Back to Journals » Medical Devices: Evidence and Research » Volume 8
In vivo measurements of regional hemoglobin oxygen saturation values and limb-to-arm ratios of near-infrared spectroscopy for tissue oxygenation monitoring of lower extremities in healthy subjects
Authors Boezeman R, Kelder J, Waanders F, Moll F, de Vries J
Received 22 August 2014
Accepted for publication 1 October 2014
Published 29 December 2014 Volume 2015:8 Pages 31—36
DOI https://doi.org/10.2147/MDER.S73103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Reinout PE Boezeman,1 Johannes C Kelder,2 Frans GJ Waanders,3 Frans L Moll,4 Jean-Paul PM de Vries1
1Department of Vascular Surgery, St Antonius Hospital, Nieuwegein, the Netherlands; 2Department of Research and Developments, St Antonius Hospital, Nieuwegein, the Netherlands; 3Department of Perfusion, St Antonius Hospital, Nieuwegein, the Netherlands; 4Department of Vascular Surgery, University Medical Center Utrecht, Utrecht, the Netherlands
Objective: Near-infrared spectroscopy (NIRS) is a noninvasive technique that allows monitoring of regional hemoglobin oxygen saturation (rSO2) values and might have a role in the diagnosis of peripheral arterial disease. We assessed the reproducibility and inter-subject variability of rSO2 values and rSO2 limb-to-arm ratios (LARs) in lower extremities of healthy subjects.
Methods: The rSO2 values and rSO2 LARs were calculated in eight healthy subjects without peripheral arterial disease. The rSO2 values were measured at rest at six fixed spots at each lower limb and a reference spot at each upper arm. NIRS provided the rSO2 values without involvement of any other processing technique. After measurements were completed, rSO2 LARs were calculated by dividing the rSO2 value of a lower extremity spot by the rSO2 value of the arm. Measurements were performed twice on 1 day and repeated on 4 different days.
Results: Mean coefficients of variation of measurements of rSO2 values and rSO2 LARs at the same spot in the same subject were respectively less than 6% and 8% for every measurement spot over time. Coefficients of variation of measurements at the same spot between different subjects were less than 15% and 19% for every measurement spot respectively.
Conclusion: NIRS is an easily applicable, noninvasive tool for measurement of tissue oxygenation of lower extremities in healthy subjects. The reproducibility of rSO2 values and rSO2 LARs at the same measurement spot in the same subject is good.
Keywords: NIRS, rSO2, lower extremities, tissue oxygenation
Introduction
None of the noninvasive diagnostic methods available today has been able to measure tissue oxygenation accurately at each desired spot of the lower limb. Ankle-brachial indices (ABIs), toe-brachial indices, computed tomography arteriographies, magnetic resonance arteriographies, and digital subtraction angiographies are used in daily vascular practice, but do not specifically measure tissue oxygenation at the transtibial and femoral level, or, for instance, around ischemic ulcers.1–4 Near-infrared spectroscopy (NIRS) is a noninvasive method that monitors the regional hemoglobin oxygen saturation (rSO2) of several cubic cm of tissue underneath the NIRS optode.5 It can be applied to the skin at most of the spots of the upper and lower extremities. Therefore, rSO2 values at rest and rSO2 limb-to-arm ratios (LARs) might be a useful tool to determine lower limb oxygenation and might have a role in the diagnosis of peripheral arterial disease (PAD). Only if NIRS has good reproducibility and low inter-subject variability it can be used to compare tissue oxygenation between subjects.
Recently, research groups focused on NIRS measurements for monitoring peripheral oxygenation, but most studies focused on individual rSO2 curves and did not compare rSO2 values between patients.6–9 The subject of this study concerns the assessment of reproducibility and inter-subject variability of rSO2 values and rSO2 LARs in lower extremities of healthy subjects. Assessment of the reproducibility and inter-subject variability of NIRS measurements is essential before its widespread clinical use for monitoring lower limb oxygenation.
Methods
Subjects
NIRS measurements were performed in eight healthy subjects (four males and four females). The eight subjects had a mean age of 37 years with a standard deviation (SD) of 15 years. None of the healthy subjects suffered from cardiovascular disease, pulmonary disease or diabetes mellitus. All had ABI ≥1 at rest and no clinical complaints of PAD.
The NIRS technique
NIRS is a noninvasive technique that determines the degree of oxy-hemoglobin saturation in tissue sampled under the optode.10 The arterial and venous contribution to the total signal has been determined for cerebral measurements and is estimated at a 1:3 ratio, respectively.11 The measurements in this study were performed with an INVOS 4100 system (Somanetics Corp, Troy, MI, USA) with software version 11.16.16 provided by Covidien (Mansfield, MA, USA). The INVOS optode consists of a light source and two receiving detectors. The light source consists of two light-emitting diodes generating wavelengths of light at 730 and 810 nm (red and near-infrared light, respectively). The first photodetector is separated from the light source by a distance of 3 cm, and the second photodetector by a distance of 4 cm. The maximum penetration depth of photons passing through the sampled tissue from source to the photodetectors is approximately 2 cm.12
NIRS calculates the absorption of photons by oxygenated and deoxygenated hemoglobin molecules. The light absorption spectra of oxygenated and deoxygenated hemoglobin are different in the near-infrared region, and therefore the relative concentration of oxygenated hemoglobin can be determined.6,13
Experimental protocol
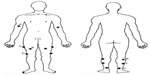
All healthy subjects were asked to lie supine for 30 minutes before the rSO2 values were measured. The measurement spots were the same for each subject and are shown in Figure 1. The rSO2 values were measured at rest and while supine at one reference spot on each upper arm (biceps 8 cm proximally of elbow joint [A]) and at six fixed spots at each lower limb (rectus femoris 15 cm distally of major trochanter [B], vastus lateralis 10 cm distally of major trochanter [C] and 5 cm proximally of knee joint [D], gastrocnemius lateralis 5 cm distally of knee joint [E] and 15 cm proximally of ankle joint [F] and soleus 5 cm proximally of ankle joint [G]). The duration of each measurement was 30 seconds. Measurements at the 14 spots were performed twice on 1 day and were repeated on 4 different days by the same observer. To ensure accurate replication of the measurement spot during each measurement, measurement spots were marked with a waterproof marker. The NIRS probe was attached to the extremities with an elastic bandage enfolded loosely around the extremity. All measurements were performed in the same examination room at a room temperature of 20°C. This study is approved by the investigational review board of St Antonius Hospital, Nieuwegein. Written informed consent was obtained from all subjects.
Statistical analysis
Measurements at the same spot in the same subject over time
The mean, the SD, and the coefficient of variation (CV) of rSO2 values and rSO2 LARs of ten measurements were calculated for every measurement spot of all eight subjects. CVs were calculated by dividing the SD by the mean, expressed in %. The mean of eight mean rSO2 values and rSO2 LARs (average mean), the mean of eight SDs and the mean of eight CVs of all eight subjects were calculated for every measurement spot.
Measurements at the same spot between different subjects
The SD and the CV of eight mean rSO2 values and rSO2 LARs of all eight subjects were calculated for every measurement spot.
At each spot on the lower extremity, an rSO2 LAR was calculated by dividing the rSO2 value of a lower extremity spot by the rSO2 value of the arm (reference spot), analogous to the ABI. This study can be considered a pilot study with the main objective being “to describe”. This means that there was no a priori goal for the width of the confidence interval and we could not make an educated guess about the intra- and inter-individual variability due to a lack of data in literature. This precluded a formal power calculation. However, for a general impression when describing a mean of a normally distributed variable with a confidence level of 95% and an acceptable difference of 50% of the mean and assuming a CV of 50%, the required sample size would be six. To be safe, we chose to measure eight subjects at ten times on 14 measuring points each. Measurements within subjects and between subjects appeared to be normally distributed after evaluation by means of visual inspection. NIRS showed in three (of the four) women no rSO2 value for point B (rectus femoris) and point C (proximal vastus lateralis) in several measurements. Therefore, these measurement spots were excluded in the results of all eight subjects.
Results
Measurements at the same spot in the same subject over time
The mean, the SD, and the CV of rSO2 values and rSO2 LARs of ten measurements of the first subject are listed for every measurement spot as an example in Table 1. The mean of eight CVs of all eight subjects was calculated per measurement spot, resulting in mean CVs ranging from 3.6% to 5.6% (only lower limb measurement spots) for rSO2 values and from 4.6% to 7.2% for rSO2 LARs, as shown in Table 2.
Measurements at the same spot between different subjects
The CV of eight mean rSO2 values of all eight subjects was calculated per measurement spot, resulting in CVs ranging from 6.0% to 14.3% (only lower limb measurement spots), as shown in Table 3. The CV of eight mean rSO2 LARs of all eight subjects was also calculated per measurement spot, resulting in CVs ranging from 8.3% to 18.0%, as shown in Table 3.
Discussion
The NIRS technique has already been investigated for monitoring peripheral oxygenation, but these studies dealt with individual rSO2 curves. Assessment of the reproducibility and inter-subject variability of rSO2 values and the rSO2 LARs for the lower extremities is essential to define the accuracy of NIRS measurements and to interpret NIRS outcomes. Besides, it is important to know whether rSO2 values can be compared between individuals like the ABI, or toe-brachial index.
This study showed that reproducibility of NIRS is good among healthy subjects with mean CVs of measurements at the same spot in the same subject over time ranging from 3.6% to 5.6% for rSO2 values and from 4.6% to 7.2% for rSO2 LARs. For example, this means that subject one may have an rSO2 value of 65% 1 day and 70% another day. CVs of measurements at the same spot between different subjects ranged from 6.0% to 14.3% for rSO2 values and from 8.3% to 18.0% for rSO2 LARs. These results suggest that NIRS seems to be less suitable for comparing lower limb oxygenation between individuals with rSO2 values and rSO2 LARs. Ubbink and Koopman14 already reported excellent reproducibility of NIRS measurements in 20 healthy subjects at the calf muscle (intra-class correlation coefficient at rest of 0.91) but moderate reproducibility at the dorsum of the foot (intra-class correlation coefficient at rest of 0.53), using an InSpectra Tissue Spectrometer Model 325. Komiyama et al15 found excellent reproducibility of a Shimadzu OM-220 device at the calf muscle (intra-class correlation coefficients at rest of 0.88) in eleven patients with claudication. Cooper et al16 provided results concerning the inter-variability of NIRS measurements. In 90 healthy subjects, a mean of 62.9% was found with an SD of 5.9% for the vastus lateralis, using a NIRO-200 device.
An explanation for the widespread inter-individual variation of rSO2 values is that the local tissue composition of the sampled tissue for each individual differs by the fat-to-muscle ratio, heterogeneity of skeletal muscle tissue and adipose tissue, scarring, and collagen etc. Cooper et al16 and Geraskin et al17 showed that an increase of thickness of adipose tissue of a measurement spot results in increase of oxygen saturation. Inter-variability of tissue composition also means that the amount of light scattering and therefore optical pathlengths differ per measurement spot. This makes quantification of hemoglobin molecules very difficult.10,18,19 LARs were calculated to decrease the influence of inter-individual tissue variability. Unfortunately, LARs did not improve the widespread inter-individual variation of NIRS results. Moving the optode from one measurement spot to another in an individual may give different rSO2 values even if oxygenation is normal. The differences in rSO2 values of the same measurement spots between individuals and the differences in rSO2 values between measurement spots in the same individual might rather be dependent on tissue composition and its variability in scattering and thus variability in sampling volume than on the actual local muscle tissue oxygenation.
This study has limitations. NIRS values could not be determined in three of four women at the rectus femoris (B) and proximal vastus lateralis (C), probably because women comprise more adipose tissue at these measurement spots. Spots at the rectus femoris and proximal vastus lateralis might therefore not be used in clinical practice for monitoring peripheral tissue oxygenation. Second, we did not measure adipose tissue thickness of the measurement spots. However, we created rSO2 LARs to correct for variability of adipose tissue thickness. Unfortunately, LARs did not improve the widespread inter-individual variation of NIRS results. Algorithms are currently being developed to account for adipose tissue thickness during SO2 measurements with NIRS.20
Future perspectives
Future NIRS studies of lower extremities might focus on individual trend measurement. A future application of NIRS might be to survey effects of percutaneous transluminal angioplasty and bypass surgery on lower limb oxygenation peri-procedurally. This can be of help to determine the extent of lower limb revascularization procedures for treatment of critical limb ischemia including ulcers. Another role of NIRS could be to use it as a continuous postoperative surveillance method of complex peripheral bypass surgery to detect early postoperative failure. Vardi and Nini6 already described the added value of NIRS measurements to asses PAD in patients using trend measurement.
Conclusion
NIRS is an easily applicable, noninvasive tool for monitoring tissue oxygenation of the lower extremities in individuals. The reproducibility and inter-subject variability of rSO2 values and rSO2 LARs have been assessed at the lower extremities in healthy subjects. The reproducibility of rSO2 values and rSO2 LARs at the same measurement spot in the same subject are good.
Disclosure
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article. The authors received no financial support for the research and/or authorship of this article.
References
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–S67. | |
Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128(20):2241–2250. | |
Peach G, Griffin M, Jones KG, Thompson MM, Hinchliffe RJ. Diagnosis and management of peripheral arterial disease. BMJ. 2012;345:e5208. | |
Begelman SM, Jaff MR. Noninvasive diagnostic strategies for peripheral arterial disease. Cleve Clin J Med. 2006;73 Suppl 4:S22–S29. | |
Kooijman HM, Hopman MT, Colier WN, van der Vliet JA, Oeseburg B. Near infrared spectroscopy for noninvasive assessment of claudication. J Surg Res. 1997;72(1):1–7. | |
Vardi M, Nini A. Near-infrared spectroscopy for evaluation of peripheral vascular disease. A systematic review of literature. Eur J Vasc Endovasc Surg. 2008;35(1):68–74. | |
Harel F, Denault A, Ngo Q, Dupuis J, Khairy P. Near-infrared spectroscopy to monitor peripheral blood flow perfusion. J Clin Monit Comput. 2008;22(1):37–43. | |
Bonaros N, Wiedemann D, Nagiller J, et al. Distal leg protection for peripheral cannulation in minimally invasive and totally endoscopic cardiac surgery. Heart Surg Forum. 2009;12(3):E158–E162. | |
Boezeman RP, Kelder JC, Waanders FG, de Vries JP. Continuous surveillance of lower limb perfusion during aortic surgery with near-infrared spectroscopy: A pilot study. Vasc Endovascular Surg. 2011;45(5):407–411. | |
Simonson SG, Piantadosi CA. Near-infrared spectroscopy. clinical applications. Crit Care Clin. 1996;12(4):1019–1029. | |
Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Stoddart HF. Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg. 1996;82(2):269–277. | |
Homma S, Fukunaga T, Kagaya A. Influence of adipose tissue thickness on near infrared spectroscopic signal in the measurement of human muscle. J Biomed Opt. 1996;1(4):418–424. | |
Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput. 2000;16(3):191–199. | |
Ubbink DT, Koopman B. Near-infrared spectroscopy in the routine diagnostic work-up of patients with leg ischaemia. Eur J Vasc Endovasc Surg. 2006;31(4):394–400. | |
Komiyama T, Onozuka A, Miyata T, Shigematsu H. Oxygen saturation measurement of calf muscle during exercise in intermittent claudication. Eur J Vasc Endovasc Surg. 2002;23(5):388–392. | |
Cooper CE, Penfold SM, Elwell CE, Angus C. Comparison of local adipose tissue content and SRS-derived NIRS muscle oxygenation measurements in 90 individuals. Adv Exp Med Biol. 2010;662:177–181. | |
Geraskin D, Boeth H, Kohl-Bareis M. Optical measurement of adipose tissue thickness and comparison with ultrasound, magnetic resonance imaging, and callipers. J Biomed Opt. 2009;14(4):044017. | |
Matcher SJ, Cooper CE. Absolute quantification of deoxyhaemoglobin concentration in tissue near infrared spectroscopy. Phys Med Biol. 1994;39(8):1295–1312. | |
Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by near-infrared spectroscopy. Eur J Vasc Surg. 1994;8(3):294–296. | |
Grieger S, Geraskin D, Steimers A, Kohl-Bareis M. Analysis of NIRS-based muscle oxygenation parameters by inclusion of adipose tissue thickness. Adv Exp Med Biol. 2013;789:131–136. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.