Back to Journals » International Journal of Nanomedicine » Volume 12
In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery
Authors Pokrowiecki R , Zaręba T, Szaraniec B, Pałka K, Mielczarek A, Menaszek E, Tyski S
Received 27 December 2016
Accepted for publication 24 February 2017
Published 6 June 2017 Volume 2017:12 Pages 4285—4297
DOI https://doi.org/10.2147/IJN.S131163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Rafał Pokrowiecki,1,2 Tomasz Zaręba,3 Barbara Szaraniec,4 Krzysztof Pałka,5 Agnieszka Mielczarek,6 Elżbieta Menaszek,7 Stefan Tyski3,8
1Center for Cranio-Maxillo-Facial Surgery, Voivodeship Children’s Hospital, Olsztyn, 2Department of Oral Surgery, Jagiellonian Medical University, Kraków, 3Department of Antibiotics and Microbiology, National Medicines Institute, Warsaw, 4Faculty of Material Science and Ceramics, AGH University of Science and Technology, Kraków, 5Department of Materials Engineering, Lublin University of Technology, Lublin, 6Department of Conservative Dentistry, Medical University of Warsaw, Warsaw, 7Department of Cytobiology, Collegium Medicum, Jagiellonian University, Kraków, 8Department of Pharmaceutical Microbiology, Medical University of Warsaw, Warsaw, Poland
Abstract: The addition of an antibacterial agent to dental implants may provide the opportunity to decrease the percentage of implant failures due to peri-implantitis. For this purpose, in this study, the potential efficacy of nanosilver-doped titanium biomaterials was determined. Titanium disks were incorporated with silver nanoparticles over different time periods by Tollens reaction, which is considered to be an eco-friendly, cheap, and easy-to-perform method. The surface roughness, wettability, and silver release profile of each disc were measured. In addition, the antibacterial activity was also evaluated by using disk diffusion tests for bacteria frequently isolated from the peri-implant biofilm: Streptococcus mutans, Streptococcus mitis, Streptococcus oralis, Streptococcus sanguis, Porphyromonas gingivalis, Staphylococcus aureus, and Escherichia coli. Cytotoxicity was evaluated in vitro in a natural human osteoblasts cell culture. The addition of nanosilver significantly increased the surface roughness and decreased the wettability in a dose-dependent manner. These surfaces were significantly toxic to all the tested bacteria following a 48-hour exposure, regardless of silver doping duration. A concentration of 0.05 ppm was sufficient to inhibit Gram-positive and Gram-negative species, with the latter being significantly more susceptible to silver ions. However, after the exposure of human osteoblasts to 0.1 ppm of silver ions, a significant decrease in cell viability was observed by using ToxiLight™ BioAssay Kit after 72 hours. Data from the present study indicated that the incorporation of nanosilver may influence the surface properties that are important in the implant healing process. The presence of nanosilver on the titanium provides an antibacterial activity related to the bacteria involved in peri-implantitis. Finally, the potential toxicological considerations of nanosilver should further be investigated, as both the antibacterial and cytotoxic properties may be observed at similar concentration ranges.
Keywords: biomaterials, dental plaque, peri-implantitis, peri implant mucositis, silver, nanotechnology, nanomedicine
Introduction
In the last 40 years, titanium oral implants have become a commonly used alternative to fixed and removable dentures. However, peri-implant infections (PIIs) still form a threat for a successful treatment.1,2 PIIs are characterized by the inflammatory destruction of the implant-supporting tissues, as a result of biofilm formation on the surface of the implant.3,4 Dental implants are at an increased risk of contamination because the oral cavity is a dynamic ecosystem continuously colonized by microorganisms. In addition, peri-implant tissues are susceptible to infection due to the restricted blood supply, the presence of scar tissue, and the lack of periodontal space at the implant–tissue interface.5 The formation of this biofilm, commonly known as dental plaque, is initiated by oral streptococci and followed by cocci, motile bacilli, and spirochetes.6,7 Inflammation leads to progressive shifts in plaque composition, which gradually accumulates anaerobic bacteria of high virulence. Porphyromonas gingivalis, Escherichia coli, and Staphylococcus aureus are usually involved in advanced infections.8,9 These species cause the resorption of circumfluent bone and hence may result in implant loss.10 At present, there is no reliable and well-defined treatment protocol available for already-established infections.
A recent approach uses the antimicrobial properties of medical implants, which is of great interest. However, the criteria for a material that could resist bacterial attachment, especially when used in the oral cavity, have yet to be fully defined.11 In the last decade, silver nanoparticles (SNPs) have gained much attention in biomedical industry. It was assumed that SNPs, when used in controlled amounts, exhibited significant antimicrobial properties with almost no toxic effects to the host tissue. Therefore, it was expected that SNPs would be used in a broad range of applications.12 Many studies regarding SNPs have been published,13–18 although there has been little discussion on their application in oral implantology.19 Most of the published works lack in comprehensive evaluation of the properties of the described materials, such as roughness,15,17,20 wettability,15,17,21,22 or biocompatibility.14–17 Moreover, antibacterial activities in oral applications have been evaluated only in a few studies.16,19
The aim of the present work was to assess the impact of SNPs on titanium surface properties and to verify the antimicrobial properties and the biocompatibility of such biomaterials in order to evaluate their potential for application in oral implantology.
Materials and methods
Titanium sample preparation, scanning electron microscopy (SEM), and surface elemental analysis
In this study, commercially available pure titanium disks were used (Grade 2; 10×1 mm; Torresin Titanio srl, Limena, Italy). The samples were ultrasonically cleaned with 70% ethanol and distilled water and then sterilized in an autoclave. The disks were modified by double acid etching in a mixture of 5.4 mL 40% hydrofluoric acid, 9 mL 65% nitric acid, and 81.5 mL H2O solutions (Avantor Performance Materials, LLC., Center Valley, PA, USA) for 5 min to increase the surface roughness (Control Group 1 – K1).
Then, some samples were immersed in a 10 M solution of sodium hydroxide (Avantor Performance Materials Poland S.A., Gliwice, Poland) for 24 hours at 60°C to develop a nanometric, hydrophilic surface topography (Control Group 2 – K2).
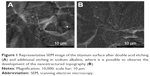
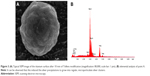
In order to incorporate the SNPs, the K2 samples were immersed in Tollens reagent formula (50 mL of 0.1 M AgNO3, conc. NH4OH, 25 mL of 0.8 M KOH, 5 mL of dextrose solution) for 30 s and 2, 5, and 10 minutes, respectively. The disks were rinsed with distilled water and dried. The samples containing SNPs were named according to the duration of the silver deposition process. Table 1 describes the disks used in the present study. The SEM analysis (Nova NanoSEM™; FEI, Hillsboro, OR, USA) and surface chemical analysis (energy dispersive spectroscopy; EDAX Phoenix system, Mahwah, NJ, USA) at an accelerating voltage of 18 kV were performed.
  | Table 1 Description of the titanium disks used in the study |
Surface roughness and wettability
The surface morphology of the sample surfaces was measured by using a surface profilometer (Tester T 4000, Hommelwerke GmbH, VS-Schwenningen, Germany). The surface roughness (right angle [Ra]) was measured at various locations on the disks (n=15). The average values and standard deviations (SDs) were calculated.
The surface wettability was measured with a sessile drop technique by using a 0.20-μL droplet size (DSA10; KRÜSS GmbH, Hamburg, Germany). The measurements of contact angle (CA; Θc) values were taken at various locations on the disks (n=15), and the average values and SDs were calculated.
Silver release
In order to examine the release of Ag+ from the surface of the titanium disks, the specimens were immersed in 10 mL of phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 7.0 mM Na2HPO4×2 H2O, 1.5 mM KH2PO4 ) at 37°C. The buffer solution was replaced at subsequent time intervals and analyzed by inductively coupled plasma mass spectroscopy (Optima 7300DV, Perkin Elmer, Waltham, MA, USA). The data were expressed as the mean ± SD from 3 measurements.
Antibacterial assay
A periodontal pathogen collection was used as a source of three clinical isolates of each of the following: Streptococcus mutans, Streptococcus salivarius, Streptococcus oralis, Streptococcus mitis, S. aureus, E. coli, and P. gingivalis.23 Each clinical strain of bacteria was named accordingly: Cl-1, Cl-2, or Cl-3.
The strains were stored at −80°C as per the requirement for the analysis. Prior to material testing, the streptococcal strains, S. aureus and E. coli, were subcultured twice on Columbia Blood Agar (CBA) with a 5% sheep blood (bioMérieux SA, Marcy l’Etoile, France) medium for 24–48 h at 37°C to ensure viability. Bacteria suspensions of 0.5 density units on the McFarland Scale, containing 1–2×108 colony-forming units/1 mL, were prepared and spread on the surfaces of the CBA plates. The titanium disks were placed on the inoculated plates and incubated under aerobic conditions at 37°C for 48 hours. P. gingivalis were spread on the Schaedler agar with hemin, vitamin K1, and 5% sheep blood (bioMerieux). The plates were incubated at 37°C under anaerobic conditions by using a GENbag analyzer (bioMerieux) for 48 hours.
The antibacterial activity was evaluated by measuring the zones of bacterial growth inhibition (ZIs) around the titanium disks (Kirby–Bauer diffusion tests). A total of 7 measurements were taken for each clinical strain cultured in the presence of each sample.
Cell culture
For the cell culture studies, the samples were washed in 70% ethanol and sterilized with ultraviolet irradiation (30 minutes for each side). The sterile samples were placed onto the wells of 48-well culture plates (Nunc, Thermo Fisher Scientific, Waltham, MA, USA). The natural human osteoblasts (NHOs; Lonza Group, Basel, Switzerland) were routinely grown in 75-mL flasks (Nunc) in the complete culture medium, OGM BulletKit (Lonza) in a 37°C, humidified CO2 incubator (ThermoSci, Germany). The medium was changed every 2–3 days. Once a 70% confluent cell monolayer had developed, the cells were rinsed with PBS (GE Healthcare Life Sciences, Logan, UT, USA) and brought into suspension with 0.5% trypsin plus ethylenediaminetetraacetic acid (HyClone). Following trypsinization, the cells were washed and resuspended in a fresh culture medium at the concentration of 1.5×104 cells/mL. Next, 1 mL of the cell suspension was added to each well of a 48-well culture plate (Nunc) containing the sterile samples: K1, K2, and Ag0.5 (n=5). The culture studies were conducted for 3 or 7 days at 37°C in 5% CO2 and a 95% air atmosphere.
Cytotoxicity assay
In order to determine the cytotoxicity and proliferation of the NHO cells (Lonza) in contact with the K1, K2, and Ag0.5 titanium disks, a ToxiLight™ BioAssay Kit and ToxiLight™ 100% Lysis Reagent Set (Lonza) were used according to the manufacturer’s instructions. Cytotoxicity was expressed in relation to the total cell number. The readings of bioluminescence were taken by using a microplate reader, POLARstar Omega (BMG Labtech, Ortenberg, Germany).
Cell morphology
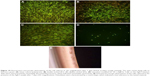
The titanium disks were stained with 0.01% acridine orange in PBS for 1 min and washed with PBS; then, the cell cultures were observed under a fluorescence microscope at 10× magnification to evaluate their density and morphology (Olympus CX41; Olympus Corporation, Tokyo, Japan).
Statistical analysis
All numerical data were analyzed by using the STATISTICA PL 12 software. The results of the roughness, wettability, and antibacterial assays were statistically analyzed by using the analysis of variance and Tukey’s honest significant difference. Any significant effects of cytotoxicity were determined by using an unpaired Student’s t-test. A P-value of <0.05 was considered statistically significant in all the tests.
Results
Surface morphology and elemental analysis
The alkaline etching produced a nanotopography, which was not obtained on the samples treated with double acid etching (Figure 1A and B). The titanium disks modified with Tollens for 30 seconds showed single granular silver structures of <500 nm across with a uniform morphology (Figure 2A–C). When the surface was treated with Tollens for ≥2 minutes, closely spaced silver clusters were developed (Figure 3A and B), increasing the amount of silver (Figure 4).
Surface roughness and wettability
Figure 5 shows the Ra values of the tested samples. The differences between the specimens were statistically significant (P<0.000). A significant increase in surface roughness associated with silver incorporation was observed (P=0.000; Figure 5).
Figure 6 shows the CA values of the tested samples. The differences between the specimens were statistically significant (P=0.000). Only the Ag0.5 samples preserved a highly hydrophilic surface (14°±1.6°) in comparison with K1 (P=0.000) and K2 (P=0.999). When Tollens was applied for ≥2 minutes, significant hydrophobization and progressing CA hysteresis were observed (P=0.000).
Silver release
Figure 7A shows the noncumulative Ag+ release profile from the tested samples. In general, the largest amounts of silver ions were released initially, with a burst release (BR) after 6 hours of immersion. Then, the number of ions decreased gradually, finally reaching a steady value at 24 hours of immersion. The Ag10 samples exhibited a considerable BR at 6 hours of immersion in comparison with the other titanium disks. These samples also exhibited steady release just after 72 hours of incubation (Figure 7A). The Ag10 disks were also characterized by a significant release rate (Figure 7B) and the total concentration of the released silver (Figure 7C). The samples with more Ag+ on the surface exhibited a more significant BR and total accumulation of silver in the medium.
Antibacterial assay
Figure 8A–G shows the ZIs after 48 hours of incubation. Only Ag-containing disks provided sufficient antibacterial properties, compared with the K1 and K2 samples (P=0.000). However, the differences between the tested pairs of Ag-modified disks were statistically insignificant (P=1; Figure 9A).
The differences in growth inhibition between the bacterial species were significant (P<0.05; Figure 9B). Gram-negative bacteria were more susceptible to nanosilver than Gram-positive bacteria (P<0.05). The inhibition of P. gingivalis was most pronounced, in comparison with other species (P<0.05) except E. coli (P=1). In the group of Gram-positive bacteria, S. aureus was the most susceptible to SNPs (P<0.05). The ZI of these bacteria was significantly wider than those of S. mutans (P=0.000) and S. oralis (P=0.028). In the streptococcal subgroup, S. sanguis exhibited the largest inhibition zones, whereas S. mutans was the most resistant to SNPs. However, the differences between the tested pairs of streptococci (P>0.05; Figure 9B) and the clinical strains (P=1; Figure 9C) were not statistically significant.
Cytotoxicity assay
After 3 and 7 days of culturing, the K1 and K2 samples and the positive control (tissue culture polystyrene [TCPS]) were fully settled by viable cells. The osteoblasts were spindle-like, polygonal, elongated, and spread out, generally with numerous filamentous extensions at the leading edges, which indicated good tolerance to the tested materials (Figure 10A–C). Good tolerance was not observed in the Ag0.5 samples (Figure 10D). The cells formed agglomerates consisting of individual cells that formed compact clusters with a round shape (Figure 10E).
The analysis of cell proliferation and cytotoxicity after 3 and 7 days demonstrated significant differences between the tested materials. After 7 days of incubation, the total cell number was significantly increased on the TCPS (P=0.000), K1 (P=0.002), and K2 (P=0.000) samples (Figure 11A). This indicated the good tolerance of NHO cells to the tested materials and confirmed cell proliferation. In the samples modified with Tollens, no cell proliferation was observed (P=0.996; Figure 9A). The cytotoxicity of K1, K2, and TCPS did not exceed 10%. In contrast, the titanium disks containing Ag were significantly cytotoxic to osteoblasts: 88% after 3 days and >90% after 7 days (Figure 11B).
Discussion
The aim of the present work was to examine the potential role of SNPs as an antibacterial additive to titanium biomaterials for oral and maxillofacial implantology. Recently, several methods of silver deposition have been described, including ion implantation, electrochemical ion exchange, sol–gel, sputtering, and plasma spray.24
The Tollens reaction was used in the present study as it is considered eco-friendly, simple, and cost-effective. The addition of silver resulted in the formation of silver precipitations, which were uniform, but heterogeneously displaced on the surface. These were irregular in shape and <500 nm in size when immersed in the Tollens solution for 30 seconds. These results were consistent with the current literature, as Tollens-mediated AgNP synthesis provides average particle size of 25–450 nm.25 However, the longer the specimens were immersed in the solution, the larger the silver particles on the surface became. After an immersion period of 10 minutes, the silver was present in the regular clusters of sizes exceeding 2 μm. These observations were congruent with the current literature. The size of the particles was related to ammonia concentrations, pH conditions, differences in the structure of the reducing agent, pH,25 the addition of magnesium salts, and buffering conditions,26 as well as the reaction time.27
Silver incorporation had a remarkable influence on the surface properties. The data indicated that the addition of SNPs significantly increased the titanium surface roughness and decreased its wettability, leading to an increase in CA hysteresis. The impact of the SNPs on the surface roughness after SNP deposition was described by Mei et al,28 Cabal et al,16 and Ewald et al.22 A decrease in surface roughness was described by Nakajo et al.14 No differences were noticed by Zhao et al29 and Li et al.30 An increase in surface wettability associated with nanosilver incorporation was described by Mei et al28 and Unosson et al,13 although Gao et al did not observe significant changes in surface wettability after silver incorporation.20 In the present study, the silver formed clusters resembling microparticulate silver, and this probably increased the surface hydrophobicity, as in the study by Kasraei and Azarsina,31 and resulted in the increase of surface roughness. The incorporation method influences the size and location of the formed silver granular structures.
Roughness is a major parameter influencing implant osseointegration. It is well known that rough surfaces increase bone-to-implant contact.32 However, rough surfaces are also more likely to facilitate biofilm formation in the oral cavity;33 therefore, a significant increase in surface roughness is not desirable, especially for the trans-gingival parts of an implant. The aspect of surface wettability is regarded as the second most relevant factor in the dynamics of cell adhesion to the surface.34,35 Some authors have described hydrophobic surfaces as accumulating more bacterial plaque than hydrophilic surfaces.36,37 The explanation given for this phenomenon is usually based on the simplified principles of thermodynamics related to adhesion forces calculated with the Derjaguin and Landau, Verwey, and Overbeek theory.38 Most species of oral bacteria possess hydrophobic cell walls and can easily adhere to single-sign surfaces. However, the extensive heterogeneity of the microbial population phylotypes in the oral cavity should be taken into consideration, including their ability to switch from hydrophobic to hydrophilic properties in response to the changes in the environment.39,40 Recent studies indicated that human osteoblasts and fibroblasts adhere more sufficiently to hydrophilic surfaces. In the present study, the samples modified with Tollens reagents for 30 seconds were hydrophilic, which seemed beneficial in terms of osseointegration. The surface hydrophobization observed after longer immersion should be considered unfavorable for both implant integration and biofilm formation. Moreover, the significant roughness that accelerates biofilm formation may neutralize any potential beneficial effect of surface wettability. Therefore, the effect of SNPs on the roughness and wettability of a material should be meticulously evaluated, as these are crucial factors influencing implant integration with bone and gingiva.41,42
The activity of SNPs in relation to microbes has been comprehensively discussed in numerous studies and reviews.12,43–45 The accumulation and dissolution of nanoparticles leading to the disruption of the bacterial membrane and release of bacterial intracellular biomolecules have also been discussed.46,47 Another mechanism is based on the generation of reactive oxygen species and damage to cellular structures, unregulated cell signaling, changes in cell motility, and apoptosis.48,49 The uptake of nanoparticles by bacterial cells also leads to the depletion of intracellular adenosine triphosphate production, disruption of DNA replication, and damage.47 Moreover, the exposure of bacteria to the silver ions released from nanoparticles leads to cellular respiration arrest and renders them in a state defined as “active, but nonculturable” in which they fail to grow.44,47
The titanium disks containing SNPs provided antibacterial properties against all clinical strains of the bacteria. The toxicity was not related to the duration of silver incorporation, as all the samples treated with Tollens were comparably antibacterial. The concentration of 0.05 ppm of silver ions released from the samples modified for 30 s was sufficient to exhibit antibacterial properties in the Kirby–Bauer diffusion test after 48 h of bacterial exposure. These results were in agreement with those from the study by Morita et al, where the concentration of 0.043 ppm sufficiently inhibited oral streptococci and P. gingivalis.19 In this study, P. gingivalis, E. coli, and S. aureus appeared the most susceptible to SNPs. Oral streptococci were the least susceptible to nanosilver, with S. mutans being the most resistant, but the overall differences in growth inhibition among streptococcal group were not statistically significant. Morita et al observed wider ZIs in the streptococcal group compared with P. gingivalis. The authors clarified that Gram-negative bacteria were more resistant to SNPs due to their slower metabolism. Consequently, they suggested that this was due to the decreased accumulation of silver ions in bacteria cells.19 In the present study, Gram-negative bacteria were more susceptible to silver ions, which was in agreement with the results of other researchers.50,51 The higher activity of SNPs against Gram-negative bacteria was most probably caused by the differences in the thicknesses of the peptidoglycan layer.44 This layer may form an obstacle to the silver ions acting through the bacterial cell wall and is significantly thinner in Gram-negative species. This is promising, as advanced PIIs are commonly caused by mixed, anaerobic microbiota.52 No significant differences were also shown in the susceptibility to silver ions between the clinical species of bacteria. The findings from the present study were consistent with those of the study of Cabal et al, where two clinical isolates of S. oralis were comparably inhibited by soda-lime glass containing SNPs. However, in their study, materials with nanosilver proved to be antiadhesive.16 In the present study, the disks with SNPs were antibacterial, which is even more favorable in terms of PII pathobiology.
Both the antiadhesive and antimicrobial effects are related to silver ion release,15 which is often excessive and uncontrolled in the first hours/days of incubation in the medium. This phenomenon, known as BR, was observed during the 6th hour of the incubation. The silver release rate, total ions released, and BR were related to the percentage weight of the silver in a dose-dependent manner. Silver BR from titanium was also observed in the study by Mei et al28 and Unosson et al.13 It is probably related to the methods used for the nanosilver incorporation, which do not provide stable ion release.24 Zhao et al suggested that a certain nanotopography could help to prevent excessive ion leakage.29 In contrast, Zhang et al proposed that the level of hydrophobicity protected the material from excessive silver release.53 Nevertheless, no reliable technique for Ag+ release stabilization has been proposed so far. BR may, however, provide high concentrations of antibacterial agent in the peri-implant space over a short period of time. On the other hand, it could contribute to the cytotoxicity of the material observed in the present study. The total amount of silver ions did not exceed 0.03 ppm after 24 hours of immersion in PBS and 0.1 ppm after 72 hours. The latter was sufficient to induce cytotoxicity in the range up to 88% in the NHO cell line. SNPs were described as an innovative antibacterial agent with a relatively insignificant toxicity.43 Panacek et al reported that the concentration of silver ions >30 ppm is exclusively cytotoxic to human fibroblasts;54 based on this, Cabal et al determined their materials as biocompatible.16 In their study, the total concentration of Ag+ after 24 hours of immersion in PBS was only 7 ppm. However, they did not confirm their conclusions regarding biocompatibility in a cell culture assay.16 The materials described by Morita et al released 0.04 ppm of silver ions after 24 hours of immersion in PBS,19 although they did not observe any cytotoxic effects in the human gingival fibroblast cell line. On the other hand, the cells were cultured for 24 hours. It is well established that silver uptake by cells is dependent on incubation time;55 therefore, such short culturing could be insufficient to observe any toxic effect in the cell line.19 In another study, Ferraris et al observed some cytotoxic effects in human osteosarcoma cell cultures already >0.15 ppm,56 which is far less than the safe dose proposed in the other studies. The cytotoxicity of SNPs is attributed to several mechanisms, including the dissolving of ions from the nanoparticles, disruption of cell membrane integrity, oxidative stress, protein or DNA binding and damage, generation of reactive oxygen species, and apoptotic cell death.27,57 It also depends on the nanoparticle properties, such as surface area, size and shape, capping agent, surface charge, purity, structural distortion bioavailability,58 and dissolution.59 Moreover, the mechanisms of toxicity induced by the nanosilver are connected with the type of cells used in the study.60 The cytotoxicity of nanomaterials containing Ag− evaluated in vitro may also show other effects related to protein adsorption, metal ion release, and suspension stability.61 Nanoparticles are exposed to different environments in living organisms from those mentioned in in vitro studies. Unbound SNPs are surrounded by proteins in the body fluid, which form complexes known as protein coronas (PCs)52 and are used in phagocytic cells. When the PC is degraded in the cell, it releases the toxic Ag+-damaging DNA, hindering cell respiration and causing cell death.62,63 It is possible that the salivary proteins in oral cavity may also form a corona on the SNPs, which would have an effect on both the bactericidal effects (via ionic Ag dissolution) and cellular effects. Therefore, the interaction of SNPs or silver ions with the other compounds and elements in human saliva should be better evaluated,64 especially as the increased production of materials containing nanoparticles presents an important toxicological concern.65,66
The results obtained in the present study showed that the materials modified with SNPs have a time-limited ion release. Similar results were obtained by Ferraris et al.56 Such materials will most probably exhibit antibacterial properties in the first weeks/months after implantation, until the silver concentration provides minimal bactericidal concentrations. The present study also proved that the antibacterial and cytotoxic effects of SNPs may occur at similar concentration ranges that were observed in the study by Greulich et al.12
Conclusion
The most significant element of the work presented here was the discovery and discussion of the potential blind spots of nanosilver-modified biomaterials for oral implantology. For the first time, three different clinical strains of seven bacterial species have been comprehensively tested. The titanium doped with SNPs via Tollens reaction exhibited an antibacterial activity against bacteria frequently isolated from infected peri-implant sites. A concentration of 0.05 ppm of silver ions was sufficient to obtain this effect, although prolonged silver deposition may significantly affect the surface properties. The longer the silver incorporation process, the more the increase in roughness and hydrophobicity; this relativity is probably unfavorable, as bacteria in the oral cavity are more likely to adhere to rough and hydrophobic surfaces. It was also observed that a concentration of 0.1 ppm of silver ions was highly toxic to NHOs, and this concentration is far less than the previously proposed safe dose of SNPs. Therefore, more studies are required to tailor a sufficient, prolonged silver release that provides antibacterial properties without harmful effects to the surrounding tissues in the oral cavity.
Acknowledgments
This work was jointly supported by Statutory Research 11.11.160.616 of the Faculty of Material Science and Ceramics, AGH University of Science and Technology, Kraków, Poland, PhD grant (No K/DSC/003105, 2015–2016), Jagiellonian University, Collegium Medicum, Kraków, Poland and KNOW research grant Jagiellonian University, Collegium Medicum, Kraków, Poland. The authors would like to thank Andrzej Stanisz, MSc, for the statistical analysis and also Prof Frank A Witzmann for the consultations regarding the formation of SNP PC in the oral cavity.
Disclosure
The authors report no conflicts of interest in this work.
References
Persson GR, Renvert S. Cluster of bacteria associated with peri-implantitis. Clin Implant Dent Relat Res. 2014;16(6):783–793. | ||
Sakka S, Baroudi K, Nassani MZ. Factors associated with early and late failure of dental implants. J Investig Clin Dent. 2012;3(4):258–261. | ||
Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282–285. | ||
Zhuang LF, Watt RM, Mattheos N, Si MS, Lai HC, Lang NP. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin Oral Implants Res. 2014;27(1):1–9. | ||
Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. | ||
Belibasakis GN. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch Oral Biol. 2014;59(1):66–72. | ||
Pye AD, Lockhart DEA, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72(2):104–110. | ||
Koyanagi T, Sakamoto M, Takeuchi Y, Maruyama N, Ohkuma M, Izumi Y. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol. 2013;40(3):218–226. | ||
Koyanagi T, Sakamoto M, Takeuchi Y, Ohkuma M, Izumi Y. Analysis of microbiota associated with peri-implantitis using 16S rRNA gene clone library. J Oral Microbiol. 2010;2:1–7. | ||
Romanos GE, Weitz D. Therapy of peri-implant diseases. Where is the evidence? J Evid Based Dent Pract. 2012;12(3 Suppl):204–208. | ||
Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–13880. | ||
Greulich C, Braun D, Peetsch A, et al. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012;2:6981–6987. | ||
Unosson E, Rodriguez D, Welch K, Engqvist H. Reactive combinatorial synthesis and characterization of a gradient Ag-Ti oxide thin film with antibacterial properties. Acta Biomater. 2015;11(1):503–510. | ||
Nakajo K, Takahashi M, Kikuchi M, et al. Inhibitory effect of Ti-Ag alloy on artificial biofilm formation. Dent Mater J. 2014;33(3):389–393. | ||
Fordham WR, Redmond S, Westerland A, et al. Silver as a bactericidal coating for biomedical implants. Surf Coatings Technol. 2014;253:52–57. | ||
Cabal B, Cafini F, Esteban-Tejeda L, et al. Inhibitory effect on in vitro Streptococcus oralis biofilm of a soda-lime glass containing silver nanoparticles coating on titanium alloy. PLoS One. 2012;7(8):e42393. | ||
Gasquères C, Schneider G, Nusko R, Maier G, Dingeldein E, Eliezer A. Innovative antibacterial coating by anodic spark deposition. Surf Coatings Technol. 2012;206(15):3410–3414. | ||
Liao J, Anchun M, Zhu Z, Quan Y. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int J Nanomedicine. 2010;5(1):337–342. | ||
Morita Y, Imai S, Hanyuda A, Matin K, Hanada N, Nakamura Y. Effect of silver ion coating of fixed orthodontic retainers on the growth of oral pathogenic bacteria. Dent Mater J. 2014;33(2):268–274. | ||
Gao A, Hang R, Huang X, et al. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35(13):4223–4235. | ||
Martinez A, Guitián F, López-Píriz R, et al. Bone loss at implant with titanium abutments coated by soda lime glass containing silver nanoparticles: a histological study in dogs. PLoS One. 2014;9(1):e86926. | ||
Ewald A, Glückermann SK, Thull R, Gbureck U, Lew D. Antimicrobial titanium/silver PVD coatings on titanium. Biomed Eng Online. 2006;5(1):22. | ||
Pokrowiecki R, Zareba T, Mielczarek A, et al. Ocena bakteriobójczej aktywności koloidalnego roztworu nanocząstek srebra w stosunku do bakterii próchnicotwórczych [Evaluation of the biocidal properties of silver nanoparticles against cariogenic bacteria]. Med Dosw Mikrobiol. 2013;65(3):197–206. Polish. | ||
Ferraris S, Spriano S. Antibacterial titanium surfaces for medical implants. Mater Sci Eng C Mater Biol Appl. 2016;61:965–978. | ||
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385–406. | ||
Dondi R, Su W, Griffith GA, Clark G, Burley GA. Highly size-and shape-controlled synthesis of silver nanoparticles via a templated Tollens reaction. Small. 2012;8(5):770–776. | ||
Behra R, Sigg L, Clift MJD, et al. Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective. J R Soc Interface. 2013;10(87):20130396. | ||
Mei S, Wang H, Wang W, et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35(14):4255–4265. | ||
Zhao B, van der Mei HC, Subbiahdoss G, et al. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater. 2014;30(7):716–727. | ||
Li B, Liu X, Meng F, Chang J, Ding C. Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings. Mater Chem Phys. 2009;118(1):99–104. | ||
Kasraei S, Azarsina M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz Oral Res. 2012;26(6):505–510. | ||
Jemat A, Ghazali MJ, Razali M, Otsuka Y. Surface modifications and their effects on titanium dental implants. Biomed Res Int. 2015;2015:791725. | ||
das Neves PBA, Agnelli JA, Kurachi C, de Souza CW. Addition of silver nanoparticles to composite resin: effect on physical and bactericidal properties in vitro. Braz Dent J. 2014;25(2):141–145. | ||
Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8(4):270–280. | ||
Gittens RA, Scheideler L, Rupp F, et al. A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater. 2014;10(7):2907–2918. | ||
Thewes N, Loskill P, Jung P, et al. Hydrophobic interaction governs unspecific adhesion of staphylococci: a single cell force spectroscopy study. Beilstein J Nanotechnol. 2014;5:1501–1512. | ||
Yamada Y, Yamada M, Ueda T, Sakurai K. Reduction of biofilm formation on titanium surfaces with ultraviolet-C pre-irradiation. J Biomater Appl. 2014;29(2):161–171. | ||
Boks NP, Norde W, van der Mei HC, Busscher HJ. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology. 2008;154(10):3122–3133. | ||
Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:112. | ||
Gallardo-Moreno AM, Navarro-Pérez ML, Vadillo-Rodríguez V, Bruque JM, González-Martín ML. Insights into bacterial contact angles: difficulties in defining hydrophobicity and surface Gibbs energy. Colloids Surf B Biointerfaces. 2011;88(1):373–380. | ||
Sartoretto SC, Alves ATNN, Resende RFB, Calasans-Maia J, Granjeiro JM, Calasans-Maia MD. Early osseointegration driven by the surface chemistry and wettability of dental implants. J Appl Oral Sci. 2015;23(3):279–287. | ||
Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Br Dent J. 2015;218(5):E9. | ||
Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chemie Int Ed. 2013;52(6):1636–1653. | ||
Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(7):2171–2178. | ||
Sadeghi B, Garmaroudi FS, Hashemi M, et al. Comparison of the anti-bacterial activity on the nanosilver shapes: nanoparticles, nanorods and nanoplates. Adv Powder Technol. 2012;23(1):22–26. | ||
Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32. doi:10.1186/2228-5326-2-32 | ||
Das B, Dash SK, Mandal D, et al. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem. In press 2015. | ||
Applerot G, Lellouche J, Lipovsky A, et al. Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small. 2012;8(21):3326–3337. | ||
Ikegami A, Chung P, Han YW. Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun. 2009;77(7):3075–3079. | ||
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668. | ||
Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. | ||
Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2(13):1599–1607. | ||
Zhang L, Zhang L, Yang Y, et al. Inhibitory effect of super-hydrophobicity on silver release and antibacterial properties of super-hydrophobic Ag/TiO2 nanotubes. J Biomed Mater Res B Appl Biomater. 2016;104(5):1004–1012. | ||
Panácek A, Kolár M, Vecerová R, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30(31):6333–6340. | ||
Lu W, Senapati D, Wang S, et al. Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem Phys Lett. 2010;487(1):92–96. | ||
Ferraris S, Venturello A, Miola M, Cochis A, Rimondini L, Spriano S. Antibacterial and bioactive nanostructured titanium surfaces for bone integration. Appl Surf Sci. 2014;311:279–291. | ||
McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–127. | ||
Zhang XF, Shen W, Gurunathan S. Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int J Mol Sci. 2016;17(10):pii:E1603. | ||
Ivask A, Kurvet I, Kasemets K, et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One. 2014;9(7):e102108. | ||
Sahu SC, Zheng J, Graham L, et al. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J Appl Toxicol. 2014;34(11):1155–1166. | ||
Horie M. The impact of the physiochemical properties of manufactured nanoparticles on in vitro and in vivo evaluation of particle toxicity. J Phys Chem Biophys. 2014;2(2):2–5. | ||
Shannahan JH, Brown JM, Chen R, et al. Comparison of nanotube-protein corona composition in cell culture media. Small. 2013;9(12):2171–2181. | ||
Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA. Silver nanoparticle protein corona composition in cell culture media. PLoS One. 2013;8(9):e74001. | ||
Medina C, Inkielewicz-Stepniak I, Santos-Martinez MJ, Radomski MW. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int J Nanomedicine. 2014;9:1677–1687. | ||
Armstead AL, Li B. Nanotoxicity: emerging concerns regarding nanomaterial safety and occupational hard metal (WC-Co) nanoparticle exposure. Int J Nanomedicine. 2016;11:6421–6433. | ||
Sierra MI, Valdés A, Fernandez AF, Torrecillas R, Fraga MF. The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome. Int J Nanomedicine. 2016;11:6297–6306. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.