result, proper wound management is critical. In addition to contemporary medicine, medicinal herbs serve an essential role in the treatment of wounds and bacterial infections. Z. scabra is a medicinal plant that has traditionally been used to treat wounds. However, there are no scientific reports on solvent fraction wound healing activities. As a result, the current study presents a scientific assessment of the wound healing ability of the solvent fractions of Z. scabra leaves.
Methods: The leaves were crushed and macerated three times in 80% methanol. Chloroform, ethyl acetate, and aqueous fractions of simple ointment at 5% w/w and 10 percent w/w strengths were prepared using the fusion technique based on the British Pharmacopoeia. Excision and incision wound models were used to assess the solvent fractions’ wound healing activities. The anti-inflammatory efficacy of crude and solvent fractions was tested in mice utilizing a carrageenan-induced hindpaw edema model.
Results: In rats, a test dose of 2000 mg/kg of the 10% w/w crude extract ointment was found to be safe. Groups treated with the 5% and 10% ethyl acetate fractions of the extract experienced significant (p< 0.05 and p< 0.01) wound reduction in the excision wound model. When compared to the negative control, the length of epithelization in groups treated with 10% ethyl acetate fraction and aqueous fractions of Z. scabra was statistically significant (p 0.001). By lowering the amount of carrageenan-induced paw edema, the leaf extract and the chloroform fraction of Z. scabra demonstrated a dose-dependent anti-inflammatory effect.
Conclusion: The extract showed remarkable wound healing and anti-inflammatory activity and might be recommended for the treatment of many types of human wounds.
Keywords: wound healing, anti-inflammatory excision, wound model, Zehneria scabra
Introduction
The term “wound” refers to the breakdown of tissue’s anatomic and cellular integrity as a result of chemical, physical, thermal, microbiological, or immunological harm, resulting in a change in normal skin structure and function.1 Wound healing is the body’s natural process of regenerating dermal and epidermal tissue through a coordinated physiological response which provides hemostasis and initiates the processes of inflammation, proliferation, and remodeling.2 Free-radical-mediated oxidative damage, prolonged inflammation, delayed granulation and tissue formation, reduction in angiogenesis, and decrease in collagen reorganization can all have a negative impact on wound healing, resulting in delayed wound healing, increased patient morbidity and mortality, and a poor cosmetic outcome.3 Irrigation, debridement, antibiotics, proteolytic enzymes, and tissue grafts, which are currently accessible wound treatment procedures, have been linked to severe downsides such as invasiveness and cost.4
The emergence of resistant bacteria, as well as a lack of, high cost, and slow rate of development of new antibiotics, all contribute to a rise in wound-related mortality and morbidity. The rise of resistant bacteria strains, particularly those that cause wounds, is a public health issue around the world.5 As more than just a result, infection usually continues to be the most common cause of non-healing wounds and continues to be a considerable burden for both patients and caregivers. Various investigations are being conducted around the world in order to identify compounds that can aid healing and so reduce healthcare costs while also saving the patient from amputation or other serious problems. However, treating persistent wounds with integrative therapy and current medicine is ridiculously expensive.6 Most of the drugs employed in wound treatment are not only costly but also have issues such as hypersensitivity reaction and drug resistance. In addition, among the drugs listed in Western Pharmacopoeia only 1–3% of them are recommended to be used on the skin and wounds; however, approximately one third of herbal medicines are intended for such use.7
A variety of medicinal plants can be used to cure wounds. According to the World Health Organization (WHO), eighty percent of the world’s population still uses plant-based treatments for basic health care, and skin-related disorders, particularly wounds, are the most common reason for medical visits in developing countries.8 Z. scabra L.F. Sond., also known as “Areg-resa” in Amharic, “Etse-sabieq” in Ge’ez, “Daaymii” in Afan Oromo, “Hafaflo” in Tigrigna, and “Kiete” in Gedeo, is one of the most important medicinal climbers in the Cucurbitaceae family. It has received increased attention due to its high medicinal value in herbal folklore practices.9–12 Z. scabra, inhabits forest and on forest margins across 900–2100m above sea level. It is native to South America and widespread in tropical Africa, Ethiopia, South Africa, Arabia, India, Java and the Philippines.13 Z. scabra is a climbing herb that can reach a height of 10 meters, with stem inter-node lengths of 6 to 7 cm and woody, corky-ridged bark as the plant matures. Flowers are greenish-white; fruits are oval, finely reticulate, red when ripe, and seeds are ovate-oblong; leaves are ovate-suborbicular with a cordate base, angular or 3–5 lobed; and seeds are ovate-oblong.14,15
Z. scabra is a traditional Ethiopian medicinal plant that is often used to cure a variety of ailments. For example, the Amhara ethnic community uses the leaf juice to treat fever and headaches.16 The leaves are used to treat paralysis, michi, and external wounds by the people of Tigray’s region, while the root is used to treat abdominal pain and ascariasis. Its blossoms have been mixed with other herbals to treat alopecia, wounds, and eczema on a topical basis. The root is used to treat anemia in the Agew-Awi zone of the Amhara Regional State. Traditional healers in southwest Ethiopia said that the plant’s root contained antimalarial powers. In addition, the 80% methanolic leaves of extract have been proven to have anti-diarrheal, anti-secretary and anti-malarial activities.17 The present experimental plant is traditionally claimed to have wound healing activity, which further investigated by solvent fraction using different solvents polarity index.
Methodss
Collection of Plant Materials
Z. scabra leaves were obtained in the Benchi-Sheko zone, about 561 kilometers far from Addis Ababa, south west of Ethiopia. Botanists at Mizan-Tepi University recognized and authenticated the plant, and a specimen was placed with a voucher number for future reference with the voucher number WWH-001/2021.
Experimental Animals
Swiss albino mice of both sexes (25 ±5 g, 6–8 weeks old) were procured from the Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia, and kept in the Mizan-Tepi University Laboratory of the Department of Pharmacy. The animals were kept in cages in conventional settings, with 12 hours of light and 12 hours of darkness. They were fed a regular pellet diet and had unlimited access to water. They were given a week to acclimate to the laboratory environment before the experiment began.18
Preparation of the Crude Extract
The leaves of Z. scabra were dried and ground into a fine powder that could be extracted using a mortar and pestle. Z. scabra leaves powder sample was weighed (1 kg) and macerated in 80% methanol (1:5) for 3 days at room temperature using a conical flask and periodic shaking. Whatman filter paper was used to filter the macerated plant material. To remove the organic solvent, the filtrates were collected and concentrated using a Rota vapor at 40°C. To eliminate the watery content, the concentrated extract was frozen in a deep freezer and dried in a lyophilizer. Finally, the dried crude extract was placed in a sealed container and preserved in the refrigerator to be used in ointment compositions.19
Solvent Fraction of the Crude Extract
To entirely combine with the solvent Z. scabra leaf crude extract was suspended in distilled water and gently shaken. A separating funnel was used to transfer the mixture. After that, an equal volume of chloroform was added. The new mixture was gently shaken to mix it, then left to settle for a while until it formed two layers, after which the chloroform portion was collected; the method was then performed twice more as described above. To obtain the ethyl acetate fraction, the aqueous residue was separated three times with ethyl acetate. Ethyl acetate was isolated from the aqueous component of the top layer. As the third fraction, the aqueous solution was collected. The residue of ethyl acetate and chloroform was dried in a dry oven at 40°C. The aqueous fraction was frozen overnight in the refrigerator before being dried with a lyophilizer. All fractions were kept in screw cap vials in the refrigerator at −4 °C until they were utilized to make ointments.
Ointment Formulation
The fusion process based on the British Pharmacopoeia was used to prepare chloroform, ethyl acetate, aqueous fractions simple ointment at 5% w/w and 10% w/w strength. Hard paraffin (10 g) was melted in a beaker over a water bath to make the 200 g simple ointment base. After removing the materials from the melting point, cetostearyl alcohol (10 g), white soft paraffin (170 g), and wool fat (10 g) were added in descending order of melting point. All of the components were melted in a water bath while being constantly stirred until they were completely homogenous. The liquid was taken off the fire and swirled until it was completely cool.19
To create chloroform fraction ointment, 10 g and 20 g of powdered fractions were portioned into 190 g and 180 g of simple ointment base to make 5% and 10% (w/w) ointment, respectively, by levigation on the surface of the ointment slab. Finally, throughout the experiment, the fraction ointment was transferred to a clean container for topical application. The control ointment, consisting of 100 gm of the complete basic ingredients, was ingested and handled in the same way as the active ingredient ointment. The ointment preparation for acetate and aqueous fractions followed the same process.
Grouping and Dosing
Eight groups of mice (each containing six) and a circular excision wound model were utilized to assess the wound healing activity of solvent fractions. Group I received simple ointment (served as a negative control); groups II and III received 5% w/w percent and 10% w/w aqueous ointment, respectively; groups IV and V received 5% w/w and 10% w/w ethyl acetate ointment, respectively; groups VI and VII received 5% w/w and 10% w/w chloroform ointment, respectively; and group VIII received 0.2% (served as a positive control).20
There were five groups of six mice each for evaluating anti-inflammatory activity. Group I received normal saline solution; group II received 100 mg/kg body weight solvent fraction solution in saline; group III received 200 mg/kg body weight solvent fraction solution in saline; group IV received 400 mg/kg body weight solvent fraction solution in saline; and group V received 5 mg/kg body weight indomethacin.18
Acute Dermal Toxicity Study
Under the investigation by Tekleyes et al, the acute cutaneous toxicity test of the 80% methanol extract of Z. scabra was performed according to the OECD 404 guideline.19
Preliminary Phytochemical Analyses
Hydroalcoholic crude extract and solvent fraction of Z. scabra leaves will be screened for
the presence of alkaloids, saponins, flavonoids, terpenoids, phenols, steroids, sterols, glycosides and tannins according to standard test procedures.21,22
Evaluation of Wound Healing
Excision and incision wound healing models were used to assess the wound healing activity of chloroform, ethyl acetate, and aqueous fractions.
Excision Wound Model
The mice were given 50 mg/kg ketamine and 5 mg/kg diazepam intraperitoneally. The animals’ dorsal furs were shaved with a shaving machine prior to wound preparation. The fur on their dorso-thoracic area was then removed. A permanent marker was used to create a 314 mm2 circular mark. The circular mark was then removed in full thickness with forceps and scissors to make a wound. This was the first day of the week. The mice were administered as specified in the grouping and dosing from day one.
The same wound area was produced and ointments were applied as stated in the grouping and dosing for the study of wound healing activities of the chloroform, ethyl acetate, and aqueous fractions. All of the preparations were applied to the wound area on a regular basis until the wounds in the test groups healed completely. Every two days, the wound area was measured with a transparent sheet and a permanent marker. The transparent sheet was traced out on to 1 mm2 size graph paper. The duration of epithelialization and percentage of wound contraction were used to evaluate the wound healing activities of solvent fractions.19 The percentage of wound contraction calculated for solvent fractionation is as follows:
Where n, the days when measurement was taken.
Linear Incision Wound Model
All of the mice were anesthetized and shaved in the same way that the mice in the excision wound model were. At a distance of 1 cm from the midline, a three-centimeter-long linear paravertebral incision will be created through the full thickness of the skin on either side of the vertebral column. The skin was kept together and sewn at 1 cm intervals with non-absorbable surgical thread and a curved needle. Day 0 was a really terrible day. The first four groups were treated similarly to the groups in the excision wound model from day one, while the fifth served as an untreated control. The medications were given topically once a day for nine days.
On the eighth day after the wound, the sutures were removed. The extent of healing was measured using tensile strength (the force required to open the healing skin). On day ten, the tensile strength of the groups treated with extracts was compared to the standard, basic ointment, and untreated control groups using a continuous water flow approach, taking into account the gram of water required to break the skin. The tensile strength was calculated as follows.23
Where so=simple ointment and lu=left untreated.
Evaluation of in vivo Anti-Inflammatory Activity
Carrageenan-induced paw edema in mice was used to test the crude extract’s anti-inflammatory efficacy. The mice were fasted overnight and given free access to water, and the basal volume of each mouse’s right hind paw was measured using a plethysmometer before any medicine was given orally. The therapies were then delivered according to the grouping and dose that had been established. Edema was generated in all groups of mice by injecting 0.05 mL of a newly made 1% carrageenan solution in normal saline into the left hind paws. A plethysmometer was used to measure the change in volume of the paw after 1-, 2-, 3-, and 4-hours following carrageenan injection. The average foot swelling in extract-treated mice was compared to the negative control, and the percent inhibition (anti-inflammatory activity) of edema was calculated using the formula below.24
Where Co is the average inflammation of the control group at a given time and Ct is the average inflammation of the plant extract or indomethacin-related mice at the same time
Statistical Analysis
The data was presented as a mean with a standard deviation (SEM). A one-way analysis of variance (ANOVA) was used to compare the results of the different treatments. The results were deemed substantially different at p<0.05. The SPSS software was used to process all of the data. The percentage of wound shrinkage was determined as a percentage of the (original) wound area at 0 day (mm2).
Result
Acute Dermal Toxicity Study
Acute Dermal Toxicity Study was carried out on solvent fractions in accordance with the OECD 404 guideline.19 Mice treated with the extracts up to a level of 2000 mg/kg showed no toxicity or death effects. From the first to the fourteenth day, the animals were watched for tremors, convulsions, salivation, diarrhea, lethargy, sleep irregularities, clinical abnormalities, gross lesions, changes in body weight, and coma. The animals were discovered to be physically active and to be regularly ingesting food and water.
Preliminary Phytochemical Analyses
According to preliminary phytochemical analyses, the hydroalcoholic crude extract of leaves from Z. scabra did not contain anthraquinone or saponins. However, the ethyl acetate fraction included every secondary metabolite that was evaluated. Alkaloids, anthraquinones, saponins, and terpenoids, however, are absent from the chloroform fraction depicted in Table 1.
 |
Table 1 Preliminary Phytochemical Screening of the Hydro Alcoholic Crude Extract and Solvent Fractions of Z. Scabra L.F. Sond (Cucurbitaceae) the Leaves |
Assessing Wound Healing Activity
Excision Wound Model
From day 6 onward, groups treated with 5% and 10% EAFZS experienced significant (p<0.05, p<0.01) wound shrinkage in the excision wound model. When compared to the negative control, mice treated with 10% (w/w) CFZS only began to exhibit appreciable (P<0.05) wound shrinkage on the fourth day. Starting on day 2, the wound contraction rate in the groups treated with 5 and 10% (w/w) AQZS was statistically significantly higher than in the negative control (p<0.01, p<0.001, respectively). The second day forward, there was a comparable diminution in wound area among the groups treated with NF, 5%, and 10% (w/w) AQZS, as shown in Figure 1. The percentage of wound closure was higher in groups treated by 10% (w/w) CEO compared to the 5% (w/w) CEO on all post wounding days but not significant. On the sixteenth day following the injury, a similar proportion of wound closure was seen in the 10% EAF and NF treated groups shown in Figure 2.
Epithelization Period
The duration of epithelization in groups treated with 10% EAFZS and AQFZS was statistically significant (p<0.001) when compared to the NC. Mice treated with 5% EAFZS and AQFZS had a significantly (p<0.01) shorter epithelization period when compared to the negative control. In comparison to other fraction ointments, the group treated with 10% EAFZS and AQFZS had the shortest period of epithelization and the highest percentage decrease in epithelization period Figure 3.
Incision Wound Model
In comparison to the NC shown in Figure 4, the tensile strength of groups treated with 5% (w/w), 10% (w/w), and NF statically significantly (p<0.001) increased. In comparison to the other fractions, the groups treated with 10% (w/w) AQFZS and NF displayed higher percentages of tensile strength.
Zehneria Scabra’s Hydro-Methanolic Leaf Extract and Solvent Fraction of Anti-Inflammatory Activities
When compared to mice fed with distilled water, the hydro-methanolic leaf extract and the chloroform fraction of ZS showed a dose-dependent anti-inflammatory activity by reducing the volume of carrageenan-induced paw edema. As shown in Table 2, the test doses of the hydro-methanolic extract of ZS (100 mg/kg, 200 mg/kg, and 400 mg/kg) demonstrated statistically significant anti-inflammatory actions (p<0.05, p<0.01 and p<0.001 respectively) beginning at the second hour of observation in comparison to the negative control.
 |
Table 2 Effect of Hydro Alcoholic Crude Extract and Solvent Fractions of Z. Scabra on Carrageenan-Induced Paw Edema Model in Mice |
At the fourth time of observation after carrageenan induction, the standard anti-inflammatory drug (indomethacin 25mg/kg) produced a percentage edema inhibition value of 61.5%. At the fourth time of observation, each test dose of the hydro-methanolic extract (100mg/kg, 200mg/kg, and 400mg/kg) demonstrated a maximum percentage of edema inhibition, with values of 37.88%, 48.45%, and 52.8%, respectively. The chloroform fraction also showed a dose-dependent edema inhibition impact on the test doses used (100 mg/kg, 200 mg/kg, and 400 mg/kg) as compared to the negative control groups, with statistical significance (p<0.05, p<0.01, and p<0.001 correspondingly).
The effects of each chloroform fraction test dose peaked during the third time of observation, as shown in Table 2, and began to wane at the fourth. In contrast, at doses of 200 mg/kg and 400 mg/kg, the queues fraction inhibited mice paw thickness statistically significantly (p< 0.05 and p <0.01, respectively), whereas the chloroform fraction inhibited paw edema statistically significantly (p< 0.05).
Discussion
A wound poses a serious risk to one’s health in terms of morbidity and mortality. In order to restore the skin’s functional state and altered anatomical stability, wounds must be properly healed. Rapid wound contraction, a shorter epithelization phase, and a sufficient gain in tensile strength are necessary for rapid wound healing. Due to their powerful biological and therapeutic qualities, herbal medicines are extremely important for improving primary healthcare in both developed and developing nations.25
In comparison to the untreated group, treatment with solvent fractions from Z. scabra leaves demonstrated quicker recovery. Z. scabra leaf may have intervened in one or more stages of the healing process, allowing for alterations in the wound contraction, duration of epithelization, and wound breaking strength. In this investigation, mice treated with 10% (w/w) of the solvent fraction topical preparations demonstrated enhanced wound healing effects in terms of accelerated wound contraction, shortened epithelialization duration, and increased wound tensile strength. The experimental plant’s ability to heal wounds so appears to be treatment related. Additionally, these effects are brought on by the presence of secondary metabolites.
The shorter epithelialization times caused by the 5, 10% CFZS and AQFZS solvent fractions in these studies compared to the negative control might be the result of facilitated epithelial cell proliferation and migration and/or higher viability. Because wound contraction minimizes the size of the wound and the amount of extracellular matrix required to fix the defect, it speeds up the healing process. In addition, by reducing the distance that migrating keratinocytes must cover, contraction promotes re-epithelization.26
When compared to the negative control, incisional wounds treated with the solvent fraction of Z. scabra in 10% AQFZS demonstrated higher tensile strength, which may be a result of increased collagen synthesis per cell and facilitated protein cross-linking.27 The availability of secondary metabolites is what causes these effects.
The simple release of polar molecules from the straightforward ointment may be related to the aqueous fraction’s higher healing efficacy. In comparison to substantially fewer polar molecules, the most polar compounds in the aqueous portion are better liberated from non-polar bases.28
Past studies on Z. scabra revealed a sizable amount of wound healing activity. Studies showed that leaf extract of Z. scabra have wound healing19 anti-inflammatory,29,30 antibacterial,19,29,33 antifungal19,31 and antioxidant32 activities.
According to a study on the wound-healing properties of the extract,19 phytochemical components of Z. scabra leaf extract in 80% methanol were directly accountable for antioxidant, antibacterial, and antifungal activities through several mechanisms. Because of their anti-microbial and free radical scavenging properties, flavonoids and triterpenoids, for instance, are known to hasten the healing of wounds. These properties appear to be responsible for wound contraction and an accelerated rate of epithelialization.26,35
By avoiding or delaying cell necrosis and enhancing vascularity, flavonoids reduce lipid peroxidation, which raises the viability of collagen fibrils by enhancing circulation19 therefore supplying the vital nutrients and oxygen needed for the wound site to recover,36 limiting cell damage, and encouraging DNA synthesis. Contrarily, tannins encourage wound healing by chelating free radicals and reactive oxygen species, decreasing proteins because of their astringent impact, promoting wound contraction, and stimulating the production of capillary vessels and fibroblasts.19 Since, free radicals and the byproducts of oxidative reactions that result from lipid peroxidation can exacerbate tissue damage.34 Terpenoids reinforce skin, boost antioxidant concentration in wounds, have antimicrobial characteristics, and aid in wound healing. They also help recover inflamed tissues by improving blood flow.37 Additionally, saponins, terpenoids, phenols, and steroids have antioxidant, anti-inflammatory, and antibacterial properties.26 Our results suggest that phytoconstituents found in Z. scabra may act singly or in concert to initiate or promote host defense mechanisms involved in wound healing. The extract component(s) responsible for this wound healing effect are still unknown. More phytochemical research is needed to determine the active component(s) causing these pharmacological effects.
Conclusion
Z. scabra leaf extracts encourage the activity of wound healing and anti-inflammatory activity. Its solvent fraction showed exceptional wound healing activity and might be recommended for the management of various types of human wounds. The extract’s capacity for wound healing may be due to the presence of bioactive metabolites. Additional research with purified ingredients is required to fully realize the mechanism of Z. scabra’s wound healing effect.
Abbreviations
ANOVA, analysis of variance; AQZS, aqueous fraction of Z. scabra; CFZS, chloroform fraction of Z. scabra; EAFZS, ethyl acetate fraction of Z. scabra; EPHI, Ethiopian Public Health Institute; OECD, Organization for Economic Co-operation and Development; SEM, standard error of mean; SPSS, statistical software for social science; WHO, World Health Organization; ZS, Zehneria scabra.
Data Sharing Statement
All data pertaining to this study are within the manuscript.
Ethical Approval
The ethical review committee of Mizan-Tepi University’s School of Pharmacy in Mizan Teferi, Ethiopia, examined and approved the application with the approval number (SOP3/114/2021). The experimental animals were cared for and used in accordance with OECD guidelines for laboratory animal care and use.38
Acknowledgments
Mizan-Tepi University’s financial help was warmly welcomed.
Author Contributions
All authors contributed significantly to the work that was compiled, whether it be in ideation, study design, execution, data collection, analysis, and interpretation, or in all of these areas. They also all participated in writing, revising, or critically reviewing the article and gave their final approval before it was published. They also all agreed on the journal to which the article was submitted and agreed to be responsible for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Masson‐Meyers DS, Andrade TA, Caetano GF, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. doi:10.1111/iep.12346
2. Nguyen DT, Orgill DP, Murphy GF. The pathophysiologic basis for wound healing and cutaneous regeneration. In: Biomaterials for Treating Skin Loss. Woodhead Publishing; 2009:25–57.
3. Nahla Saeed IA Evaluation of gastro protective effect and wound healing potential of cibotium barometz and vitex pubecsens in an animal model/Nahla Saeed Ibraheem Alwajeeh Doctoral dissertation. University of Malaya; 2017.
4. Muluye AB, Desta AG, Abate SK, et al. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar J. 2019;18:261. doi:10.1186/s12936-019-2887-7
5. Atkin L. Understanding methods of wound debridement. Br J Nurs. 2014;23(12):S10–S5. doi:10.12968/bjon.2014.23.sup12.S10
6. García-Villén F, Faccendini A, Aguzzi C, et al. Montmorillonit- norfloxacin nanocomposite intended for healing of infected wounds. Int J Nanomedicine. 2019;14:5051–5060. doi:10.2147/IJN.S208713
7. Hamdan S, Pastar I, Drakulich S, et al. Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci. 2017;3(3):163–175. doi:10.1021/acscentsci.6b00371
8. Agyare C, Boakye YD, Bekoe EO, Hensel A, Dapaah SO, Appiah T. African medicinal plants with wound healing properties. J Ethnopharmacol. 2016;11(177):85–100. doi:10.1016/j.jep.2015.11.008
9. Gedif T, Hahn HJ. The use of medicinal plants in self-care in rural central Ethiopia. J Ethnopharmacol. 2003;87(2–3):155–161. doi:10.1016/S0378-8741(03)00109-0
10. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in kilte awulaelo district, tigray region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):1–23. doi:10.1186/1746-4269-9-65
11. Tekle Y. An ethno-veterinary botanical survey of medicinal plants in Kochore district of Gedeo zone, southern nations nationalities and peoples regional state (SNNPRs), Ethiopia. J Sci Innov Res. 2014;3(4):433–445. doi:10.31254/jsir.2014.3407
12. Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–28. doi:10.1186/s13002-015-0014-6
13. Abew B, Sahile S, Moges F. In vitro antibacterial activity of leaf extracts of Z. scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus. Asian Pac J Trop Biomed. 2014;4(10):816–820. doi:10.12980/APJTB.4.201414B16
14. Agogbua J, Ekeke C, Okoli BE. Morpho-anatomical characters of Zehneria capillacea (Schumach) C. Jeffrey and Z. scabra (LF) Sond Cucurbitaceae. Afr J Plant Sci. 2015;9(12):457–465. doi:10.5897/AJPS2015.1306
15. Murthy KS, Ravindranath D, Sandhya RS, Pullaiah T. Ethnobotany and distribution of wild and cultivated genetic resources of Cucurbitaceae in the Eastern Ghats of Peninsular India. Top Class J Herbal Med. 2013;2(6):149–158.
16. Ragunathan M, Abay SM. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, Northwestern Ethiopia; 2009.
17. Nureye D, Tekalign E, Fisseha N, Tesfaye T, Hammeso WW. Evaluation of antiplasmodial activity of hydroalcoholic crude extract and solvent fractions of Z. scabra roots against plasmodium berghei in Swiss albino mice. Infect Drug Resist. 2021;14:2583. doi:10.2147/IDR.S314262
18. Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC, USA: National Academies Press; 2010.
19. Tekleyes B, Huluka SA, Wondu K, Wondmkun YT. Wound healing activity of 80 percent methanol leaf extract of Z. scabra (Lf) sond (cucurbitaceae) in mice. J Exp Pharmacol. 2021;13:537. doi:10.2147/JEP.S303808
20. Beshir K. Evaluation of wound healing activity of 70 percent ethanol leaf extract of becium grandiflorum lam. (Lamiaceae) in Mice. Addis Ababa, Ethiopia: Addis Ababa University; 2017.
21. Geetha TS, Geetha N. Phytochemical screening, quantitative analysis of primary and secondary metabolites of Cymbopogan citratus (DC) Stapf. Leaves from Kodaikanal Hills, Tamilnadu. Int J Pharmtech Res. 2014;6(2):521–529.
22. Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2(4):1–4. doi:10.4172/2161-1009.1000144
23. Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):341. doi:10.1186/s12906-015-0878-y
24. Belachew TF, Asrade S, Geta M, Fentahun E. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of hagenia abyssinica (Bruce) J.F. Gmel in Mice. Evid-Based Complement Altern Med. 2020;2020:1–12. doi:10.1155/2020/9645792
25. Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of vernonia auriculifera hiern (asteraceae) in mice. J Exp Pharmacol. 2021;13:677. doi:10.2147/JEP.S308303
26. Ambikar D, Tsegaw A, Belayneh YM, Belayneh YM. Wound healing activity of 80 percent methanolic crude extract and solvent fractions of the leaves of justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in Mice. J Exp Pharmacol. 2022;14:167. doi:10.2147/JEP.S340177
27. Upadhyay A, Chattopadhyay P, Goyary D, Mitra Mazumder P, Veer V. Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. ISRN Pharmacol. 2014;2014:w356. doi:10.1155/2014/751824
28. Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646. doi:10.1016/j.jep.2012.12.002
29. Assefa A, Urga K, Melaku D, Guta M, Mekonnen W. Bronchodilator and anti-inflammatory activities of Adhatoda schimperiana. Ethiop J Health Sci. 2008;18:2.
30. Robert JN, Els N, Danny EC, Petra GB, Klaske N, Paul L. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi:10.1093/ajcn/74.4.418
31. Mekonnen NA, Atnafie AS, Atta WM. Evaluation of the anti-inflammatory activities of 70 percent ethanol leaves extract and solvent fractions of Z. scabra (Lf) Sond (Cucurbitaceae) in rodents. Evid-Based Complement Altern Med. 2020;2020:11.
32. Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48(4):461–468. doi:10.3109/13880200903173593
33. Sagbo IJ, Afolayan AJ, Bradley G. Antioxidant, antibacterial and phytochemical properties of two medicinal plants against the wound infecting bacteria. Asian Pac J Trop Biomed. 2017;7(9):817–825. doi:10.1016/j.apjtb.2017.08.009
34. Ali A, Alam S, Ahmad S, Ali S, Ahsan M, Siddiqui W. Wound healing activity of alcoholic extract of Tamarix aphylla L. on animal models. Biomed Pharmacol J. 2019;12(1):41–48. doi:10.13005/bpj/1611
35. Arun M, Satish S, Anima P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna J Phytome. 2016;6(3):295.
36. Samanta R, Pattnaik AK, Pradhan KK, Mehta BK, Pattanayak SP, Banerjee S. Wound healing activity of silibinin in mice. Pharmacognosy Res. 2016;8(4):298. doi:10.4103/0974-8490.188880
37. Samejo MQ, Sumbul A, Shah S, Memon SB, Chundrigar S. Phytochemical screening of Tamarix dioica Roxb. ex Roch. J Pharm Res. 2013;7(2):181–183. doi:10.1016/j.jopr.2013.02.017
38. Institute of Laboratory Animal Resources (US). Committee on Care, Use of Laboratory Animals. In: Guide for the Care and Use of Laboratory Animals. US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.